Abstract
Background
We have recently reported that the susceptibility vessel sign (SVS) at the proximal portion of the horizontal (M1) middle cerebral artery (MCA) on T2*-weighted MRI is a strong predictor for no early recanalization after intravenous recombinant tissue plasminogen activator (t-PA) therapy. However, it is unclear whether the presence of the SVS at other locations, such as distal M1, the vertical portion (M2) of the MCA, and distal branches (MCA distal), is a predictor for no early recanalization in acute ischemic stroke patients.
Methods
The SVS was defined as a hypointense signal of the MCA on T2*-weighted MRI on admission. The locations of the SVS were classified as M1 proximal, M1 distal, and MCA distal. M1 proximal SVS was defined as an SVS at the origin of the M1. M1 distal SVS was any M1 SVS not including the origin of the M1. MCA distal SVS was an SVS further away from M1. Early recanalization was defined as a new appearance of at least one of the distal branches on MRA within 1 h after t-PA therapy. A good outcome at 3 months was defined as a modified Rankin Scale (mRS) score of 0-1.
Results
Consecutive acute stroke patients admitted to our stroke center and treated with t-PA between October 2005 and October 2012 were enrolled. There were 158 patients [median age, 78 (71-84) years; 84 (53%) males; median National Institutes of Health Stroke Scale score, 16 (10-20)]. Internal carotid artery occlusion was seen in 18 (72%) of the 25 patients with M1 proximal SVS, in 3 (14%) of the 22 patients with M1 distal SVS, in 4 (9%) of the 44 patients with MCA distal SVS, and in 18 (27%) of the 67 patients with No SVS (p < 0.001). Twenty-four (96%) of the 25 patients with M1 proximal SVS had no early recanalization, while 16 (73%) of the 22 patients with M1 distal SVS, 25 (57%) of the 44 patients with MCA distal SVS, and 36 (54%) of the 67 patients with No SVS had no early recanalization (p < 0.001, 0.140, and 0.846, respectively, compared to the patients with No SVS). Multivariate analysis showed that only M1 proximal SVS was significantly associated with no early recanalization (odds ratio 16.80, 95% confidence interval 2.04-138.17, p = 0.009). Among the 95 patients with a premorbid mRS score of 0-1, none (0%) of the 16 patients with M1 proximal SVS, 5 (36%) of the 14 patients with M1 distal SVS, 12 (48%) of the 25 patients with MCA distal SVS, and 13 (33%) of the 40 patients with No SVS achieved a good outcome (p = 0.011, 1.000, and 0.295, respectively, compared to the patients with No SVS).
Conclusion
M1 proximal SVS on T2*-weighted MRI is a strong predictor for no early recanalization, and all patients with it had a poor outcome. However, M1 distal SVS and MCA distal SVS were not predictors for no early recanalization, and half of the patients had a poor outcome.
Key Words : Acute ischemic stroke, Susceptibility vessel sign, Thrombolysis
Introduction
The aim of the intravenous recombinant tissue plasminogen activator (t-PA) therapy for acute stroke is to recanalize the occluded artery and reperfuse the penumbral tissue. Early recanalization, defined as recanalization during the first 1-2 h of t-PA therapy, is expected to triple or quadruple the favorable outcome rate [1,2,3]. In patients without early recanalization, intra-arterial intervention is a promising approach. The RECANALISE (Recanalisation Using Combined Intravenous Alteplase and Neurointerventional Algorithm for Acute Ischemic Stroke) study showed that combined t-PA therapy and intra-arterial intervention was associated with higher recanalization than t-PA alone, and a better outcome was associated with time from symptom onset to recanalization [4].
We have recently reported that the susceptibility vessel sign (SVS) seen on T2*-weighted MRI as a hypointense signal at the proximal portion of the horizontal (M1) middle cerebral artery (MCA) served as the only independent factor related to no early recanalization and a poor outcome [5,6]. Thus, we considered that patients with M1 proximal SVS are exactly those who need immediate combined intravenous and intra-arterial treatment. In our previous reports, however, we did not evaluate the impact of the SVS at other locations, such as distal M1, the vertical portion (M2) of the MCA, and distal branches, on early recanalization and patient outcomes. Although some studies reported patients' characteristics and radiological findings based on different SVS sites [7], their effects on early recanalization and clinical outcome remain unknown.
