Abstract
The distinction between processes used to perceive and understand the self and others has received considerable attention in psychology and neuroscience. Brain findings highlight a role for various regions, in particular the medial prefrontal cortex (mPFC), in supporting judgments about both the self and others. We performed a meta-analysis of 107 neuroimaging studies of self- and other-related judgments using Multilevel Kernel Density Analysis (MKDA; Kober & Wager, 2010). We sought to determine what brain regions are reliably involved in each judgment type, and in particular, what the spatial and functional organization of mPFC is with respect to them. Relative to non-mentalizing judgments, both self and other judgments were associated with activity in mPFC, ranging from ventral to dorsal extents, as well as common activation of the left temporoparietal junction (TPJ) and posterior cingulate. A direct comparison between self and other judgments revealed that ventral mPFC (vmPFC), as well as left ventrolateral PFC and left insula, were more frequently activated by self-related judgments, whereas dorsal mPFC (dmPFC), in addition to bilateral TPJ and cuneus, were more frequently activated by other-related judgments. Logistic regression analyses revealed that ventral and dorsal mPFC lay at opposite ends of a functional gradient: the z-coordinates reported in individual studies predicted whether the study involved self- or other-related judgments, which were associated with increasingly ventral or dorsal portions of mPFC, respectively. These results argue for a distributed rather than localizationist account of mPFC organization and support an emerging view on the functional heterogeneity of mPFC.
Introduction
The ability to discern and act upon one’s own feelings, thoughts, and desires across time, place, and varying situational demands serves an evolutionarily-adaptive purpose (Sedikides & Skowronski, 1997). For example, it’s adaptive to differentiate how the thoughts and feelings of oneself are different from those of other people, and vice versa. As such, philosophers and psychologists have long debated the nature of the mental representations that separate the self from others (Goldman, 1992; Stich & Nichols, 1992), and addressing this question has generated an extensive experimental literature (Klein & Kihlstrom, 1986; Rogers, Kuiper, & Kirker, 1977; Symons & Johnson, 1997).
In particular, one focus has been on asking whether information that is relevant to the self is processed in a fundamentally different manner than information that pertains to other people. Put simply: is information about self and others processed in categorically distinct ways and subserved by discrete neuroanatomical substrates, or is the distinction more a matter of degree? The answer to this question could have important basic and translational implications. For example, self-focus – and in particular ruminative self-focus – has been shown to be positively associated with the prevalence of negative affect in a meta-analysis of 226 effect sizes (Mor & Winquist, 2002). What's more, numerous clinical disorders are characterized by deficits in self-perception, self-knowledge and the ability to understand others' beliefs, intentions and feelings as well as potential mPFC dysfunction. Indeed, disorders ranging from autistic spectrum disorder (ASD) (Di Martino et al., 2009), to schizophrenia (Whitfield-Gabrieli et al., 2009), PTSD (Bremner, 2002; Etkin & Wager, 2010; Liberzon & Sripada, 2008), and depression (Drevets, 2001; Savitz & Drevets, 2009) all have shown abnormal patterns of mPFC activity and/or structure. Our ability to draw inferences about the meaning of these data is limited, however, by the fact that there is as of yet no clear model of the functional organization of mPFC with respect to processes that support judgments of self and others.
Over the past few decades two types of data have supported the idea that the “self” is a unique mental construct and that self-relevant information enjoys privileged processing, at least in Western, independent cultural contexts (Markus & Kitayama, 1991). The first type comes from behavioral studies showing that recall of self-relevant information is often better than recall of other types of information (Bower & Gilligan, 1979; Rogers et al., 1977) and is associated with relatively greater accessibility (i.e. ease of generation), greater confidence, and reduced response time relative to other-relevant information (Kuiper & Rogers, 1979). While some took these data to indicate a special status for self-related information, this conclusion was called into question by studies showing that the self-reference effect is reduced or not present under certain conditions, such as when the information is negative rather than positive (Ferguson, Rule, & Carlson, 1983; for a review see Higgins & Bargh, 1987). In addition, some have argued that the self-reference effect may be attributable to non-self-specific mechanisms, such as depth of processing (Symons & Johnson, 1997).
The second type of data comes from neuroimaging studies asking what brain systems support self- and other-related judgments. Across studies, numerous regions have been associated with attributions about the self or others, including the ventral and dorsal portions of the medial PFC (mPFC), anterior and posterior cingulate cortices (ACC and PCC), medial parietal cortex, precuneus and the temporal poles (Kelley et al., 2002; Mitchell, Macrae, & Banaji, 2006; Ochsner et al., 2005; Ochsner et al., 2004). Despite this apparent consistency, there has also been a great deal of variability in the specific regions and subregions activated – and a clear answer about whether dedicated neural systems support the access and use of self-knowledge has yet to emerge. To help make sense of this variability, meta-analyses have begun asking what neural systems are associated with judgments about the self and/or others and whether the neural representation of information about the self is categorically distinct or “special” in some way (Gillihan & Farah, 2005).
For example, Northoff and colleagues (2006) analyzed 27 functional magnetic resonance imaging (fMRI) studies related to judgments about the self and found evidence that self judgments are associated with a group of cortical midline structures, including the ventral and dorsal mPFC, ACC and PCC, and medial parietal cortex. Hierarchic cluster and factor analyses indicated a 3-cluster solution incorporating vmPFC/pre-genual ACC, dmPFC, and PCC/precuneus, all of which were associated with self-related judgments without functional specificity (i.e. across verbal, facial, and emotional functional domains, among others). Consistent with this, Gilbert and colleagues (2006) reported findings from a meta-analysis of 104 functional neuroimaging studies activating one subregion of mPFC: Brodmann Area 10 (BA 10). They found that studies involving mentalizing (i.e. attention to and/or judgments about one’s own mental states or the mental states of another person) were more associated with medial BA 10 activation, in contrast to lateral BA 10, which was more strongly associated with other cognitive tasks such as working memory and episodic memory retrieval.
