The association of chromosomal abnormalities with cancer has been known for over a century, as has Boveri’s hypothesis that these abnormalities drive carcinogenesis. Most human cancers are associated with some forms of genetic abnormalities that range from single chromosome translocations to examples containing multiple translocations, deletions, amplifications, inappropriate numbers of chromosomes (aneuploidy) and point mutations. Even karyotypically stable cancers, such as mismatch repair defective cancers, are genetically abnormal due to high frequencies of point mutations. Despite our current understanding of cancer genomes, the role of many genetic abnormalities as a cause or consequence of carcinogenesis has been difficult to test. Increased mis-segregation of whole chromosomes to generate aneuploidy can promote tumorigenesis in some genetic contexts in mice (1), as Boveri initially proposed >100 years ago. Recent findings from Sheltzer et al. (2) and Solomon et al. (3) on pages XXXand XXXof this issue identify aneuploidy to enhance mitotic recombination and defective DNA damage repair, thereby providing a mechanistic linkage between aneuploidy and genomic instability.
While it is clear that cancers accumulate mutations (including single base changes and insertions and deletions), chromosomal rearrangements, and aneuploidy during the development and progression of cancer, it is less clear how, and in what order, these mutations arise. One prominent idea, the mutator hypothesis, posits that cancers must acquire a mutator phenotype with increased rates of mutations and genome rearrangements (4). An alternative hypothesis is that selection alone can account for the mutations and genome rearrangements seen in different cancers, which is supported by the current failure to identify common mutations in genome stability pathways in many cancers and the possibility that genome rearrangements could result during crisis after telomere erosion.
The mutator hypothesis is supported by the observation of numerous familial cancer predisposition syndromes caused by defects in DNA repair or DNA damage response pathways. For example, the common cancer susceptibility syndrome, Lynch Syndrome is caused by inherited mismatch repair defects that cause increased rates of accumulating base substitution and frameshift mutations and a significant fraction of sporadic cancers are associated with acquired mismatch repair defects resulting in a mutator phenotype. Highermutation rates alone can drive carcinogenesis in mice (5,6). Similarly, human homologs of some genes identified as causing increased genome rearrangements in the yeast Saccharomyces cerevisiae are mutated in inherited cancer predisposition syndromes (7). But despitethis evidence, mutator phenotypes and defects underlying these phenotypes have only been identified in a fraction of human cancers to date.
Origins of aneuploidy in cancer and effects on the development and progression of cancer are even less clear. Aneuploidy is common in cancer and can correlate with poor prognosis (8). Paradoxically, gains of single chromosomes in both mouse (9) and yeast (10) cells causes cells to grow more slowly. Remarkably, aneuploidy in mice can both enhance the formation of certain cancers while suppress others (1). Studies in model organisms have identified many genes and pathways that prevent aneuploidy, including genes that function in mitotic checkpoints and homologous recombination (11). The relationship between defects in such genes and cancer has proven complex as mutations in such genes in cancers are rare (12), and over-expression and reduced expression of such genes in mice result in both increased and decreased incidence of cancer.
In the current issue of Science, Sheltzer et al (2) use yeast to examine the consequences of a single extra copy of a chromosome. Surprisingly, the presence of an extra chromosome produced modest but significant increases in the rates of point mutations, mitotic recombination, and loss of whole chromosomes, as well as a range of DNA repair and recombination defects. Unresolved is whether these phenotypes were due to unbalanced protein expression or some other stress due to aneuploidy. In an accompanying paper, Solomon et al (3) identified mutations in the STAG2 gene (encoding a cohesion complex component), as well as reduced STAG2 protein levels, in a diversity of human cancer cell lines, xenografts and primary tumors. They further showed that in cell lines, inactivation of STAG2 results in a sister chromatid cohesion defect accompanied by a modest increase in aneuploidy.
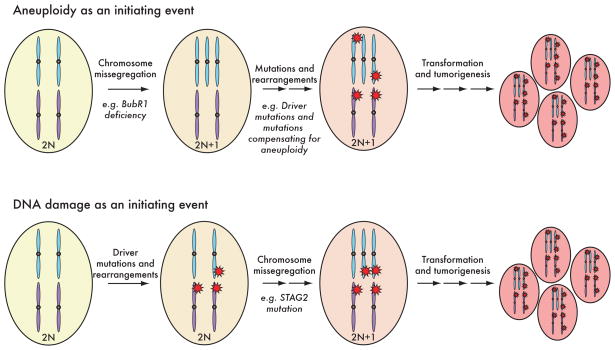
These new results have important implications for understanding the genetics of human cancers (Figure 1). First, aneuploidy, or even modest copy number changes in key regions of chromosomes, can causea modest mutator phenotype and may promote the accumulation of mutations in some cancers even in the absence of initiatingmutations (i.e., aneuploidy as an initiator event). One such example is the mosaic variegated aneuploidy cancer predisposition syndrome. Second, aneuploidy may also be caused by mutations accumulated during cancer progression (i.e., aneuploidy as a later event), as suggested by the acquisition of somatic STAG2 mutations (3). Third, that aneuploidy can result in increased mutation rates provides a mechanism for the observation that low levels of aneuploidy due to reduced levels of components that directly affect chromosome segregationcan drive tumorigenesis, whereas even higher rates of aneuploidy can suppress it by causing slow growth or cell death (1). In a similar vein, defects in STAG2, which likely result in mildly increased aneuploidy, would be predicted to drive tumorigenesis through induction of a mutator phenotype. The broader implication is that mutator phenotypes in cancer could result from deregulation of genes that are not immediately recognized as acting to directly prevent mutations. Itwill be interesting to see if aneuploidy can also drive the accumulation of genome rearrangements, including translocations and copy number changes, that can also contribute to tumorigenesis.
Figure 1.
Aneuploidy as an initiator event and as a later event during cancer progression.
Contributor Information
Richard D. Kolodner, Email: rkolodner@ucsd.edu.
Don W. Cleveland, Email: dcleveland@ucsd.edu.
Christopher D. Putnam, Email: cdputnam@ucsd.edu.
References
- 1.Weaver BA, et al. Cancer Cell. 2007;11:25. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Sheltzer JM, et al. Science. 2011 [Google Scholar]
- 3.Solomon DA, et al. Science. 2011 [Google Scholar]
- 4.Loeb LA. Cancer Res. 2001;61:3230. [PubMed] [Google Scholar]
- 5.Albertson TM. PNAS. 2009;106:17101. doi: 10.1073/pnas.0907147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang G. Cancer Cell. 2004;6:139. doi: 10.1016/j.ccr.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Kolodner RD. Science. 2002;297:552. doi: 10.1126/science.1075277. [DOI] [PubMed] [Google Scholar]
- 8.Carter SL, et al. Nature Genet. 2006;38:1043. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 9.Torres EM, et al. Cell. 2010;143:71. doi: 10.1016/j.cell.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams BR, et al. 2008;322:703. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen KWY, et al. PNAS. 2007;104:3925. [Google Scholar]
- 12.Perez de Castro, et al. Carcinogenesis. 2007;28:899. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]