Abstract
A novel liposomal formulation of docetaxel targeting the folate receptor (FR) was synthesized and characterized. Liposomal formulations are less toxic and can provide longer systemic circulation time than the Tween 80 and ethanol based clinical formulation of docetaxel. Folate receptor-α (FR) is frequently over-expressed on epithelial cancer cells. Therefore, FR targeted liposomes can potentially enhance tumor cell uptake and antitumor efficacy of encapsulated drugs. The formulation studied had the compositions of egg phosphatidylcholine/cholesterol/methoxy-polyethylene glycol (PEG)2,000-distesroylphnosphatidylethanolamine/folate-PEG3,350-cholesteryl hemisuccinate (ePC/Chol/mPEG-DSPE/folate-PEG-CHEMS) at ratios of (80:15:4.5:0.5, mol/mol) and a drug-to-lipid ratio of 1:20, wt/wt. Sucrose was used as a lyoprotectant. The liposomes were prepared by thin-film hydration, polycarbonate membrane extrusion, followed by lyophilization. They remained stable for more than 5 months when stored as lyophilized powder and for 72 h at 4 °C following rehydration. The mean particle size of reconstituted liposomes ranged from 110 to 120 nm. FR-targeted liposomes of the same lipid composition entrapping calcein were shown to be efficiently taken up by FR + KB oral carcinoma cells. FR-targeted liposomes containing docetaxel showed 4.4-fold greater cytotoxicity compared to non-targeted liposomes in KB cells. Plasma clearance profiles of FR-targeted and non-targeted liposomeal docetaxel were evaluated and compared with that of docetaxel in Tween 80/ethanol formulation. The liposomal formulations showed much longer terminal half lives (4.92 h and 6.75 h for FR-targeted and non-targeted, respectively) than docetaxel in Tween 80/ethanol solution (1.09 h). FR-targeted liposomes are promising tumor cell-selective nanocarriers for docetaxel with potential for therapeutic applications.
Keywords: Docetaxel, Liposomes, Folate Receptor, Targeted Drug Delivery, Cancer
1. Introduction
Docetaxel is a microtubule stabilizing drug and a potent chemotherapeutic agent that has shown substantial clinical efficacy for ovarian, breast, colon, head and neck, and non-small cell lung cancers.1–3 Due to its poor aqueous solubility, the current clinical formulation, Taxotere™, consists of a solution of Tween 80/ethanol.4,5 This vehicle has been associated with several hypersensitivity reactions and has shown incompatibility with common PVC tubings used for intravenous administration.6 It interferes with the normal binding of docetaxel to serum proteins in a concentration dependent-manner and can modulate the pharmacokinetics of docetaxel in vivo.7 In order to eliminate the toxicity related to Tween 80-based vehicle, alternative vehicles such as liposomes have been investigated.8–10
Tumor cell targeting is a promising strategy for enhancing the therapeutic potential of chemotherapy agents. Folate receptor (FR) is a high affinity folate binding protein that has two glycosylphosphatidylinositol (GPI)-anchored isoform, α and β. Normal tissues generally lack FR expression. In contrast, FR-α is frequently over-expressed in epithelial cancers including over 90% of ovarian carcinomas.11–14 A number of FR-targeted therapeutic and imaging agents have been evaluated in preclinical studies, including liposomal agents, with promising results.15–19 Targeting of liposomes with phospholipid-anchored folate conjugates is an attractive approach for the delivery of chemotherapeutic agents to FR expressing tumors. The use of polyethylene glycol (PEG)-coated liposomes with folate attached to the distal termini of a small fraction of phospholipid-anchored PEG molecules appears to be the most appropriate way to combine long-circulating properties critical for liposome deposition in tumors and binding of liposomes to FR on tumor cells.20
In this study, a lyophilized FR-targeted liposomal formulation of docetaxel was synthesized and characterized in terms of its stability, FR-dependent cytotoxicity, and pharmacokinetic properties in mice.
2. Materials and Methods
2.1. Chemicals
Docetaxel was a gift from Jiangsu Hengrui Medicine Co., Ltd (China). Monomethoxy polyethylene glycol 2,000-distearoyl phosphatidylethanolamine (mPEG-DSPE) was purchased from Avanti Polar Lipids (Alabaster, AL). Polyoxyethylene bisamine (M.W. 3350, H2N-PEG-NH2), cholesteryl hemisuccinate (CHEMS), N-hydroxysuccinimide (NHS), dicyclohexylcarbodiimide (DCC), disuccinimidyl suberate (DSS), N-hydroxysuccinimide (NHS), folic acid (FA) and triethylamine (TEA) were all purchased from Sigma Chemical Co. (St. Louis, MO, USA). All reagents and solvents were of analytical or HPLC grade and were used without further purification.