Several additional recent meta-analyses have likewise reported an important role for mPFC in mentalizing about self and others (Legrand & Ruby, 2009; Qin & Northoff, 2011; van der Meer, Costafreda, Aleman, & David, 2010; Van Overwalle, 2009, 2011; Van Overwalle & Baetens, 2009). In particular, a meta-analysis by van der Meer and colleagues provided evidence that vmPFC is associated with self-relevant cognition, but that dmPFC is associated with both self and other evaluation and decision-making processes (van der Meer et al., 2010). Qin and Northoff (2011) also report overlapping activity for self and other processing (in particular, familiar other processing) within mPFC. Van Overwalle (2011) found that the extent to which mentalizing is involved in a functional neuroimaging study is positively predictive of whether mPFC is recruited, further substantiating our focus on this region in the present meta-analysis.
Taken together, these meta-analyses highlight the centrality of mPFC for mentalizing in particular, and for processing information about self and others more generally. However, it is not yet clear whether judgments about the self and others are supported by overlapping or distinct neural systems. Indeed, none of the prior meta-analyses quantitatively assessed the nature of the spatial organization of self and other judgment processing in mPFC, and making inferences about this organization was a principal aim in the current study.
With this in mind, this quantitative meta-analysis aimed to address two fundamental questions concerning the neural systems supporting self and other judgments. First, we asked what brain regions are reliably involved in making such judgments? Second, and more importantly, we asked what is the spatial and functional organization of the mPFC with respect to self and other judgments, given its putative role in subserving both? Here we performed the first direct, quantitative investigation of the spatial and functional organization of self and other judgment-related processing within mPFC.
Two hypotheses could be proposed for the latter aim. First, the mPFC could be organized in discrete, localized modules. If this hypothesis is correct one might expect to see a double dissociation between self and other-related regions (Mitchell et al., 2006; Qin & Northoff, 2011; van der Meer et al., 2010). Alternatively, the neural systems supporting self and other-related judgments could be distributed and largely overlapping. If this hypothesis is correct, one might expect to see a spatial processing gradient within mPFC (Amodio & Frith, 2006; Ochsner, 2004; Olsson & Ochsner, 2008; Zaki & Ochsner, 2011).
The question of how different brain systems support judgments about the self as opposed to others is of fundamental interest to both psychology and neuroscience. While the results of numerous individual studies have been equivocal with respect to this question, meta-analyses offer the chance to aggregate across these studies to identify the most reliable patterns, given that individual studies may vary substantially with respect to the localization of self versus other-related judgments and the existence and type of multiple comparison correction employed (Kober & Wager, 2010). To address our hypotheses about the neural differences between self and other-related judgments across the brain, and within mPFC in particular, we had a threefold plan. First, we examined contrasts of self or other judgments vs. non-mentalizing baseline conditions. Second, we examined contrasts of self and other judgments vs. each other. Third, we focused specifically on mPFC and used logistic regression analyses to predict the specific spatial locations of activations related to self and other judgments. This final analysis allowed us to make inferences about the localized vs. distributed nature of the functional organization of self and other judgments within mPFC.
Methods
Identification of Studies
We used several sources to find reports on the neural correlates of self and other-related judgments (including theory of mind) that were published between January 1995 and February 2008. First, we searched peer-reviewed journals using online indexes such as MEDLINE. We then reviewed the reference lists of these articles to find additional reports. Finally, we searched for additional publications by the same authors. The datasets and associated contrasts included in this meta-analysis met the following inclusion criteria: (1) They involved self, other, or theory-of-mind-related cognition; (2) They involved a judgment (i.e. a mental state judgment or other non-mentalizing judgment) from the participant; (3) They involved unmedicated, healthy adults; (4) They measured regional cerebral blood flow (e.g. PET) or blood oxygenation (e.g. fMRI); (5) They used the image subtraction method to determine activation foci; (6) They provided standard Talairach (Talairach & Tournoux, 1988) or Montreal Neurologic Institute (MNI) coordinates, allowing for comparison of results across different studies and different laboratories. Contrasts were excluded if they met any of the following criteria: (1) They involved patients with major psychiatric disease; (2) They involved participants on a therapeutic regimen; (3) They involved children under 18 years of age; (4) They were non-contrast analyses (e.g. parametric analyses). This process yielded 107 published reports, which involved 307 individual contrasts (summarized in Supplementary Tables 1 & 2). As detailed in Supplementary Table 1, 29 studies were coded but did not contribute contrasts to the meta-analysis due to our conservative coding algorithm, described below. The meta-analytic database included all individual contrast foci that were reported as significant as designated by the criteria of the individual studies. Coordinates were verified by at least 2 independent investigators.
Study Coding
Included contrasts were coded by multiple coders in the Social Cognitive Affective Neuroscience Unit at Columbia University. All contrasts were coded by at least 2 trained coders. Any disagreements between coders were arbitrated after independent coding in order to any resolve any discrepancies (Kober et al., 2008). Coders made binary (yes/no) coding judgments on the basis of the individual experimental conditions that comprised the included contrasts for the agent involved in the experimental condition (e.g. the target of a mental state judgment): Self, Non-Self, Other (normative/people in general), Other (specific group), or Other (individuated). Coders also made ratings of the nature of the judgment performed in the contrast (i.e. whether it was a mental state judgment or not). Finally, coders specified how conditions were grouped and subtracted from one another to form contrasts.
This procedure then yielded binary contrast codes for each included contrast of an included study that corresponded to whether that contrast was reflective of activation pertaining to Self, Other (i.e. any one of the three Other options) and whether it was reflective of a mental state judgment. The contrast code algorithm was designed to exclude contrasts that did not isolate self; for example, a contrast of assessing the self-relevance of positive versus negative trait words would not count as a “self” contrast, given that the self target was involved in both parts of the contrast. Further, contrasts that were reflective of both Self and Other-related judgments were excluded from further analysis.
