Abstract
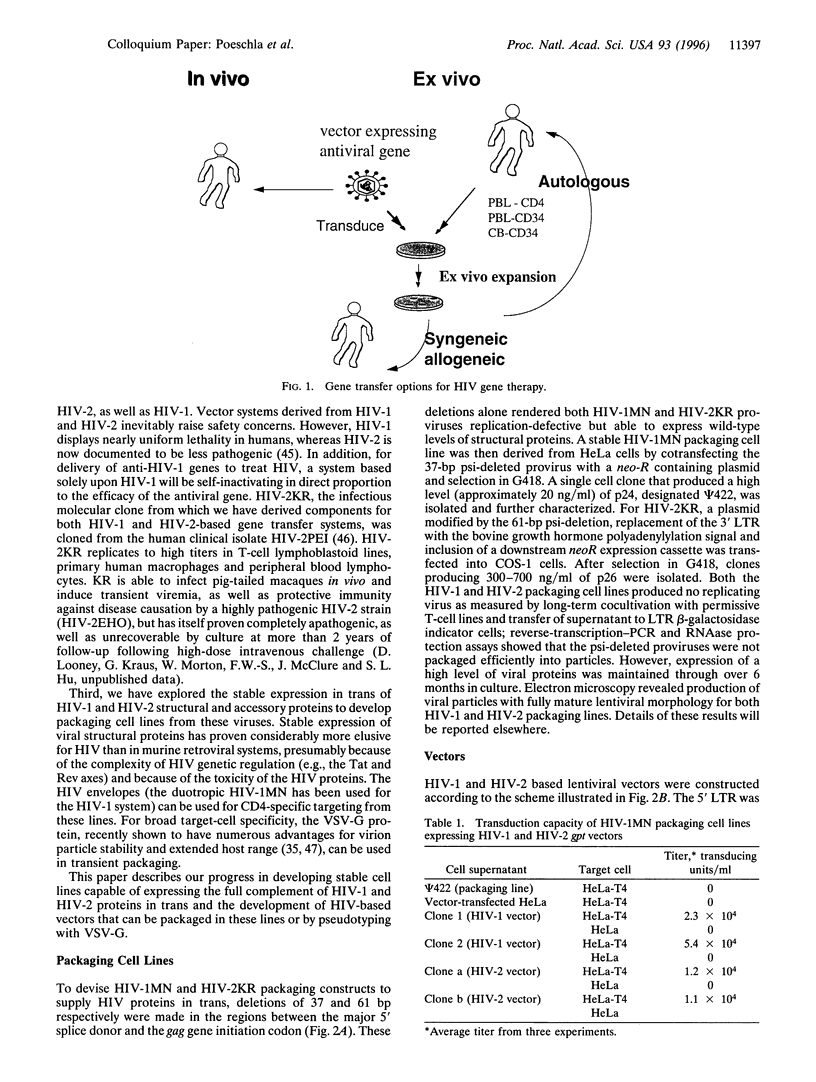
Current gene therapy protocols for HIV infection use transfection or murine retrovirus mediated transfer of antiviral genes into CD4+ T cells or CD34+ progenitor cells ex vivo, followed by infusion of the gene altered cells into autologous or syngeneic/allogeneic recipients. While these studies are essential for safety and feasibility testing, several limitations remain: long-term reconstitution of the immune system is not effected for lack of access to the macrophage reservoir or the pluripotent stem cell population, which is usually quiescent, and ex vivo manipulation of the target cells will be too expensive and impractical for global application. In these regards, the lentivirus-specific biologic properties of the HIVs, which underlie their pathogenetic mechanisms, are also advantageous as vectors for gene therapy. The ability of HIV to specifically target CD4+ cells, as well as non-cycling cells, makes it a promising candidate for in vivo gene transfer vector on one hand, and for transduction of non-cycling stem cells on the other. Here we report the use of replication-defective vectors and stable vector packaging cell lines derived from both HIV-1 and HIV-2. Both HIV envelopes and vesicular stomatitis virus glycoprotein G were effective in mediating high-titer gene transfer, and an HIV-2 vector could be cross-packaged by HIV-1. Both HIV-1 and HIV-2 vectors were able to transduce primary human macrophages, a property not shared by murine retroviruses. Vesicular stomatitis virus glycoprotein G-pseudotyped HIV vectors have the potential to mediate gene transfer into non-cycling hematopoietic stem cells. If so, HIV or other lentivirus-based vectors will have applications beyond HIV infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkina R. K., Walton R. M., Chen M. L., Li Q. X., Planelles V., Chen I. S. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996 Apr;70(4):2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M., Prasad S., Dubay J. W., Hunter E., Jeang K. T., Rekosh D., Hammarskjöld M. L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchschacher G. L., Jr, Panganiban A. T. Human immunodeficiency virus vectors for inducible expression of foreign genes. J Virol. 1992 May;66(5):2731–2739. doi: 10.1128/jvi.66.5.2731-2739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R., Lin J. T., Dacquel E. J., Mosca J. D., Burke D. S., St Louis D. C. A human immunodeficiency virus type 1 (HIV-1)-based retroviral vector system utilizing stable HIV-1 packaging cell lines. J Virol. 1994 Sep;68(9):6047–6051. doi: 10.1128/jvi.68.9.6047-6051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genetic diversity and evolution of retroviruses. Curr Top Microbiol Immunol. 1992;176:143–164. doi: 10.1007/978-3-642-77011-1_10. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995 Jan 27;267(5197):483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- Dropulić B., Lin N. H., Martin M. A., Jeang K. T. Functional characterization of a U5 ribozyme: intracellular suppression of human immunodeficiency virus type 1 expression. J Virol. 1992 Mar;66(3):1432–1441. doi: 10.1128/jvi.66.3.1432-1441.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Goodman S., Xiao X., Donahue R. E., Moulton A., Miller J., Walsh C., Young N. S., Samulski R. J., Nienhuis A. W. Recombinant adeno-associated virus-mediated gene transfer into hematopoietic progenitor cells. Blood. 1994 Sep 1;84(5):1492–1500. [PubMed] [Google Scholar]
