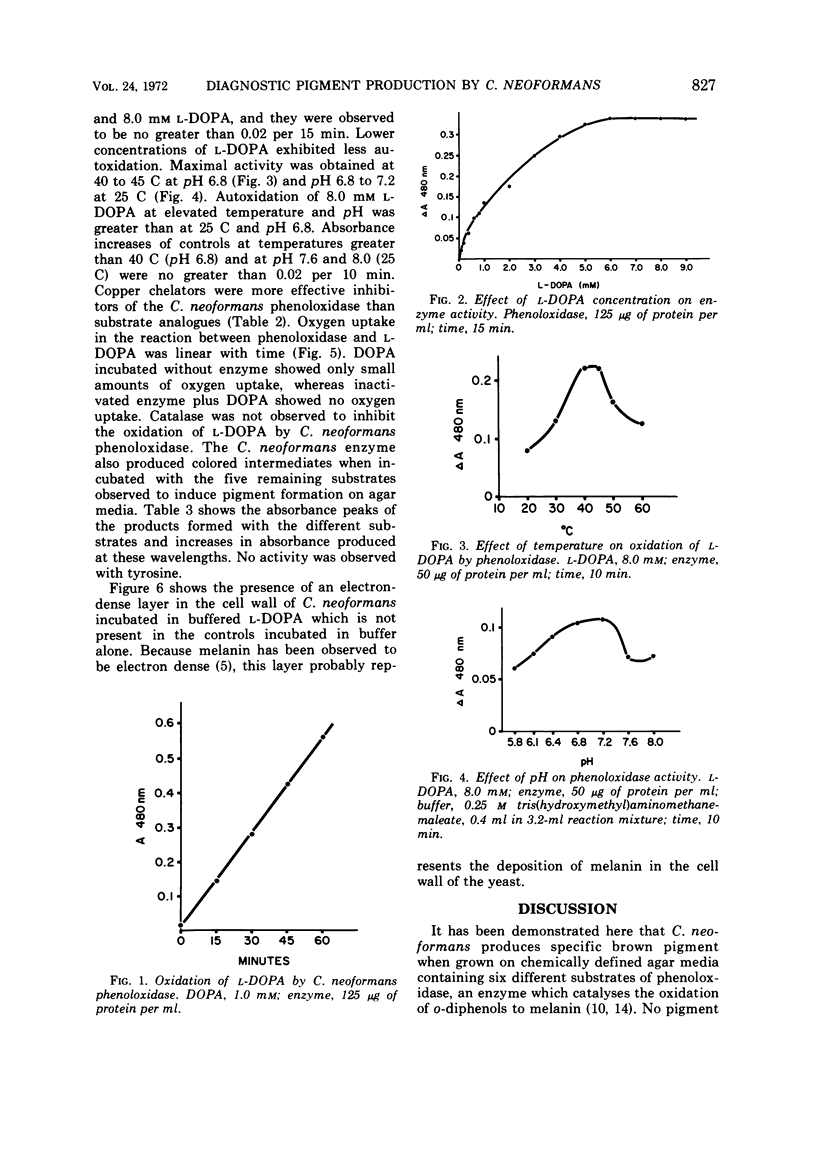
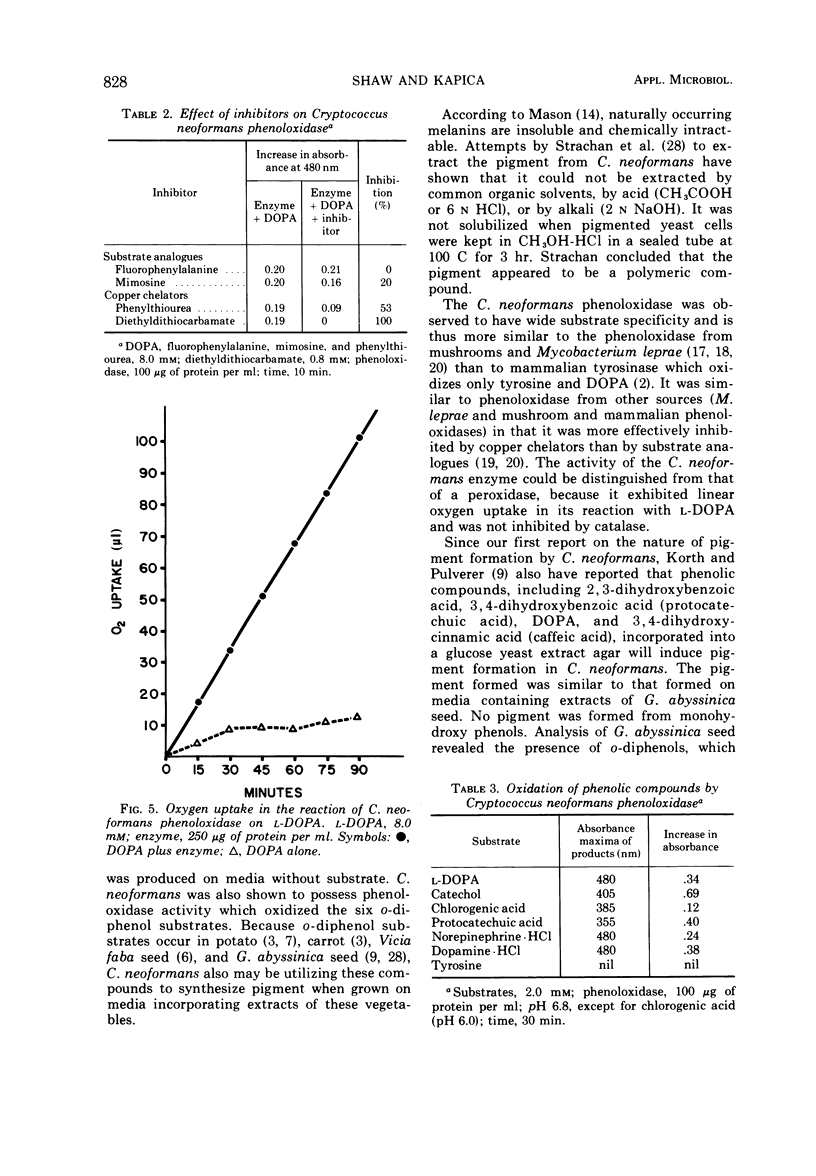
Abstract
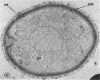
Cryptococcus neoformans produces brown pigmented colonies when grown on agar media made from an extract of potatoes and carrots, broad beans (Vicia faba), or Guizotia abyssinica seeds. Since other yeasts do not produce the pigment, these media are useful as differential isolation media for C. neoformans. Similar specific pigment was produced by C. neoformans on chemically defined agar media which contained six different substrates of phenoloxidase (o-diphenol: oxygen oxidoreductase EC 1.10.3.1) an enzyme which catalyses the oxidation of o-diphenols to melanin. Substrates were incorporated singly into the media and included L-3, 4-dihydroxyphenylalanine (L-DOPA), chlorogenic acid, protocatechuic acid, catechol, norepinephrine, and 3-hydroxytyramine hydrochloride (dopamine). No pigment was produced on media without substrate. Phenoloxidase activity in (NH4)2SO4 precipitates of C. neoformans cell-free extract was assayed by measuring increases in absorbance at 480 nm produced in solutions of L-DOPA. This reaction showed oxygen uptake and was effectively inhibited by copper chelators, but not by catalase. The enzyme also oxidized the five other substrates which induced pigment formation. Electron micrographs of cells incubated in L-DOPA showed deposition of the pigment in the cell wall.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botard R. W., Kelley D. C. Modified Littman oxgall agar to isolate Cryptococcus neoformans. Appl Microbiol. 1968 May;16(5):689–690. doi: 10.1128/am.16.5.689-690.