We also created a baseline contrast designation that applied across the entire database, defined as contrasts that were not reflective of Self-related or Other-related judgments, were not reflective of making any mental state judgment, and were positively coded as being reflective of a non-mental state judgment. Examples of baseline contrasts included contrasts that were reflective of general semantic processing (e.g. letter judgment) and non-mentalizing object description.
The total number of contrasts that pertained to each condition were as follows: 74 for Self, 76 for Other, and 24 for Baseline. These contrasts were drawn from 48 studies for Self, 48 studies for Other, and 19 studies for Baseline (Supplementary Tables 1 & 2).
Across Self and Other judgment conditions the nature of the mental state judgment varied, although for each judgment a comparable number of contrasts were coded as being affectively-relevant (17 for Self, 19 for Other), relating to trait judgments (11 for Self, 14 for Other), and relating to a target’s intentions (9 for Self, 20 for Other). Contrasts coded as cognitive, such as theory-of-mind judgments, were more often associated with Other (22 contrasts) rather than the Self (2 contrasts). A given contrast could be associated with multiple mental state judgments, and some contrasts are not represented in the mental state judgment totals above if the contrast involved the same type of judgment in each compared condition. For example, if a given contrast compared the same type of mental state judgment (e.g. trait adjective judgment) across two targets (e.g. Self and Other), it would be represented as a Self contrast, but not a trait contrast. That said, given the relatively small number of studies within each Self/Other x judgment type condition, drawing inferences about differences between types of mental state judgments was not the focus of the present analysis.
Data analysis overview
To answer the questions posed above, we used the following analysis methods. First, we investigated which brain regions were reliably associated with self-related judgments, other-related judgments, or both (as assessed via comparing self and other-processing to a non-mentalizing baseline, defined above). To do this, we used Multi-level Kernel Density Analysis (Kober et al., 2008; Kober & Wager, 2010; Wager, Lindquist, & Kaplan, 2007), as described below. This approach treats contrasts as the unit of analysis. A thorough explanation of MKDA and its relationship with other meta-analytic techniques has been previously given by Wager and colleagues (2007; 2008; Kober & Wager, 2010). Second, focusing on mPFC specifically, we sought to draw inferences about the distribution of self-other representations across space in this region by performing a logistic regression analysis, described below (Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, in press).
I. Multi-level kernel density analyses
First, activation peak coordinates from each of the included contrasts included in the meta-analytic database were registered to a standard brain from the Montreal Neurologic Institute (MNI), avg152T1.img, as distributed with SPM5 software (Wellcome Department of Imaging Neuroscience, London, UK). Coordinates originally reported in Talairach stereotactic space (Talairach & Tournoux, 1988) were converted to MNI space (Matthew Brett, http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/tal2mni.m). Peaks were then convolved with a 10 mm spherical kernel, a consensus kernel size for MKDA (Kober & Wager, 2010; Salimi-Khorshidi, Smith, Keltner, Wager, & Nichols, 2009; Wager, Jonides, & Reading, 2004; Wager et al., 2007). This produced Comparison Indicator Maps (CIMs) in which “active” voxels are given a value of 1 (e.g., “this contrast activated within 10mm”).
Once CIMs were constructed for every contrast, a density map across all contrasts reflected the proportion of contrasts that activate near each voxel by taking a weighted average of the indicator maps. The weights for each study were the square root of the sample size, which weights larger studies more heavily (similar to effect size measurements). CIMs from one condition (e.g. Self) could then be compared to CIMs from another condition (e.g. Other). The meta-analysis statistic at each voxel (P, or “density”) was the proportion difference (P) of contrasts that activated within 10 mm of that voxel, weighted by the sample size of the study.
Finally, to threshold the results, P was compared with a null-hypothesis density P0 established through Monte Carlo simulation. The null hypothesis was a uniform random distribution of peaks within each comparison in gray matter in the standard brain. For each CIM, we identified contiguous activation blobs of suprathreshold voxels. In each of 5000 Monte Carlo iterations, the locations of the activation blobs were selected at random within a gray-matter mask (smoothed to include an 8 mm border, derived from segmentation of the avg152T1.img template using SPM2). The search volume contained 231,202 2mm isotropic voxels. Shape of the activation blobs was held constant (i.e. we condition on activation blob size and shape within each CIM). After each iteration, the maximum across-study density statistic (P) over the whole brain is saved. The critical Familywise Error Rate (FWER)-controlled threshold is the proportion that exceeds the whole-brain maximum in 95% of the Monte Carlo maps — controlling for the chance of seeing false positives anywhere in the brain at p<.05 corrected.
After each Monte Carlo iteration, the largest cluster of contiguous voxels was saved, and a cluster extent threshold was set at the 95th percentile of these values across iterations, following the concept behind “cluster extent-based” multiple comparison correction implemented in SPM software (Friston, Worsley, Frackowiak, Mazziotta, & Evans, 1994). Results survived whole-brain correction if they either met height-based criteria alone (i.e. sufficiently large activation proportion differential, regardless of extent), or if they met cluster extent-based criteria. Height (i.e. activation proportion) and extent thresholds varied for each MKDA, but all were significant at p<0.05, FWE-corrected. Results were visualized using NeuroElf software (neuroelf.net).
II. Logistic regression analysis for mPFC
In order to draw direct inferences about the spatial distribution of self vs. other representations that are unbiased by potential smoothing artifacts, we performed a binary logistic regression incorporating three continuous predictors (MNI-standardized x, y, and z coordinates for each point located within an independently-defined mPFC boundary) and one binary dependent variable (activation point associated with a self vs. other-specific contrast). The mPFC boundary was defined as follows: |x|<25, y>15, z>−5. We imposed the restriction on the z coordinate in order to exclude the orbitofrontal cortex area, which was not of interest to the present analyses (cf. Van Overwalle, 2009, 2011). The resulting analysis was performed on 47 Self contrasts and 43 Other contrasts that reported activation points within this mPFC region. These contrasts were drawn from 33 studies for Self and 34 studies for Other.