The aim of the present study was to investigate, whether the impact of the SVS on early recanalization and patient outcomes differs based on their MCA location in patients treated with t-PA.
Methods
Consecutive patients with acute ischemic stroke and treated with t-PA admitted to our stroke center between October 2005 and October 2012 were prospectively enrolled; t-PA therapy was generally administered based on the Japan Alteplase Clinical Trial [8,9]. In addition, in our hospital, acute ischemic stroke patients with unknown onset time were also treated with t-PA based on diffusion-weighted imaging (DWI)/fluid-attenuated inversion recovery (FLAIR) mismatch [10]. The interval from onset to t-PA therapy was automatically calculated as 180 min in those patients. Only patients with an occlusion of the internal carotid artery (ICA), M1, and M2 were enrolled in the present study. A single t-PA dose of 0.6 mg/kg (not exceeding 60 mg) was administered intravenously, with 10% given as a bolus, followed by a continuous infusion of the remainder of the dose over 1 h. The institutional Review Board at the Kawasaki Medical School approved this study.
The following clinical information was obtained: age, sex, time from onset to treatment, neurological deficits, modified Rankin Scale (mRS) score before stroke, vascular risk factors, atrial fibrillation, past antithrombotic medication, blood pressure before intravenous thrombolysis, stroke etiology, and the presence of cardiac sources of emboli. Neurological deficits were assessed using the National Institutes of Health Stroke Scale (NIHSS) score before thrombolysis. Vascular risk factors were identified as: (1) hypertension, a history of using antihypertensive agents, a systolic blood pressure ≥140 mm Hg, or a diastolic blood pressure ≥90 mm Hg at hospital discharge; (2) diabetes mellitus, the use of hypoglycemics, random glucose level ≥200 mg/dl, or glycosylated hemoglobin >6.4% on admission, and (3) hyperlipidemia, the use of antihyperlipidemic agents or serum cholesterol level >220 mg/dl. Medications taken before admission were also documented. Anticoagulation was performed using warfarin sodium. Aspirin, ticlopidine, clopidogrel, and cilostazol were given as antiplatelet therapy.
Stroke etiology was determined at hospital discharge using Trial of ORG 10172 in Acute Stroke Treatment criteria: (1) small-vessel occlusion, (2) large-artery atherosclerosis, (3) cardioembolism, or (4) other or undetermined etiology of stroke [11]. Duplex ultrasonography was performed in all patients to assess the common carotid artery and ICA. To determine the presence of cardiac sources of emboli, transcranial Doppler, transthoracic echocardiography, and Holter electrocardiography were also carried out. Transesophageal echocardiography and conventional cerebral angiography were performed if appropriate.
The MRI protocol included DWI, FLAIR, T2*-weighted MRI, and intracranial MRA, which were performed on a 1.5-tesla scanner (Signa EXCITE XL v. 11.0; GE Healthcare, Milwaukee, Wis., USA) on admission and 1 h after t-PA administration. DWI was obtained using the following parameters: repetition time (TR), 6,000 ms; echo time (TE), 78 ms; b values, 0 and 1,000 s/mm2; field of view, 24 cm; acquisition matrix, 128 × 192, and slice thickness, 6.0 mm with a 1.0-mm gap. FLAIR sequences were obtained in the axial plane using the following parameters: TR, 8,002 ms; TE, 109 ms; inversion time, 2,000 ms; field of view, 24 cm; acquisition matrix, 256 × 224, and section thickness, 6.0 mm with a 1.0-mm gap. T2*-weighted images were obtained using the following parameters: TR, 700 ms; TE, 20 ms; field of view, 24 cm; acquisition matrix, 256 × 192, and section thickness, 6.0 mm with a 1.0-mm gap. Time-of-flight MRA covered the circle of Willis with: TR, 25 ms; TE, 6.9 ms, and flip angle, 20°.