- Heim R., Cubitt A. B., Tsien R. Y. Improved green fluorescence. Nature. 1995 Feb 23;373(6516):663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Heinzinger N. K., Bukrinsky M. I., Haggerty S. A., Ragland A. M., Kewalramani V., Lee M. A., Gendelman H. E., Ratner L., Stevenson M., Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch M., Kraus G., Johnson P., Wong-Staal F. Intracellular immunization against SIVmac utilizing a hairpin ribozyme. Virology. 1996 Feb 1;216(1):241–244. doi: 10.1006/viro.1996.0055. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D. Viral counts count in HIV infection. Science. 1996 May 24;272(5265):1124–1125. doi: 10.1126/science.272.5265.1124. [DOI] [PubMed] [Google Scholar]
- Leavitt M. C., Yu M., Wong-Staal F., Looney D. J. Ex vivo transduction and expansion of CD4+ lymphocytes from HIV + donors: prelude to a ribozyme gene therapy trial. Gene Ther. 1996 Jul;3(7):599–606. [PubMed] [Google Scholar]
- Leavitt M. C., Yu M., Yamada O., Kraus G., Looney D., Poeschla E., Wong-Staal F. Transfer of an anti-HIV-1 ribozyme gene into primary human lymphocytes. Hum Gene Ther. 1994 Sep;5(9):1115–1120. doi: 10.1089/hum.1994.5.9-1115. [DOI] [PubMed] [Google Scholar]
- Lin S., Gaiano N., Culp P., Burns J. C., Friedmann T., Yee J. K., Hopkins N. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science. 1994 Jul 29;265(5172):666–669. doi: 10.1126/science.8036514. [DOI] [PubMed] [Google Scholar]
- Marlink R., Kanki P., Thior I., Travers K., Eisen G., Siby T., Traore I., Hsieh C. C., Dia M. C., Gueye E. H. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994 Sep 9;265(5178):1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Rinaldo C. R., Jr, Gupta P., White R. M., Todd J. A., Kingsley L. A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996 May 24;272(5265):1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Fox B. A., Post L., Thompson C. B., Woffendin C. A molecular genetic intervention for AIDS--effects of a transdominant negative form of Rev. Hum Gene Ther. 1994 Jan;5(1):79–92. doi: 10.1089/hum.1994.5.1-79. [DOI] [PubMed] [Google Scholar]
- Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Ojwang J. O., Hampel A., Looney D. J., Wong-Staal F., Rappaport J. Inhibition of human immunodeficiency virus type 1 expression by a hairpin ribozyme. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10802–10806. doi: 10.1073/pnas.89.22.10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Parolin C., Dorfman T., Palú G., Göttlinger H., Sodroski J. Analysis in human immunodeficiency virus type 1 vectors of cis-acting sequences that affect gene transfer into human lymphocytes. J Virol. 1994 Jun;68(6):3888–3895. doi: 10.1128/jvi.68.6.3888-3895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson A. S., Neumann A. U., Markowitz M., Leonard J. M., Ho D. D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996 Mar 15;271(5255):1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Poeschla E., Wong-Staal F. Antiviral and anticancer ribozymes. Curr Opin Oncol. 1994 Nov;6(6):601–606. doi: 10.1097/00001622-199411000-00012. [DOI] [PubMed] [Google Scholar]
- Poznansky M., Lever A., Bergeron L., Haseltine W., Sodroski J. Gene transfer into human lymphocytes by a defective human immunodeficiency virus type 1 vector. J Virol. 1991 Jan;65(1):532–536. doi: 10.1128/jvi.65.1.532-536.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. H., Kaye J. F., Child L. A., Lever A. M. Helper virus-free transfer of human immunodeficiency virus type 1 vectors. J Gen Virol. 1995 Mar;76(Pt 3):691–696. doi: 10.1099/0022-1317-76-3-691. [DOI] [PubMed] [Google Scholar]
- Richman D. D. Resistance, drug failure, and disease progression. AIDS Res Hum Retroviruses. 1994 Aug;10(8):901–905. doi: 10.1089/aid.1994.10.901. [DOI] [PubMed] [Google Scholar]