2.2. Synthesis of Folate-PEG-CHEMS
The synthesis of folate-PEG-CHEMS was conducted by reacting folate-PEG-amine with CHEMS-NHS.21 Folate-PEG-amine and CHEMS-NHS were prepared according to methods described previously by Wu et al.22 and Kempen et al.,23 respectively. These two reacting substances were then dissolved in CHCl3 and reacted overnight at 25 °C. The resulting products formed micelles in the Na2CO3 solution, and micelles were dialyzed against deionized water to remove by-products. Finally, folate-PEG-CHEMS was obtained by lyophilization.
2.3. Liposome Preparation
Liposomes were prepared by polycarbonate membrane extrusion.22 The lipid compositions of the FR-targeted liposomes and non-targeted liposomes were ePC/Chol/mPEG-DSPE/folate-PEG-CHEMS at molar ratios of 80:15:4.5:0.5 and ePC/Chol/mPEG-DSPE at molar ratios of 80:15:5, respectively. Briefly, lipid ingredients and docetaxel, at a drug-to-lipid ratio of 1:20 (wt/wt) were dissolved in CHCl3 and dried on a rotary evaporation device in a round-bottom flask and then under vacuum. The lipid film was then hydrated with 10% (w/v) lyoprotectant solution to produce multilamellar vesicles (MLV). The lipid suspension was then extruded 5 times each through 0.2 μm and then 0.1 μm pore size polycarbonate membranes on a Lipex lipid extruder from Northern Lipids Inc. driven by high pressure nitrogen. The particles size distribution was determined by dynamic light scattering using a NICOMP Sub-micron Particle Sizer Model 370 (Nicomp, Santa Barbara, CA). For lyophilization, the resulting liposome suspensions were dispensed into 10 mL vials (2 mL each vial), then rapidly transferred to a freezer at −80 °C for at least 2 h to solidify the solvent, and subsequently transferred to a Labconco Lyphlok 12 freeze-dryer using a program of 48 h at −44 °C for primary drying and 12 h at 20 °C for secondary drying. Three lyoprotectants, glucose, mannitol and surcrose, were evaluated and compared for formulation stability.
Fluorescent liposomes were prepared by the same method except that the lipid was initially hydrated in 50 mM calcein. The liposomes were then purified by size exclusion chromatography on a Sepharose CL-4B column equilibrated in phosphate buffered saline (PBS, 130 mM NaCl, 20 mM Na2HP04, adjusted to pH 7.4).
The amount of docetaxel in liposome suspensions was determined by HPLC. Briefly, HPLC analysis was performed with an isocratic solvent system (70% methanol, 30% water) at a flow rate of 1 mL/min, and docetaxel was detected by absorbance at 229 nm. A Beckman HPLC system was used, consisting of a Model 126 pump, 166 detector, 507 autosampler and System Gold software. An AllTech RP C18 column (4.1 × 300 mm) was used for analysis.
Free docetaxel was separated from the liposomal docetaxel by dialysis.8 The liposome suspensions before or after dialysis were dissolved in methanol for HPLC assay to determine the total drug content remaining in liposome. The entrapment efficiency was calculated as the ratio of drug content incorporated in liposome to the total drug content of the suspension.
2.4. Physical Stability of Liposomes
Stability of lyophilized liposomal formulations after storage of 1 to 5 months was analyzed by determining the remaining drug and the vesicle size of liposome suspensions formed upon rehydration.
For stability study on rehydrated liposome suspension, changes in mean diameters and remaining drug were also monitored after 72 h of rehydrating the lyophilized powder of liposomes.
2.5. Cell Culture
KB, a human oral carcinoma cell line, which has high FR expression was cultured as a monolayer in folate-free RPMI 1640 media (Life Technologies, Inc., Bethesda, MD), supplemented with 50 μg/ml penicillin, 50 μg/ml streptomycin, and 10% fetal bovine serum and maintained in a humidified atmosphere containing 5% CO2 at 37 °C.