The SVS defined as a hypointense signal of the MCA on T2*-weighted MRI on admission was assessed by one vascular neurologist (J.A.) who was blinded to the clinical information. M1 proximal SVS was defined as an SVS at the origin of the M1. M1 distal SVS was any SVS not including the origin of the M1. MCA distal SVS was considered as an SVS further away from M1 (fig. 1). The Alberta Stroke Program Early CT Score on DWI on admission was also assessed. A semiquantitative evaluation using the Alberta Stroke Program Early CT Score on DWI has been shown to be a reproducible method similar to that on CT [12,13,14]. The infarct volume on the initial DWI was calculated using NIH image. First, the perimeter of the area of abnormal signal intensity was traced on each DWI image, and then the NIH image calculated the total volume using the thickness and the area delineated on the slice. The presence of recanalization was assessed using MRA 1 h after t-PA therapy. Recanalization was graded as complete, partial, or no recanalization as follows: (1) complete recanalization: reappearance of the entire occluded artery and distal branch of vessels; (2) partial recanalization: new appearance of at least one of the distal branches supplied by an occluded artery, and (3) no recanalization: persistent occlusion. For patients treated with intra-arterial intervention before follow-up MRI, digital subtraction angiography was used to assess recanalization. Early recanalization was evaluated before intra-arterial intervention started. Grade 2 and more according to the Thrombolysis in Cerebral Infarction grading system was defined as early recanalization [15].
Fig. 1.
Representative cases with an SVS at the proximal (a) and distal portions of the horizontal (M1) MCA (b), and at the distal portion of the MCA (c, d).
At 3 months, the presence of a good outcome (mRS score, 0-1) was assessed.
Statistical Analysis
First, all patients were classified into 4 groups based on the presence and location of the SVS as follows: (1) M1 proximal SVS, (2) M1 distal SVS, (3) MCA distal SVS, and (4) No SVS. Clinical, radiological, and laboratory findings were compared among the 4 groups. Second, all patients were divided into 2 groups based on the presence and absence of early recanalization. Multivariate logistic regression analysis was used to identify independent factors associated with early recanalization. Variables from univariate analyses with p values <0.1 were entered into the multivariate analysis. The relative risk of early recanalization was expressed as odds ratios with 95% confidence intervals. After that, clinical outcomes were evaluated among the 4 groups. The Mann-Whitney U test was used to analyze differences in continuous variables, and Fisher's exact test was used to analyze differences in categorical variables. The data are presented as median values (interquartile ranges) or frequencies (%). All statistical analyses were performed using IBM SPSS Statistics for Windows version 19 software (Chicago, Ill., USA). Results were considered significant at p < 0.05.
Results
From October 2005 to October 2012, 266 patients were admitted to our hospital and treated with t-PA therapy. Of the 266 patients, 43 had ICA occlusion, 69 had M1 occlusion, and 46 had M2 occlusion. Thus, 158 patients [median age, 78 (71-84) years; 84 (53%) males; median NIHSS score, 16 (10-20)] were enrolled into the present study. Of the 158 patients, 23 (15%) underwent intra-arterial intervention, and of these 23 patients, the presence of early recanalization was assessed by digital subtraction angiography in 4.
An SVS was seen in 91 (58%) of the 158 patients. M1 proximal SVS (M1 proximal group) was seen in 25 (16%) patients; M1 distal SVS (M1 distal group) in 22 (14%); MCA distal SVS (MCA distal group) in 44 (28%), and 67 (42%) patients did not have an SVS (No SVS group). The patients' clinical characteristics and imaging findings according to the SVS locations are shown in table 1. The NIHSS score of the M1 proximal group was the highest (p = 0.001). The incidence of hypertension was significantly different among the 4 groups (p = 0.037). Regarding the sites of arterial occlusions, ICA occlusion was frequent (72%) in the M1 proximal group (p < 0.001), M1 occlusion was frequent (86%) in the M1 distal group (p < 0.001), and M2 occlusion was frequent (52%) in the MCA distal group (p < 0.001). The No SVS group had ICA occlusion more frequently than the MCA distal group (27 vs. 9%, p = 0.028).
Table 1.