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R., Gordon M. A., Lapa E. W., Ghiorse W. C. Micromorphology of Cryptococcus neoformans. J Bacteriol. 1967 Sep;94(3):766–777. doi: 10.1128/jb.94.3.766-777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G., Schaal L. A. Relation of Chlorogenic Acid to Scab Resistance in Potatoes. Science. 1952 Jun 6;115(2997):627–629. doi: 10.1126/science.115.2997.627. [DOI] [PubMed] [Google Scholar]
- Korth H., Pulverer G. Pigment formation for differentiating Cryptococcus neoformans from Candida albicans. Appl Microbiol. 1971 Mar;21(3):541–542. doi: 10.1128/am.21.3.541-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERNER A. B. Metabolism of phenylalanine and tyrosine. Adv Enzymol Relat Subj Biochem. 1953;14:73–128. doi: 10.1002/9780470122594.ch3. [DOI] [PubMed] [Google Scholar]
- MASON H. S. Comparative biochemistry of the phenolase complex. Adv Enzymol Relat Subj Biochem. 1955;16:105–184. doi: 10.1002/9780470122617.ch3. [DOI] [PubMed] [Google Scholar]
- MacMillan P. C., Brandt W. H. Possible role of a peroxidative system in melanin synthesis. Antonie Van Leeuwenhoek. 1966;32(2):202–211. doi: 10.1007/BF02097462. [DOI] [PubMed] [Google Scholar]
- McVeigh I., Morton K. Nutritional studies of Histoplasma capsulatum. Mycopathol Mycol Appl. 1965 Apr 14;25(3):294–308. doi: 10.1007/BF02049917. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K., Harris E. B., Kirchheimer W. F. Effect of inhibitors on phenoloxidase of Mycobacterium leprae. J Bacteriol. 1969 Nov;100(2):935–938. doi: 10.1128/jb.100.2.935-938.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K., Kirchheimer W. F., Harris E. B. Oxidation of phenolic compounds by Mycobacterium leprae and inhibition of phenolase by substrate analogues and copper chelators. J Bacteriol. 