2.6. Uptake of FR-Targeted Fluorescent Liposomes by KB Cells
KB cells were suspended by a brief exposure to trypsin-EDTA (Invitrogen, Carlsbad, CA) and rinsed with pH 3.5 saline (130 mM NaCl, 20 mM NaAc) and then PBS at 4 °C to remove receptor-bound free folate. For liposome uptake studies, the cells were incubated with FR-targeted or non-targeted control liposomes, each containing 150 nM calcein, in folate-free RPMI1640 media for 60 min at 37 °C. The same experiment was repeated using media containing 1 mM free folic acid to determine the effect of FR blockade. At the end of incubation, cells were washed 3 times with cold PBS and then visualized and photographed on a Nikon Eclipse 800 fluorescence microscope.
2.7. Cyctotoxicity Analyses
Cytotoxixity of FR-targeted liposomal docetaxel was determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolum bromide (MTT) assay.22 KB cells were transferred to 96-well tissue culture plates at 6 × 103 cells per well 24 h prior to drug treatment. The culture medium was then replaced with 200 μL of medium containing serial dilutions of docetaxel formulations in FR-targeted liposomes, non-targeted control liposomes, or Tween 80/ethanol solutions. Following 2 h incubation at 37 °C, the cells were washed twice with PBS and cultured in fresh medium for an additional 72 hr. Then, 20 μL MTT stock solution (5 mg/mL) was added to each well and the plate was incubated for 4 h at 37 °C. Medium was then removed and DMSO was added to dissolve the blue formazan crystal converted from MTT. Cell viability was assessed by absorbance at 570 nm measured on a Biorad microplate reader.
2.8. Pharmacokinetic Studies
Pharmacokinetics of docetaxel in different formulations including a FR-targeted liposomal formulation, a non-targeting PEG-liposomal formulation and drug in Tween 80/ethanol vehicle was determined. Female ICR mice (18–22 g, Charles River Lab, Wilmington, MA) were used for the study. Mice, in groups of 3, received intravenous injections of docetaxel in various formulations at 15 mg/kg body weight via tail vein. Blood samples were collected in heparin-containing tubes at various time points (5, 20, 60, 180, 480, 960, 1440 min). Plasma was isolated by centrifugation (10 min at 4,000 rpm), stored at −20 °C, and subsequently analyzed for docetaxel content by HPLC. Extraction of docetaxel was accomplished by addition of 3 mL of tert-butyl methyl ether to 0.2 mL plasma samples and vortex mixing for 3 min. The mixture was then centrifuged for 10 min at 3,000 rpm at 4 °C, after which 2.5 mL of the organic layer was transferred to a clean tube and evaporated to dryness by RapidVap Concentrator (Labconco, Kansas City, MI, USA). For HPLC sample loading, 100 μL of methanol was used to dissolve the drug, and a 50 μL aliquot was injected onto the C18 column for analysis. The retention time for docetaxel was 8.3 min and linearity was obtained in the range of 0.1 to 25 μg/ml. Pharmacokinetic parameters were determined using the Winnonlin software. Parameters, including area under the curve (AUC), mean residence time (MRT), total body clearance (CL) and plasma half life for the distribution and elimination phase, were determined.
3. Results
3.1. Synthesis and Characterization of FR-Targeted Liposomes Containing Docetaxel
The choice of lyoprotectant is shown in Table I. Lyophilization of FR-targeted liposomal formulations prepared by polycarbonate membrane extrusion in the presence of sucrose, as a lyoprotectant, only resulted in a minor increase in particle size (from 104.5 to 117.7 nm) and a slight decrease in entrapment efficiency of docetaxel (from 96.4 to 94.3%). Compared with sucrose, glucose or mannitol, as a lyoprotectant, led to a significant decrease in entrapment fraction (9.4% for glucose, 13.4% for mannitol) and a significant increase in mean diameters (20.7% for glucose, 35.4% for mannitol) following reconsitution.
Table I.