Clinical characteristics and imaging findings based on the presence and location of the SVS
| Variables | M1 proximal SVS | M1 distal SVS | MCA distal SVS | No SVS | p |
|---|---|---|---|---|---|
| (n = 25) | (n = 22) | (n = 44) | (n = 67) | ||
| Median age, years | 79 (73 – 83) | 76 (66 – 86) | 79 (74 – 84) | 77 (70 – 85) | 0.960 |
| Male sex, n | 14 (56) | 15 (68) | 21 (48) | 34 (51) | 0.431 |
| Median NIHSS score on admission | 20 (15 – 23) | 17 (12 – 22) | 13 (7 – 19) | 16 (10 – 20) | 0.001 |
| Premorbid mRS score 0 – 1, n | 20 (75) | 18 (82) | 32 (73) | 49 (73) | 0.771 |
| Interval from onset to thrombolysis, min | 153 (131 – 167) | 146 (120 – 167) | 145 (120 – 175) | 143 (115 – 172) | 0.894 |
| Intra-arterial intervention, n | 4 (16) | 4 (18) | 5 (11) | 10 (15) | 0.887 |
| Vascular risk factor, n | |||||
| Hypertension | 11 (44) | 14 (64) | 34 (77) | 47 (70) | 0.037 |
| Diabetes mellitus | 3 (12) | 3 (14) | 8 (18) | 18 (27) | 0.310 |
| Hyperlipidemia | 3 (12) | 6 (27) | 9 (21) | 15 (22) | 0.608 |
| Atrial fibrillation | 18 (72) | 14 (64) | 25 (57) | 35 (52) | 0.356 |
| Past medication, n | |||||
| Antiplatelet therapy | 7 (28) | 6 (27) | 8 (18) | 19 (28) | 0.648 |
| Anticoagulation therapy | 4 (16) | 2 (9) | 5 (11) | 5 (8) | 0.666 |
| Median systolic blood pressure, mm Hg | 152 (145 – 168) | 156 (138 – 167) | 147 (131 – 164) | 156 (139 – 170) | 0.334 |
| Median diastolic blood pressure, mm Hg | 82 (68 – 95) | 81 (70 – 98) | 81 (70 – 91) | 84 (70 – 92) | 0.860 |
| Stroke etiology, n | |||||
| Large-artery atherosclerosis | 4 (16) | 3 (14) | 2 (5) | 7 (10) | 0.430 |
| Cardioembolism | 18 (72) | 14 (64) | 28 (64) | 38 (57) | 0.587 |
| Other or undetermined | 3 (12) | 5 (23) | 14 (32) | 22 (33) | 0.205 |
| Portion of occlusion, n | |||||
| ICA | 18 (72) | 3 (14) | 4 (9) | 18 (27) | <0.001 |
| M1 | 7 (28) | 19 (86) | 17 (39) | 26 (39) | <0.001 |
| M2 | 0 (0) | 0 (0) | 23 (52) | 23 (34) | <0.001 |
| DWI findings | |||||
| Median ASPECTS | 7 (3 – 9) | 7 (5 – 9) | 8 (6 – 10) | 8 (6 – 10) | 0.095 |
| Median initial ischemic volume, ml | 12.3 (4.0 – 56.3) | 13.2 (2.1 – 42.2) | 4.1 (1.0 – 21.5) | 7.1 (1.6 – 35.8) | 0.091 |
| Early recanalization | |||||
| No | 24 (96) | 16 (73) | 25 (57) | 36 (54) | <0.001 |
| Partial | 1 (4) | 5 (23) | 12 (27) | 19 (28) | 0.088 |
| Complete | 0 (0) | 1 (4) | 7 (16) | 12 (18) | 0.071 |
| Clinical outcome at 3 months | |||||
| mRS score 0 – 1, n | 0/16 (0) | 5/14 (36) | 12/25 (48) | 13/40 (33) | 0.014 |
Figures in parentheses are percentages or interquartile ranges. M1 indicates the horizontal portion of the MCA, M2 the sylvian portion of the MCA. ASPECTS = Alberta Stroke Program Early Computed Tomography Score.
Early recanalization occurred in 57 (36%) patients (table 2). On univariate analysis, M1 proximal SVS was seen in 24 (24%) of the 101 patients with no early recanalization and in 1 (2%) of the 57 patients with early recanalization (p < 0.001). On the other hand, No SVS was more frequent in the early recanalization group than in the no early recanalization group (54 vs. 36%, p = 0.029). ICA occlusion was less frequently observed in patients in the early recanalization group than in those not in the early recanalization group (14 vs. 35%, p = 0.005).
Table 2.