Results
MKDA Analyses: Regions involved in Processing Self, Other, or Both
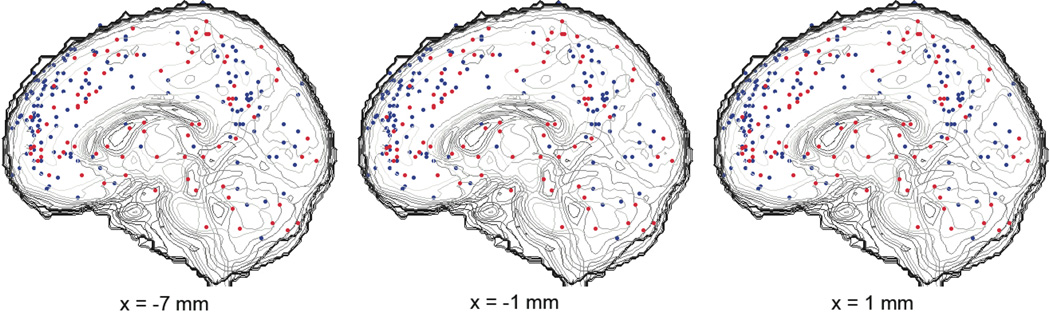
Though contrasts were the unit of analysis, we first plotted standardized individual points corresponding to each condition of interest (Self and Other target judgments) on canonical brain slices for visualization (Figure 1).
Figure 1.
Three sagittal midline point plots highlighting the self vs. other point distribution within mPFC. Red=Self-related judgment, Blue=Other-related judgment.
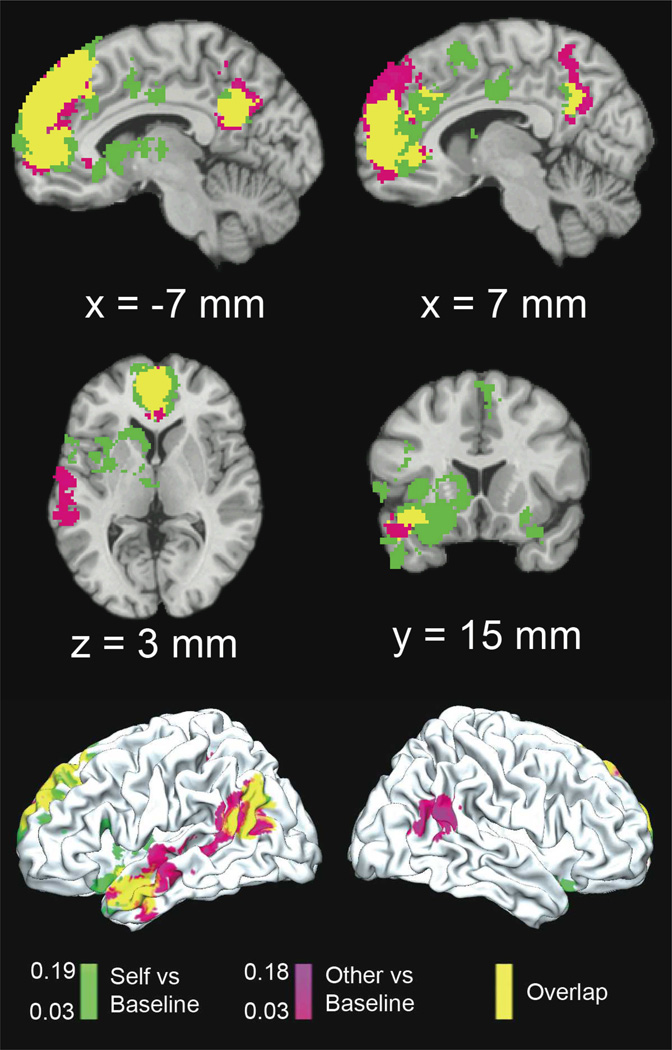
In order to address our first question regarding which regions are associated with making judgments with respect to the self, an other, or both, we performed an MKDA comparing both Self and Other to a non-mentalizing baseline. Figure 2 shows results for both Self>Baseline, Other>Baseline, and their overlap on representative brain slices, and Supplementary Figure 1 shows a whole-brain montage. Results showed broad similarity in activation patterns: both Self vs. Baseline and Other vs. Baseline involved activation of a large area of mPFC ranging from ventral to dorsal extents. While mPFC represented by far the greatest region of overlap, commonly activated regions were also seen in the left temporoparietal junction, posterior cingulate, and left middle temporal gyrus/superior temporal sulcus (Supplementary Table 3).
Figure 2.
Self vs. Baseline (Green) and Other vs. Baseline (Magenta), with Overlap shown in Yellow. Color gradients for Self vs. Baseline and Other vs. Baseline show activation proportion differentials between conditions (FWE-corrected, p<0.05). Coordinates shown are in MNI space.