- Rizvi T. A., Panganiban A. T. Simian immunodeficiency virus RNA is efficiently encapsidated by human immunodeficiency virus type 1 particles. J Virol. 1993 May;67(5):2681–2688. doi: 10.1128/jvi.67.5.2681-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. J., Elkins D., Zaia J. A., Sullivan S. Ribozymes as anti-HIV-1 therapeutic agents: principles, applications, and problems. AIDS Res Hum Retroviruses. 1992 Feb;8(2):183–189. doi: 10.1089/aid.1992.8.183. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Holodniy M., Kuritzkes D. R., O'Brien W. A., Coombs R., Poscher M. E., Jacobsen D. M., Shaw G. M., Richman D. D., Volberding P. A. HIV viral load markers in clinical practice. Nat Med. 1996 Jun;2(6):625–629. doi: 10.1038/nm0696-625. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Shimada T., Fujii H., Mitsuya H., Nienhuis A. W. Targeted and highly efficient gene transfer into CD4+ cells by a recombinant human immunodeficiency virus retroviral vector. J Clin Invest. 1991 Sep;88(3):1043–1047. doi: 10.1172/JCI115365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Brichacek B., Heinzinger N., Swindells S., Pirruccello S., Janoff E., Emerman M. Molecular basis of cell cycle dependent HIV-1 replication. Implications for control of virus burden. Adv Exp Med Biol. 1995;374:33–45. doi: 10.1007/978-1-4615-1995-9_4. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Small catalytic RNAs. Annu Rev Biochem. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]
- Talbott R., Kraus G., Looney D., Wong-Staal F. Mapping the determinants of human immunodeficiency virus 2 for infectivity, replication efficiency, and cytopathicity. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4226–4230. doi: 10.1073/pnas.90.9.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasinghe M., Liem S. E., Asad S., Read S. E., Joshi S. Resistance to human immunodeficiency virus type 1 (HIV-1) infection in human CD4+ lymphocyte-derived cell lines conferred by using retroviral vectors expressing an HIV-1 RNA-specific ribozyme. J Virol. 1991 Oct;65(10):5531–5534. doi: 10.1128/jvi.65.10.5531-5534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995 Jan 12;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Yamada O., Kraus G., Leavitt M. C., Yu M., Wong-Staal F. Activity and cleavage site specificity of an anti-HIV-1 hairpin ribozyme in human T cells. Virology. 1994 Nov 15;205(1):121–126. doi: 10.1006/viro.1994.1626. [DOI] [PubMed] [Google Scholar]
- Yamada O., Yu M., Yee J. K., Kraus G., Looney D., Wong-Staal F. Intracellular immunization of human T cells with a hairpin ribozyme against human immunodeficiency virus type 1. Gene Ther. 1994 Jan;1(1):38–45. [PubMed] [Google Scholar]
- Yu M., Leavitt M. C., Maruyama M., Yamada O., Young D., Ho A. D., Wong-Staal F. Intracellular immunization of human fetal cord blood stem/progenitor cells with a ribozyme against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):699–703. doi: 10.1073/pnas.92.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Ojwang J., Yamada O., Hampel A., Rapapport J., Looney D., Wong-Staal F. A hairpin ribozyme inhibits expression of diverse strains of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6340–6344. doi: 10.1073/pnas.90.13.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Poeschla E., Wong-Staal F. Progress towards gene therapy for HIV infection. Gene Ther. 1994 Jan;1(1):13–26. [PubMed] [Google Scholar]
- Yu M., Poeschla E., Yamada O., Degrandis P., Leavitt M. C., Heusch M., Yees J. K., Wong-Staal F., Hampel A. In vitro and in vivo characterization of a second functional hairpin ribozyme against HIV-1. Virology. 1995 Jan 10;206(1):381–386. doi: 10.1016/s0042-6822(95)80053-0. [DOI] [PubMed] [Google Scholar]