Clinical characteristics and imaging findings between patients with early recanalization and those with no early recanalization
| Variables | Early recanalization (n = 57) | No early recanalization (n = 101) | p |
|---|---|---|---|
| Median age, years | 78 (73– 84) | 78 (71 – 84) | 0.710 |
| Male sex, n | 33 (58) | 51 (51) | 0.409 |
| Median NIHSS score on admission | 15 (10 – 20) | 17 (11 – 20) | 0.420 |
| mRS score 0 – 1, n | 41 (74) | 77 (76) | 0.848 |
| Interval from onset to thrombolysis, min | 132 (113 – 166) | 153 (125 – 170) | 0.107 |
| Intra-arterial intervention, n | 3 (5) | 20 (20) | 0.017 |
| Susceptibility vessel sign, n | |||
| M1 proximal | 1 (2) | 24 (24) | <0.001 |
| M1 distal | 6 (11) | 16 (16) | 0.474 |
| MCA distal | 19 (33) | 25 (25) | 0.271 |
| No SVS | 31 (54) | 36 (36) | 0.029 |
| Vascular risk factor, n | |||
| Hypertension | 19 (33) | 33 (33) | 1.000 |
| Diabetes mellitus | 45 (79) | 81 (80) | 0.840 |
| Hyperlipidemia | 48 (84) | 77 (76) | 0.309 |
| Atrial fibrillation | 27 (47) | 39 (39) | 0.316 |
| Past medication, n | |||
| Antiplatelet therapy | 14 (25) | 26 (26) | 1.000 |
| Anticoagulation therapy | 5 (9) | 11 (11) | 0.788 |
| Median systolic blood pressure, mm Hg | 132 (113 – 166) | 152 (136 – 167) | 0.406 |
| Median diastolic blood pressure, mm Hg | 80 (70 – 90) | 84 (71 – 96) | 0.256 |
| Stroke etiology, n | |||
| Large-artery atherosclerosis | 2 (4) | 14 (14) | 0.053 |
| Cardioembolism | 32 (56) | 66 (65) | 0.306 |
| Other or undetermined | 23 (40) | 21 (21) | 0.010 |
| Portion of occlusion, n | |||
| ICA | 8 (14) | 35 (35) | 0.005 |
| M1 | 30 (53) | 39 (39) | 0.097 |
| M2 | 19 (33) | 27 (27) | 0.466 |
| DWI findings | |||
| Median ASPECTS | 8 (5 – 10) | 8 (6 – 9) | 0.828 |
| Median initial ischemic volume, ml | 5.6 (1.0 – 39.2) | 9.4 (1.9 – 37.2) | 0.588 |
Figures in parentheses are percentages or interquartile ranges. M1 indicates the horizontal portion of the MCA, M2 the sylvian portion of the MCA. ASPECTS = Alberta Stroke Program Early Computed Tomography Score.
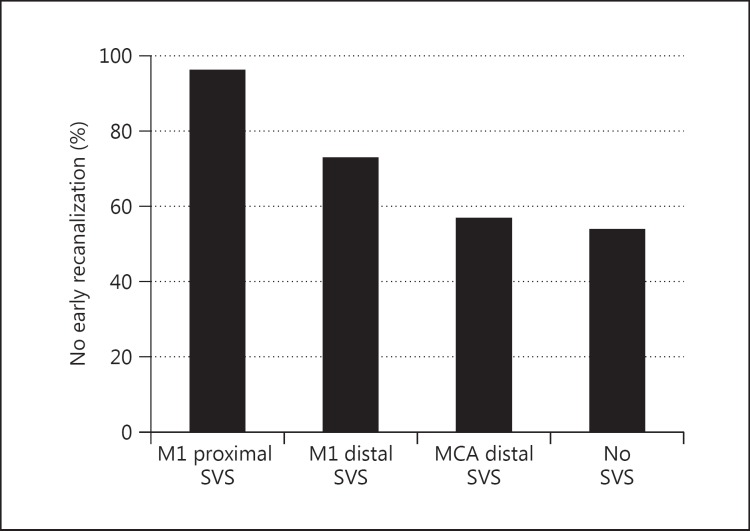
The rates of no early recanalization among the 4 groups are shown in figure 2. Twenty-four (96%) of the 25 patients in the M1 proximal group, 16 (73%) of the 22 patients in the M1 distal group, 25 (57%) of the 44 patients in the MCA distal group, and 36 (54%) of the 67 patients in the No SVS group had no early recanalization (p < 0.001). The frequency of no early recanalization was significantly higher in the M1 proximal group than in the M1 distal group, the MCA distal group, and the No SVS group (p = 0.040, 0.001, and <0.001, respectively). On the other hand, the rate of no early recanalization in the M1 distal group was not different from that in the MCA distal group and in the No SVS group (p = 0.284 and 0.140, respectively). No significant difference in the rate of no early recanalization was seen between the MCA distal group and the No SVS group (p = 0.846).
Fig. 2.