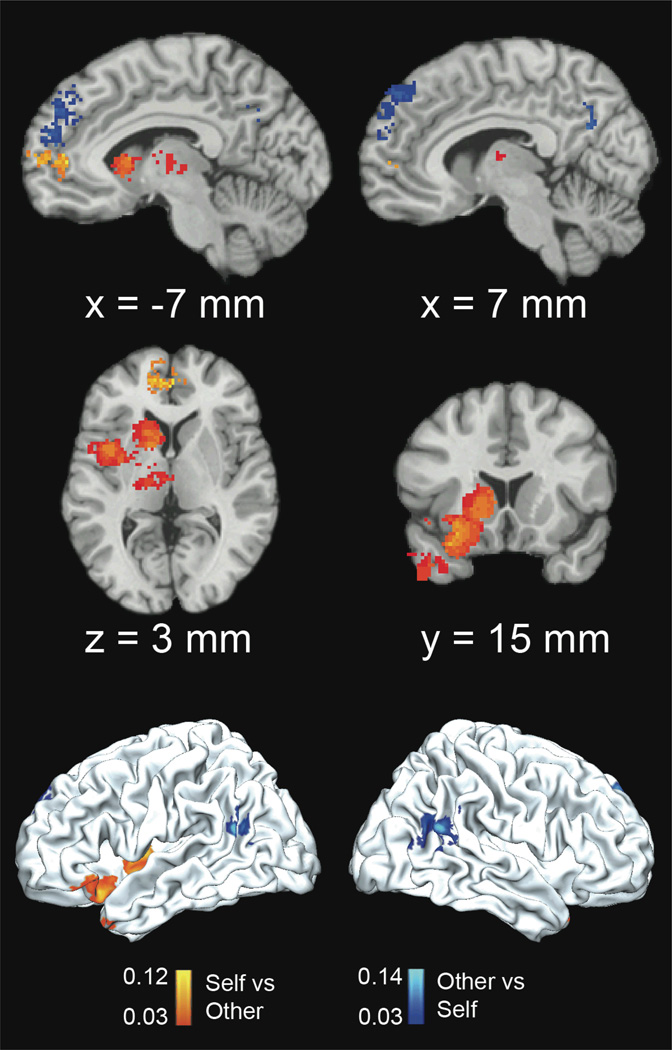
Next, we sought to directly compare self and other-related judgments using MKDA. Figure 3 shows that the Self condition, relative to the Other condition, significantly activated vmPFC, including left rostral BA 10, anterior paracingulate cortex (BA 32), left ventrolateral PFC (vlPFC), left anterior and mid-insula, left dorsal caudate, thalamus, and left temporal pole. Notably, very little right-lateralized activation was observed for the Self relative to the Other condition. For the Other condition relative to the Self condition, robust bilateral dmPFC activity was observed, in addition to bilateral TPJ and cuneus activity (Supplementary Figure 2 and Supplementary Table 4).
Figure 3.
Self vs. Other (Orange) and Other vs. Self (Blue) MKDA Results. Color gradients show activation proportion differentials between conditions (FWE-corrected, p<0.05). Coordinates shown are in MNI space.
Logistic Regression: Discerning the Spatial/Functional Organization of mPFC
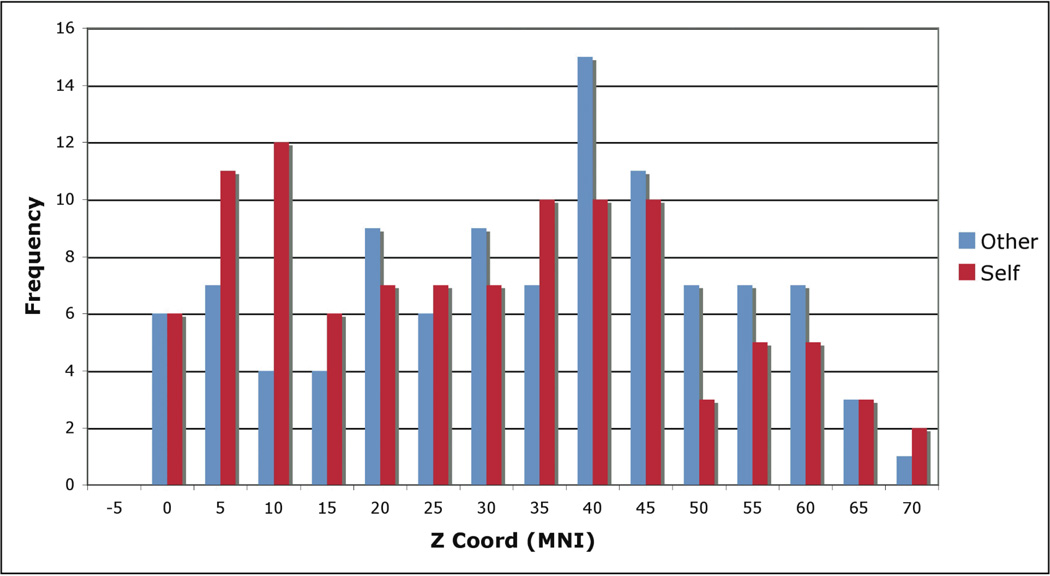
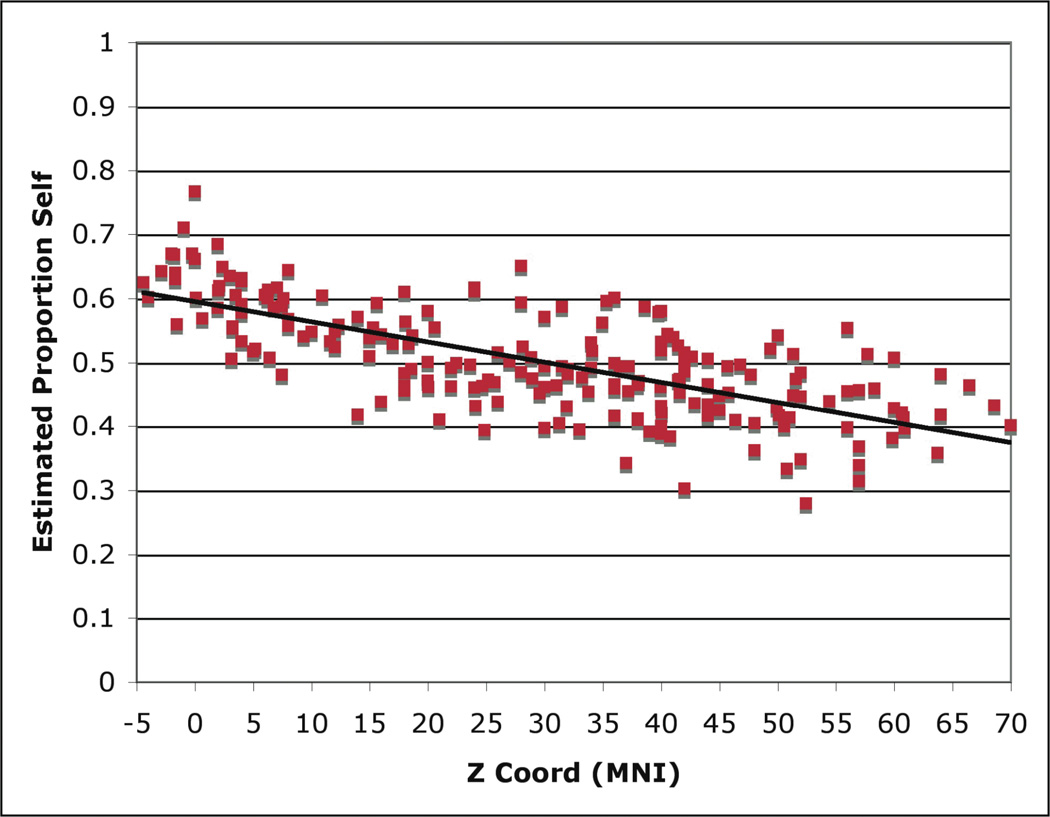
Within mPFC, logistic regression analyses showed a significant effect of z coordinate predicting Self vs. Other contrast status, while x and y coordinates were not significantly predictive (Table 1). These results reflect a spatial processing gradient, such that increasingly ventral or dorsal mPFC activation were increasingly associated with either self- or other-related judgments, respectively. A histogram showing distributions of self and other-related points as a function of z coordinate within mPFC (defined independently of the MKDA analyses) is shown in Figure 4. Further, to illustrate the logistic regression result for the z coordinates in particular, a scatterplot showing estimated self proportions as a function of z coordinate within the mPFC boundary is shown in Figure 5 (r=−0.71, p<0.01).
Table 1.
Logistic regression results.
| Logistic Regression Results | ||
|---|---|---|
| x coord: | β=−0.21, | p<0.18 |
| y coord: | β=−0.16, | p<0.19 |
| z coord: | β=−0.19, | p<0.04 |
In this analysis, self and other-related points were coded as 1 and -1, respectively.
Figure 4.
Distributions of mPFC Z Coordinates for Other and Self Points.
Figure 5.
Estimated Proportion Self as a Function of mPFC Z Coordinate
Each dot represents the z coordinate of an included point in the database corresponding to either a Self or Other contrast plotted against estimated proportion Self, derived from the logistic regression analysis.
Discussion
The question of how different brain systems support judgments about the self as opposed to others is of fundamental interest to both psychology and neuroscience. While the results of numerous individual studies have been equivocal with respect to this question, meta-analyses offer the chance to aggregate across these studies to identify the most reliable patterns. With this in mind, the meta-analysis presented here had two principal goals. First, we sought to discern which