The effect of different lyoprotects on liposomal preparations.
| Before lyophilization | After lyophilization | |||
|---|---|---|---|---|
|
|
||||
| Liposome lyoprotect | Encapsulated fraction (%) | Particle diameter (nm) | Encapsulated fraction (%) | Particle diameter (nm) |
| Glucose | 94.5±2.1 | 106.7±7.3 | 85.6±6.2 | 128.8±8.4 |
| Sucrose | 96.4±1.9 | 104.5±6.7 | 94.3±4.2 | 117.7±7.5 |
| Mannitol | 95.2±2.0 | 104.8±6.9 | 83.4±5.9 | 141.9±9.7 |
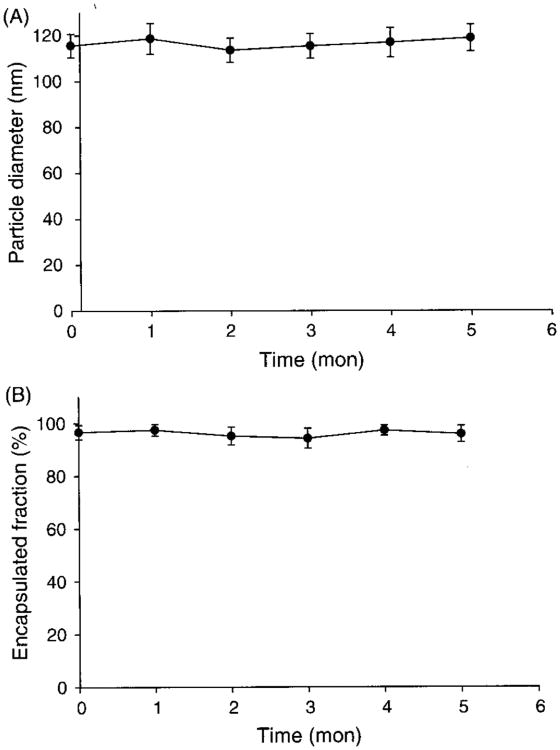
The stability of liposome formulations of docetaxel was monitored by changes in particle size and encapsulated fraction after rehydration during storage at 4 °C over a five-month period. As shown in Figure 1, the lyophilized formulation showed excellent stability during this period, and there were no visible changes to the physical appearance of the formulation or signs of drug precipitation following rehydration of the lyophilized formulation.
Fig.1.
The stability of lyophilized liposome formulation at 4 °C. Panel A shows the change of particle diameter and panel B shows the change of encapsulated fraction of liposome suspension after rehydration of lyophilized formulation (n = 5).
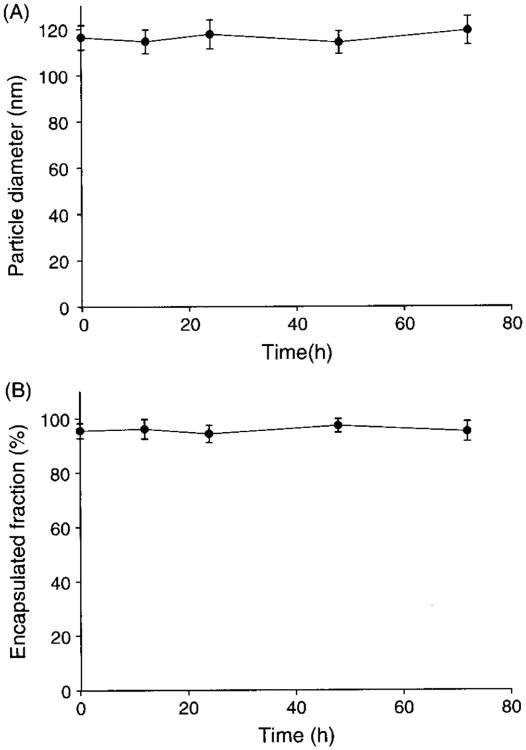
As for liposome suspension after rehydration of lyophilized formulation, there were no significant changes in mean diameter and encapsulated fraction stored at 4 °C for 72 h, as shown in Figure 2.
Fig. 2.
Colloidal stability of docetaxel liposomes. The changes of liposome suspensions after rehydration in particle diameter (A) and encapsulated fraction (B) stored at 4 °C for 72 h (n = 5).