Incidences of no early recanalization based on the presence of the SVS.
Multivariate analysis showed that only M1 proximal SVS was significantly associated with no early recanalization after adjusting for M1 distal SVS, MCA distal SVS, stroke etiologies (large-artery atherosclerosis and other or undetermined), ICA occlusion, and M1 occlusion (odds ratio, 16.80; 95% confidence interval, 2.04-138.17; p = 0.009; table 3).
Table 3.
Multivariate logistic regression analysis for factors associated with no early recanalization
| Parameters | Odds ratio | 95% Confidence interval | p |
|---|---|---|---|
| Susceptibility vessel sign | |||
| M1 proximal | 16.80 | 2.04–138.17 | 0.009 |
| M1 distal | 2.93 | 0.92 – 9.34 | 0.070 |
| MCA distal | 1.23 | 0.55 – 2.79 | 0.615 |
| Stroke etiology | |||
| Large-artery atherosclerosis | 2.64 | 0.51 – 13.73 | 0.250 |
| Other or undetermined | 0.56 | 0.26 – 1.20 | 0.134 |
| Portion of the arterial occlusion | |||
| ICA | 1.21 | 0.39 – 3.71 | 0.744 |
| M1 | 0.6 | 0.26 – 1.41 | 0.242 |
M1 indicates the horizontal portion of the MCA.
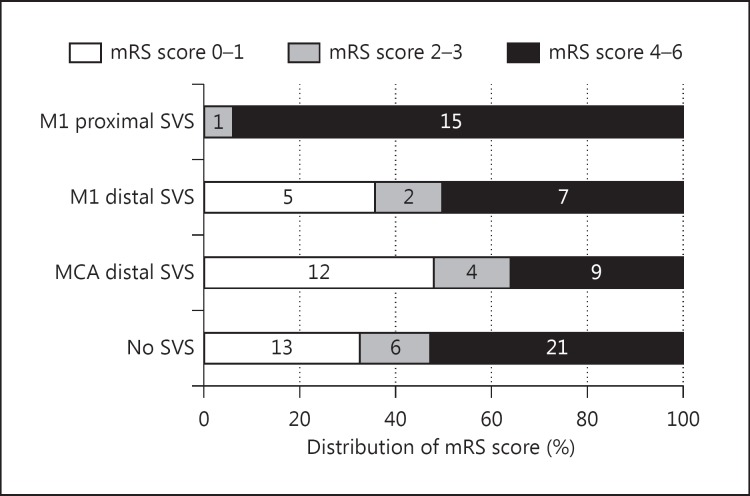
At 3 months, 95 patients with premorbid mRS scores of 0-1 and without intra-arterial intervention were eligible for the assessment of clinical outcome (fig. 3). None (0%) of the M1 proximal group, 36% of the M1 distal group, 48% of the MCA distal group, and 33% of the No SVS group achieved good outcomes at 3 months (p = 0.014). Therefore, the M1 proximal group obtained a significantly lower rate of good outcome than the M1 distal group, the MCA distal group, and the No SVS group (p = 0.014, 0.001, and 0.011, respectively). However, no significant difference in the rate of good outcome was seen between the M1 distal group and the No SVS group, nor between the MCA distal group and the No SVS group (p = 1.000 and 0.295, respectively).
Fig. 3.
Distribution of the mRS score at 3 months after stroke onset based on the presence of the SVS. Numbers of patients are given in the bars.
Discussion
The present study demonstrated that 96% of the patients with M1 proximal SVS had no early recanalization after t-PA therapy. Furthermore, no patients with M1 proximal SVS achieved a good outcome at 3 months. On the other hand, neither the rates of early recanalization nor good outcome was different among patients with M1 distal SVS, MCA distal SVS, and No SVS.
Recently, we have reported that the presence of an SVS on T2*-weighted MRI was associated with resistance to t-PA therapy. The magnetic susceptibility effect of deoxygenated hemoglobin in red thrombi may result in hypointense signals on T2*-weighted MRI. Cho et al. [16] reported that red thrombi in occluded vessels were visualized as hypointense signals within vascular cisterns on T2*-weighted MRI. They called such radiological findings the ‘SVS’. The SVS may reflect thrombus composition. Hemoglobin desaturation from oxyhemoglobin to deoxyhemoglobin occurs within a few hours. Thus, in hyperacute clot cases, the main component may still be oxyhemoglobin, and such emboli would not be identified as SVS on T2*-weighted MRI. In other words, the SVS is present in older thrombi, which may be resistant to t-PA therapy.