brain areas, in general, were reliably associated with judgments made with respect to the self, an other, or both. Second, based on the reliable association of the mPFC with both self and other processing in this and other meta-analyses, we sought to perform the first direct, quantitative investigation of the spatial and functional organization of self and other-related processing within mPFC. Using a database of 107 published neuroimaging studies we reported evidence for self- and other-related judgments being (1) supported by both common and distinct groups of regions that include many implicated in mentalizing in prior work, and (2) associated with a spatial mentalizing gradient within mPFC, with increasingly ventral activation being increasingly associated with self-related judgments and increasingly dorsal activation being increasingly associated with other-related judgments.
Whole-Brain Comparisons of Self and Other-Related Judgments
To address our initial question of what regions, in general, are involved in each type of judgment, we compared the neural correlates of self- or other-related judgments to a non-mentalizing baseline across the whole brain using Multilevel Kernel Density Analysis. We first separately compared self and other-processing to baseline activity. Then, we examined the extent of the overlap of regions that were recruited. Broad overlap was observed in both ventral and dorsal mPFC, which is consistent with prior work indicating that mPFC is involved in attending to one’s own or another’s mental state (Gilbert et al., 2006; Legrand & Ruby, 2009; Ochsner et al., 2005; Ochsner et al., 2004; Van Overwalle, 2011; Van Overwalle & Baetens, 2009). While the overlap in mPFC was the most striking and extensive, there was substantial functional overlap in other areas of the brain as well, including left temporoparietal junction (TPJ), posterior cingulate, and left middle temporal gyrus and superior temporal sulcus, all of which have been previously associated with mentalizing about the beliefs, desires, perceptions, or emotions of oneself and others (Gallagher & Frith, 2003; Northoff et al., 2006; Saxe, Carey, & Kanwisher, 2004; Van Overwalle & Baetens, 2009). As such, these results confirm the idea that this suite of regions is essential for making judgments about mental states – regardless of whose states they are.
We next directly compared self and other judgments in order to identify regions differentially associated with each type of judgment, with a particular emphasis on interrogating mPFC activity for functional specificity related to self judgments. Consistent with prior work (Gilbert et al., 2006; Mitchell, Banaji, & Macrae, 2005; Mitchell et al., 2006; Northoff et al., 2006; Qin & Northoff, 2011; van der Meer et al., 2010), we found that self-related judgments were associated with relatively ventral mPFC (BA 10) and anterior paracingulate cortex (BA 32) whereas other-related judgments were associated with relatively dorsal mPFC (BA 8 & 9).