3.2. Cellular Uptake and Cytotoxicity of FR-Targeted Liposomes
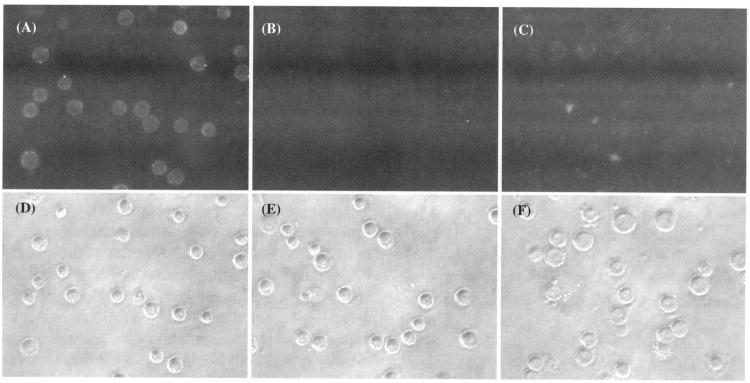
To determine whether folate might mediate the selective targeting of liposomes to cells, KB cells were incubated with FR-targeted or non-targeted liposomes encapsulating calcein, and were examined under a fluorescence microscope. As shown in Figure 3, FR-targeted liposomes showed much greater cellular uptake and liposome internalization compared to non-targeted control liposomes. The presence of excess free folic acid blocked the cell uptake of FR-targeted liposomes. These results indicated that these liposomes have efficient interactions with the cellular FR.
Fig. 3.
Uptake of FR-targeted liposomal calcein and non-targeted liposomal calcein by KB cells. The cells were treated with FR-targeted liposomal calcein, non-targeted liposomal calcein or FR-targeted liposomal calcein in medium containing 1 mM free folic acid and photographed in both the fluorescence (dark field) and phase-contrast modes (bright field) on a fluorescence microscope, as described in Section 2. (A) and (D): cells treated with FR-targeted liposomal calcein; (B) and (E): cells treated with non targeted liposomal calcein; (C) and (F): cells treated with FR-targeted liposomal calcein in medium containing 1 mM free folic acid.
Cytotoxicity studies were carried out on FR-targeted docetaxel liposomes, non-targeted docetaxel liposomes and docetaxel in Tween 80/ethanol formulation. The KB cell line was used in MTT assays and the results are summarized in Table II. The results showed that FR-targeted liposomal docetaxel had about 4.4 times lower IC50 value compared to that of non-targeted liposomal docetaxel and 2 times lower than that of docetaxel in Tween 80/ethanol. The differential in cytotoxicity was diminished in the presence of 1 mM free folate. These data suggest that the FR-targeted liposomal docetaxel exhibited FR-dependence of cytotoxicity.
Table II.
Cytotoxicity of docetaxel liposomes in KB cells determined by MTT assay.
| Administrated formulations of docetaxel | IC50 ± s.d.(nM) (n = 3) |
|---|---|
| FR-targeted liposomes | 16.4±4.8 |
| FR-targeted liposomes + 1 mM free folic acid | 48.5±10.2 |
| Non-targeted liposomes | 72.7±21.2 |
| Tween 80/ethanol solution | 33.3±9.1 |
IC50: docetaxel concentration giving 50% cell killing.
3.3. Pharmacokinetic Properties of FR-Targeted Liposomal Docetaxel
The pharmacokinetic behavior of docetaxel encapsulated in FR-targeted and non-targeted liposomes in mice after i.v. administration was assessed. Docetaxel in Tween 80/ehtanol formulation was used as a reference control.
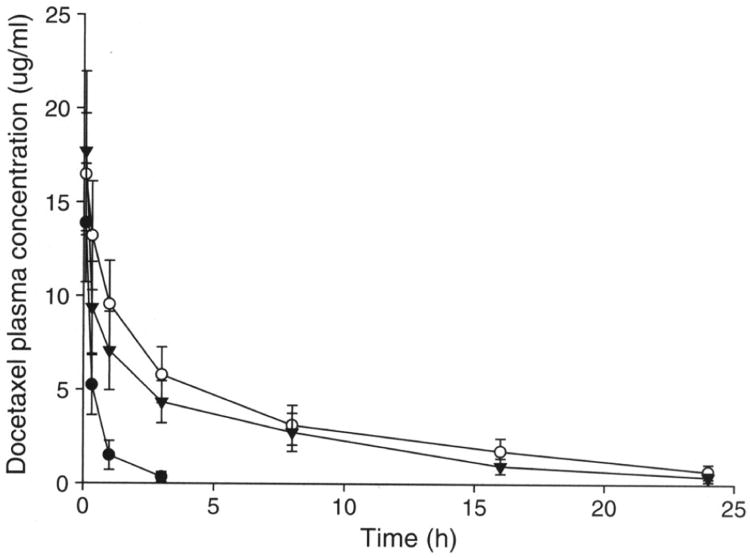
Figure 4 and Table III showed the docetaxel concentration in plasma versus time curves and the pharmacokinetic parameters obtained using Winnonlin for FR-targeted liposomes, non-targeted liposomes and drug dissolved in Tween 80/ethanol, based on the total docetaxel levels in plasma measured by HPLC.