In the present study, the early recanalization rate of M1 distal SVS and MCA distal SVS was about 50%, which was higher than that of M1 proximal SVS. In general, when a small thrombus occludes the distal artery, the embolus volume of M1 proximal SVS may be largest among M1 proximal, M1 distal, and MCA distal SVS. When a thrombus is small, the proportion of the area in contact with t-PA becomes larger compared to a large thrombus, and thus t-PA may be more effective for smaller emboli. Therefore, M1 proximal SVS is the most resistant to t-PA, and its early recanalization rate should be the lowest.
Early recanalization by t-PA administration can improve patient outcomes [2]. None of the patients with M1 proximal SVS achieved a good outcome 3 months after onset. We strongly presume that this is because t-PA cannot dissolve the M1 proximal SVS thrombus. Furthermore, the NIHSS score was higher in the M1 proximal group than in the other groups. Neurological severity before t-PA infusion affects patient outcome [17]. Therefore, patients with M1 proximal SVS most frequently had a poor outcome. After the Merci retriever and Penumbra aspiration systems were approved, the combination of t-PA therapy and intra-arterial intervention has attracted significant attention, because this combination therapy significantly increases the early recanalization rate [18,19]. In the RECANALISE study, 87% of the patients treated with the combined intravenous and intra-arterial approach achieved recanalization, compared to only 52% of the patients treated with t-PA alone [4]. With respect to medical therapy, we previously showed that the administration of edaravone, a free radical scavenger, during t-PA therapy might increase the incidence of early recanalization [20]. For patients with M1 proximal SVS, an immediate combination of these therapies may be of benefit, and this should be initiated before judging whether t-PA will be effective or not.
No SVS did not necessarily indicate patients who achieved a good outcome, i.e. only 33% of the patients with No SVS had a good outcome. Our study showed that the rates of no early recanalization were not different among patients with M1 distal SVS, MCA distal SVS, and No SVS. Thus, this result may be explained by the ICA occlusion rate, which was higher in the order of patients with M1 proximal SVS, No SVS, M1 distal SVS, and MCA distal SVS. It is reasonable to consider that large-vessel occlusion damages larger tissue samples compared to small-vessel occlusion.
There are several limitations in the present study. First, the number of patients was small. Second, our T2* protocol included a slice thickness of 6 mm and an interslice gap of 1 mm. If the artery was smaller than 6 mm, it may have been difficult to detect a small SVS, especially in the MCA branches. Third, our study did not include detailed information on occluded ICA segments. It was difficult to distinguish ICA top occlusion from ICA occlusion at other intracranial segments, when the patients showed a diminished flow signal. Even patients with proximal ICA occlusion might have tandem ICA top occlusion. This may be clarified only when CT angiography or digital subtraction angiography is performed. In addition, it also seems to be potentially difficult to discriminate a thrombus from the blood stream on T2*-weighted MRI [21]. Thus, artery occlusion must be diagnosed on MRA to assess the SVS. Finally, a 0.6-mg/kg dose of t-PA was used, based on the Japan Alteplase Clinical Trial [8]. If the internationally recommended dose had been selected, the role of M1 proximal SVS might have been different. Further, large, prospective, randomized trials are required to confirm the utility of M1 proximal SVS in patients treated with intravenous thrombolysis.