In addition, self-related judgments were associated with almost entirely left-lateralized activity, including left ventrolateral PFC (vlPFC), left anterior and mid-insula, and dorsal caudate. This stands in contrast to prior work that has associated self-related judgments with right-lateralized activity (Keenan, Ganis, Freund, & Pascual-Leone, 2000; Keenan, Wheeler, Gallup, & Pascual-Leone, 2000). Ventrolateral PFC, and in particular left vlPFC, has been associated with retrieval of information from semantic memory (Badre & Wagner, 2007; Thompson-Schill, Bedny, & Goldberg, 2005), and this represents a plausible functional explanation in this case, given task demands that required recalling personally-relevant information. The left anterior insula findings are consistent with the results of another recent self-other meta-analysis (Qin et al., 2011; Qin & Northoff, 2011) and are consistent with a role for interoception in self-related awareness that involves the anterior insula (Craig, 2009; Denny, Ochsner, Weber, & Wager, in prep.; Wager & Barrett, 2004). In addition, the caudate body activity reported here may reflect a connection between self-focused mentalizing and generalized reward processing, consistent with the notion that there are inherently rewarding aspects to the self (Enzi, de Greck, Prosch, Tempelmann, & Northoff, 2009).
Taken together, the present results are largely consistent with those of prior meta-analyses (Gilbert et al., 2006; Northoff et al., 2006; Van Overwalle, 2009, 2011; Van Overwalle & Baetens, 2009). Specifically, like Northoff and colleagues, we found evidence for self-related judgments being reliably associated with activation along the cortical midline, including mPFC, anterior cingulate, and posterior cingulate, particularly for the self vs. baseline comparison, though activation was not exclusive to the midline. Consistent with Gilbert and colleagues, we found that mentalizing (i.e. attention to mental state of either self or other) was associated with medial activation within mPFC, including BA 10. In addition, our results are consistent with those of Van Overwalle (2011), who found that mPFC activation is positively associated with degree of mentalizing content, though in the present analysis we did not characterize the degree to which mentalizing is involved in each contrast.
Further, these results are largely consistent with two meta-analyses that have directly compared self- and other-referential processing, replicating the finding that self is particularly associated with ventral rather than dorsal mPFC (Qin & Northoff, 2011; van der Meer et al., 2010). Critically, however, neither of those meta-analyses provided evidence for dmPFC being specifically associated with other vs. self processing, as we have reported here. In the case of van der Meer and colleagues, this may have been due to either the reduced number of included contrasts or the fact that only trait adjective judgment studies were included. The reason for the discrepancy is less clear in the case of Qin and Northoff given that the authors had a relatively large self database and used MKDA. One possible explanation is the fact that their Other condition was restricted to a smaller number of non-familiar Other contrasts, which likely reduced power to detect differences in dmPFC. While Qin and Northoff also compared Self contrasts to a relatively small number of familiar Other contrasts, this analysis also did not reveal dmPFC activity, nor did a contrast of familiar relative to non-familiar others.
Discerning the Spatial and Functional Organization of mPFC
Critically, though prior meta-analyses have addressed relative processing dissociations as described above, this meta-analysis represents the first direct examination of the spatial organization in mPFC of subregions supporting judgments about the self and others. Using a binary logistic regression analysis that was independent of the MKDA and independent of any spatial smoothing, we found that self or other judgment status was significantly predicted by location along the dorsal-ventral axis in mPFC. Thus, the present data argue that the representation of processes supporting judgments about the self and others in mPFC is organized as a gradient rather than discrete modules.
Strikingly, the results of this logistic regression stand in contrast to the results of the MKDA analysis directly comparing self- and other-related judgments. Recall that this comparison showed apparently discrete and separate dorsal and ventral mPFC regions associated with other- and self-related judgments, respectively. It is only with the logistic regression analysis that the spatial gradient for self- and other-judgments was observed. The notion that some apparent double dissociations may be more accurately conceptualized as gradients has been described in other domains in the physiology and neuroimaging literature (Fuster, 2003, 2006), including in an fMRI examination of the medial temporal lobe regarding response profiles to objects and scenes (Litman, Awipi, & Davachi, 2009). In all cases, contrasts comparing only the categories represented at the end-points of a gradient can only show apparent double dissociations. In order to detect a gradient, one needs a finer-grained analysis that takes into account regions spanning the end points - as we did with the logistic regression used here.
In addition, the current results are consistent with what would be expected based on prior anatomical and parametric functional investigations in mPFC. Connectivity analyses within anterior mPFC have revealed a ventrally mediated viscerolimbic affective and self-relevance network (with connections to amygdala, insula, and nucleus accumbens) and a dorsally mediated cognitive network (with connections to dorsolateral PFC and hippocampus) (Kim & Whalen, 2009; Price, Carmichael, & Drevets, 1996; Schmitz & Johnson, 2006). Thus, increasingly ventral mPFC is more associated with processing self-relevant judgments, whereas dorsal mPFC is relatively more attuned to focusing attention on making judgments about the external world. This view is also consistent with the notion, derived from a recent review of the functional neuroimaging literature, that vmPFC supports relatively stimulus-driven processes that may be important for computing the value of stimuli in a current judgment context, whereas dmPFC supports more reflective processes used for selecting higher level social and affective meanings (Olsson & Ochsner, 2008).
Relationship to Default Mode Hypothesis
The present results are also interesting when viewed in the context of the default mode hypothesis, which posits that a large network of primarily medial brain structures including mPFC subserve a neural default state, given the observation that these structures show decreased activation during performance of goal-directed tasks relative to resting state activity (Gusnard, Akbudak, Shulman, & Raichle, 2001; Mason et al., 2007; Raichle et al., 2001). The present results suggest that increasingly ventral activity is more strongly associated with self-related “default” functional activity, given that mPFC activity is also prominently observed during rest. It is notable, however, that Gusnard and colleagues (2001) addressed this question with a neuroimaging task involving internally versus externally-directed attention and came to a divergent conclusion, finding internally-directed (self-referential) attention to be more associated with dorsal mPFC activity. While this particular discrepant result could stem from multiple causes, we included the results from this study in the present meta-analysis and found that while both ventral and dorsal mPFC were active for both self and other judgments, self judgments were more strongly associated with ventral relative to dorsal mPFC activity across all studies. Critically, Qin and Northoff (2011) directly compared self and other-related activity to activity from tasks that showed greater activity to rest than to task (i.e. putative default mode network localizer tasks) and showed overlap between self-related processing and default mode network activity in perigenual anterior cingulate cortex, near the self-related vmPFC focus reported in the current MKDA.