Fig. 4.
Pharmacokinetic properties of docetaxel liposomes. Plasma concentration profiles of docetaxel after intravenous injection of Tween 80/ethanol (●), PEGylated liposome (○) or FR targeted liposome (▼) in mice that were given 15 mg/kg of docetaxel. Each data point represents the mean ± standard deviation of 3–5 mice.
Table III.
Pharmacokinetic parameters of docetaxel formulations in mice following i.v. bolus administration.
| Formulation | AUC (mg ml−1 min) | MRT (h) | t1/2α (h) | t1/2β(h) | CL(ml h−1) |
|---|---|---|---|---|---|
| Tween80/ethanol solution | 0.41±0.06 | 0.93±0.06 | 0.11±0.03 | 1.09±0.22 | 44.18±5.72 |
| Non-targeted liposomes | 4.92±0.60 | 9.00±1.31 | 0.47±0.09 | 6.75±1.03 | 3.66±0.38 |
| FR-targeted liposomes | 3.41±0.40 | 6.79±1.13 | 0.17±0.02 | 4.92±0.82 | 5.28±0.62 |
After i.v. bolus administration, docetaxel in Tween 80/ethanol followed a biphasic pattern of plasma clearance with relatively short distribution phase (tl/2α = 0.11 h) and terminal elimination phases (t1/2β = 1.09 h).
Encapsulation of docetaxel in liposomes produced significant changes in pharmacokinetic parameters. When given by i.v. bolus administration of non-targeted liposomes, the drug was distributed (tl/2α = 0.47 h) and eliminated much slower (t1/2β = 6.75 h). For FR-targeted liposomes, the drug was distributed with tl/2α = 0.17 h and eliminated slowly with t1/2β = 4.92 h. Differences between the free drug and liposomal drug were significant. The mean clearance value of free drug was 12 or 8.4 times greater than that of non-targeted liposomes or FR-targeted liposomes. This suggests that the longer half life of liposomes be a result of the reduced clearance rate.
4. Discussion
In this study we reported the preparation and characterization of a novel FR-targeted liposomal formulation of docetaxel. Folate-PEG-CHEM, a new ligand molecule targeting the FR was used as a component in the formulation, which achieved high uptake efficiency by KB cells. Previously targeting ligands such as folate-PEG-DSPE, 22 folate-PEG-Chol.20, 24–26 were reported in the studies on targeting liposomal drug delivery. Due to potentially rapid hydrolysis of the carbamate linkage of PEG-Chol, the FR targeting activities were decreased or lost gradually during storage. However, PEG-CHEMS with amide and ester linkages is more sable than folate-PEG-Chol, although the chemical structure is similar to that of folate-PEG-Chol PEG-CHEMS was firstly used by Xiang et al.21 in the liposomal formulation of doxorubicin showing high targeting efficiency by KB cells even after 3-month storage The purpose of our study expanded the application of new targeting ligand on the docetaxel liposomes.
According to previous studies,22,27,28 liposomal encapsulation of paclitaxel was generally not very efficient in liposomes composed of saturated lipids and/or containing cholesterol. Docetaxel is structurally similar to paclitaxel.29 Although docetaxel has better aqueous solubility compared to paclitaxel, unsaturation in the lipid acyl chains and the absence of high cholesterol content were found necessary to achieve improved drug incorporation.8 Cholesterol provides stability to the liposomes, which is important for prolonging liposomal systemic circulation time.30–34 It was found that incorporation of 15% cholesterol was able to provide good stability to liposomes without compromising docetaxel loading. Our liposomal docetaxel formulation contained 4.5% negatively charged phospholipids (mPEG-DSPE), which provided excellent physical stability and greater than 90% retention of initial drug content at 72 h after rehydrating the lyophilized powder. Therefore, multiple factors contributed to the stability of the formulation we have investigated: an unsaturated lipid, 15% cholesterol, and mPEG-DSPE, which is both a negatively charged and a PEGylated lipid, providing electrostatic as well as steric stabilization to the liposomal particles.35, 36
FR is a tumor marker that is consistently overexpressed in ovarian carcinomas. Results in this study are consistent with previous reports on FR-targeted liposomes.20,22,37,38 Folate-PEG-CHEMS as a ligand molecule provided good FR-targeting properties as shown by the KB cell uptake studies using fluorescent liposomes. The enhancement in cytotoxicity exhibited by FR-targeted liposomal docetaxel was 4.4-fold over the non-targeted control. The lack of a greater targeting ratio might be attributed to the docetaxel release into the media, which can be non-specifically taken up by tumor cells during the 2 hr incubation period. The results nonetheless demonstrated FR-dependence of the cytotoxicity.