In conclusion, M1 SVS at the proximal portion of the MCA, but not at other portions, is the critical determinant of no early recanalization and a poor outcome after t-PA therapy.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Kimura K, Iguchi Y, Shibazaki K, Aoki J, Uemura J. Early recanalization rate of major occluded brain arteries after intravenous tissue plasminogen activator therapy using serial magnetic resonance angiography studies. Eur Neurol. 2009;62:287–292. doi: 10.1159/000235753. [DOI] [PubMed] [Google Scholar]
- 2.Kimura K, Iguchi Y, Shibazaki K, Aoki J, Watanabe M, Kobayashi K, Sakamoto Y. Recanalization within one hour after intravenous tissue plasminogen activator is associated with favorable outcome in acute stroke patients. Eur Neurol. 2010;63:331–336. doi: 10.1159/000311736. [DOI] [PubMed] [Google Scholar]
- 3.Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Liang S, Venketasubramanian N, Rathakrishnan R, Ahmad A, Ng KW, Loh PK, Ong JJ, Wakerley BR, Chong VF, Bathla G, Sharma VK. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70:353–358. doi: 10.1001/2013.jamaneurol.547. [DOI] [PubMed] [Google Scholar]
- 4.Mazighi M, Serfaty JM, Labreuche J, Laissy JP, Meseguer E, Lavallee PC, Cabrejo L, Slaoui T, Guidoux C, Lapergue B, Klein IF, Olivot JM, Abboud H, Simon O, Niclot P, Nifle C, Touboul PJ, Raphaeli G, Gohin C, Claeys ES, Amarenco P. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–809. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 5.Kimura K, Iguchi Y, Shibazaki K, Watanabe M, Iwanaga T, Aoki J. M1 susceptibility vessel sign on T2* as a strong predictor for no early recanalization after IV-t-PA in acute ischemic stroke. Stroke. 2009;40:3130–3132. doi: 10.1161/STROKEAHA.109.552588. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Sakamoto Y, Aoki J, Iguchi Y, Shibazaki K, Inoue T. Clinical and MRI predictors of no early recanalization within 1 h after tissue-type plasminogen activator administration. Stroke. 2011;42:3150–3155. doi: 10.1161/STROKEAHA.111.623207. [DOI] [PubMed] [Google Scholar]
- 7.Rovira A, Orellana P, Alvarez-Sabin J, Arenillas JF, Aymerich X, Grive E, Molina C, Rovira-Gols A. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. 2004;232:466–473. doi: 10.1148/radiol.2322030273. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, Shinohara Y. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 h of onset: Japan alteplase clinical trial (J-ACT) Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3. [DOI] [PubMed] [Google Scholar]
- 9.Shinohara Y, Yamaguchi T. Outline of the Japanese guidelines for the management of stroke 2004 and subsequent revision. Int J Stroke. 2008;3:55–62. doi: 10.1111/j.1747-4949.2008.00178.x. [DOI] [PubMed] [Google Scholar]
- 10.Aoki J, Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Watanabe M, Kobayashi K, Sakai K, Sakamoto Y. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovasc Dis. 2011;31:435–441. doi: 10.1159/000323850. [DOI] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 13.Barber PA, Hill MD, Eliasziw M, Demchuk AM, Pexman JH, Hudon ME, Tomanek A, Frayne R, Buchan AM. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry. 2005;76:1528–1533. doi: 10.1136/jnnp.2004.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turc G, Apoil M, Naggara O, Calvet D, Lamy C, Tataru AM, Meder JF, Mas JL, Baron JC, Oppenheim C, Touze E. Magnetic resonance imaging-DRAGON score: 3-month outcome prediction after intravenous thrombolysis for anterior circulation stroke. Stroke. 2013;44:1323–1328. doi: 10.1161/STROKEAHA.111.000127. [DOI] [PubMed] [Google Scholar]
- 15.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 16.Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005;36:2379–2383. doi: 10.1161/01.STR.0000185932.73486.7a. [DOI] [PubMed] [Google Scholar]
- 17.Frankel MR, Morgenstern LB, Kwiatkowski T, Lu M, Tilley BC, Broderick JP, Libman R, Levine SR, Brott T. Predicting prognosis after stroke: a placebo group analysis from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial. Neurology. 2000;55:952–959. doi: 10.1212/wnl.55.7.952. [DOI] [PubMed] [Google Scholar]
- 18.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 19.Bose A, Henkes H, Alfke K, Reith W, Mayer TE, Berlis A, Branca V, Sit SP. The penumbra system: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29:1409–1413. doi: 10.3174/ajnr.A1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura K, Aoki J, Sakamoto Y, Kobayashi K, Sakai K, Inoue T, Iguchi Y, Shibazaki K. Administration of edaravone, a free radical scavenger, during t-PA infusion can enhance early recanalization in acute stroke patients – A preliminary study. J Neurol Sci. 2012;313:132–136. doi: 10.1016/j.jns.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Teitelbaum GP, Ortega HV, Vinitski S, Clark RA, Watanabe AT, Matsumoto AH, Rifkin MD, Barth KH. Optimization of gradient-echo imaging parameters for intracaval filters and trapped thromboemboli. Radiology. 1990;174:1013–1019. doi: 10.1148/radiology.174.3.174-3-1013. [DOI] [PubMed] [Google Scholar]