1968 Jun;95(6):2051–2053. doi: 10.1128/jb.95.6.2051-2053.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K., Kirchheimer W. F. Use of 3,4-Dihydroxyphenylalanine Oxidation in the Identification of Mycobacterium leprae. J Bacteriol. 1966 Oct;92(4):1267–1268. doi: 10.1128/jb.92.4.1267-1268.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K. Phenoloxidase of Mycobacterium leprae. Nature. 1967 Jul 22;215(5099):436–437. doi: 10.1038/215436a0. [DOI] [PubMed] [Google Scholar]
- STAIB F. MEMBRANFILTRATION UND NEGERSAAT (GUIZOTIA ABYSSINICA)-NAEHRBODEN FUER DEN CRYPTOCOCCUS NEOFORMANS-NACHWEIS (BRAUNFARBEFFEKT) Z Hyg Infektionskr. 1963 Oct 25;149:329–336. [PubMed] [Google Scholar]
- Shields A. B., Ajello L. Medium for selective isolation of Cryptococcus neoformans. Science. 1966 Jan 14;151(3707):208–209. doi: 10.1126/science.151.3707.208. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Staib F., Seeliger H. P. Un nouveau milieu sélectif pour l'isolement de C. neoformans des matières fécales et du sol. Ann Inst Pasteur (Paris) 1966 May;110(5):792–793. [PubMed] [Google Scholar]
- Strachan A. A., Yu R. J., Blank F. Pigment production of Cryptococcus neoformans grown with extracts of Guizotia abyssinica. Appl Microbiol. 1971 Sep;22(3):478–479. doi: 10.1128/am.22.3.478-479.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER PLOEG M., VAN DUIJN THE INFLUENCE OF PEROXIDASES ON THE DOPA-SYSTEM. J R Microsc Soc. 1964 Dec;83:405–414. doi: 10.1111/j.1365-2818.1964.tb00558.x. [DOI] [PubMed] [Google Scholar]
- WIEBECKE B., STAIB F. GENERALISIERTE CRYPTOKOKKOSE. Munch Med Wochenschr. 1965 Feb 19;107:361–365. [PubMed] [Google Scholar]