Conclusions
Based on results of a quantitative meta-analysis, we have found broad overlap in the neural correlates of making self and other-related judgments in multiple brain regions, including both ventral and dorsal mPFC in addition to left TPJ and posterior cingulate. Direct comparisons between judgments of self and others yielded a distributed set of regions that are relatively more active for self judgments, including vmPFC, left vlPFC, and left caudate, and another set of regions that are more active for other judgments, including dmPFC, bilateral TPJ and cuneus. Most critically, we have provided evidence for a spatial mentalizing gradient in mPFC. While both ventral and dorsal mPFC were significantly engaged for judgments about self and others, increasingly ventral mPFC regions were more strongly associated with making judgments about the self, and increasingly dorsal mPFC regions were more strongly involved in making judgments about others. Thus, we argue that these results support a distributed rather than a localizationist account (cf. Poldrack, 2008) of the neural mechanisms that support judgments about the self others. Future work may investigate whether the gradient described here is specific to certain types of judgments (e.g. affective, cognitive, or non-mentalizing judgments pertaining to the self and others, etc). Interrogating relative neural processing dissociations for evidence of gradient or distributed activation networks represents an emerging direction in social cognitive neuroscience research.
Supplementary Material
Acknowledgements
We would like to thank Sam Gershman, Josh Joseph, Jenny Liu, Chukwudi Onyemekwu, Tiffany Joy Vaglica, Jamil Zaki, Joshua Davis, Andreas Olsson, and Matthew Davidson for their assistance with preparation of the database and Jochen Weber for his assistance with data visualization. This work was supported by NIH Grant R01 MH076137 to KNO, NIH Grant R21 MH082308 to TDW, and a National Science Foundation Graduate Research Fellowship to HK.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bower GH, Gilligan SG. Remembering information related to one's self. Journal of Research in Personality. 1979;13(4):420–432. [Google Scholar]
- Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatry Rep. 2002;4(4):254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD. When preparation fails: Prefrontal activity in anticipation of the need to reappraise predicts increased amygdala activity and reduced reappraisal success. (in prep.). [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Enzi B, de Greck M, Prosch U, Tempelmann C, Northoff G. Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS One. 2009;4(12):e8429. doi: 10.1371/journal.pone.0008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Brain systems underlying anxiety disorders: a view from neuroimaging. In: Simpson HB, Schneier F, Neria Y, Lewis-Fernandez R, editors. Anxiety Disorders: Theory, Research and Clinical Perspectives. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and Mind: Unifying Cognition. New York: Oxford University Press; 2003. [Google Scholar]
- Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol. 2006;60(2):125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of 'theory of mind'. Trends Cogn Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull. 2005;131(1):76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Goldman AI. In defense of the simulation theory. Mind & Language. 1992;7(1–2):104–119. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan JP, Ganis G, Freund S, Pascual-Leone A. Self-face identification is increased with left hand responses. Laterality. 2000;5(3):259–268. doi: 10.1080/713754382. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Wheeler MA, Gallup GG, Jr, Pascual-Leone A. Self-recognition and the right prefrontal cortex. Trends Cogn Sci. 2000;4(9):338–344. doi: 10.1016/s1364-6613(00)01521-7. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Kihlstrom JF. Elaboration, organization, and the self-reference effect in memory. Journal of Experimental Psychology: General. 1986;115(1):26–38. doi: 10.1037//0096-3445.115.1.26. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Wager TD. Meta-analyses of neuroimaging data. Wiley Interdisciplinary Reviews. 2010;1:293–300. doi: 10.1002/wcs.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper NA, Rogers TB. Encoding of personal information: Self-other differences. Journal of Personality and Social Psychology. 1979;37(4):499–514. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev. 2009;116(1):252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences. 2008 doi: 10.1017/S0140525X11000446. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19(3):308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychological Review. 1991;98(2):224–253. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mor N, Winquist J. Self-focused attention and negative affect: a meta-analysis. Psychol Bull. 2002;128(4):638–662. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Current directions in social cognitive neuroscience. Curr Opin Neurobiol. 2004;14(2):254–258. doi: 10.1016/j.conb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12(2):65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, Wang Y, Duncan N, Gong Q, et al. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: A combined fMRImeta- analytic study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35(9):677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Metaanalysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45(3):810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsalventral aMPFC networks. Neuroimage. 2006;30(3):1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedikides C, Skowronski JJ. The symbolic self in evolutionary context. Personality and Social Psychology Review. 1997;1(1):80–102. doi: 10.1207/s15327957pspr0101_6. [DOI] [PubMed] [Google Scholar]
- Stich S, Nichols S. Folk psychology: Simulation or tacit theory? Mind & Language. 1992;7(1–2):35–71. [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychol Bull. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3- dimensional proportional system – An approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15(2):219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30(3):829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54(2):1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF. From affect to control: Functional specialization of the insula in motivation and regulation. PsycExtra. 2004 Retrieved July 5, 2011, from http://psych-www.colorado.edu/~tor/Papers/Wager_Feldman_Barrett_2004_Insula_meta-analysis.pdf.
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2(2):150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN. You, me, my brain: Self and other representation in social cognitive neuroscience. In: Todorov A, Fiske ST, Prentice D, editors. Social neuroscience: Toward understanding the underpinnings of the social mind. New York: Oxford University Press; 2011. pp. 14–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.