Pharmacokinetic studies on the liposomal formulations of docetaxel indicated a longer systemic circulation time comparing to that of the Tween 80/ethanol formulation, which suggested in vivo stability of the formulation following i.v. administration. The AUCs of the liposomal formulations were much greater than those of the Tween 80/ethanol formulation. Based on the size of these liposomes and the long circulation time, it can be predicted that liposomal formulations may preferentially accumulate in solid tumors due to the enhanced permeability and retention (EPR) effect via a passive targeting mechanism.39–42
The FR-targeted formulation had faster clearance compared to the non-targeted control. This was consistent with previous studies on FR-targeted liposomes, which showed that folate-liposomes were preferentially taken up by liver and spleen of mice43 or rats44 and that the increased uptake was greater in rats on a folate free diet.44 Another important factor is that tumor infiltrating macrophages are likely FR positive. Turk et al.45 showed that FR-targeted liposomes preferentially target macrophages even in the presence of FR + tumor cells. This raises the possibility that FR + tumor infiltrating macrophages may constitute the actual target cell population in solid tumors. Even with these limitations, the FR-targeted liposomes can provide much longer blood circulation time and much better cytotoxicity to KB cells comparing to the current clinic Tween 80/ethanol formulation.
5. Conclusion
A novel FR-targeted liposomal formulation for docetaxel is reported. This formulation has good drug loading properties and exhibit excellent stability either in powder or in hydration state. Furthermore, the formulation can be efficiently taken up by FR + KB cells and exhibit FR-dependent cytotoxicity in these cells. Pharmacokinetic studies showed prolonged circulation time of the liposomal docetaxel relative to the Tween 80/ethanol formulation. This formulation could serve as a promising approach for future tumor specific therapy of the FR over-expressed cancer.
Acknowledgments
This work was supported in part by National Science Foundation (NSF) grant EEC-0425626.
References and Notes
- 1.Cortes JE, Pazdur R. J Clin Oncol. 1995;13:2643. doi: 10.1200/JCO.1995.13.10.2643. [DOI] [PubMed] [Google Scholar]
- 2.Huizing MT, Misser VH, Pieters RC, Ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, Pinedo HM, Beijnen JH. Cancer Invest. 1995;13:381. doi: 10.3109/07357909509031919. [DOI] [PubMed] [Google Scholar]
- 3.van Oosterom AT, Schrijvers D. Anticancer Drugs. 1995;6:356. doi: 10.1097/00001813-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Baker SD, Zhao M, He P, Carducci MA, Verweij J, Sparreboom A. Anal Biochem. 2004;324:276. doi: 10.1016/j.ab.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Colombo T, Parisi I, Zucchetti M, Sessa C, Goldhirsch A, D'lncalci M. Ann Oncol. 1999;10:391. doi: 10.1023/a:1008309916974. [DOI] [PubMed] [Google Scholar]
- 6.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Eur J Cancer. 2001;37:1590. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 7.Baker SD, Zhao M, He P, Carducci MA, Verweij J, Sparreboom A. Anal Biochem. 2004;324:276. doi: 10.1016/j.ab.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 8.immordino ML, Brusa P, Arpicco S, Stella B, Dosio F, Cattel L. J Control Release. 2003;91:417. doi: 10.1016/s0168-3659(03)00271-2. [DOI] [PubMed] [Google Scholar]
- 9.Straubinger RM, Balasubramanian SV. Methods Enzymol. 2005;391:97. doi: 10.1016/S0076-6879(05)91005-7. [DOI] [PubMed] [Google Scholar]
- 10.Liang G, Jia-Bi Z, Fei X, Bin N. J Pharm Pharmacol. 2007;59:661. doi: 10.1211/jpp.59.5.0006. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Gunning W, Ratnam M. Cancer Epidemiol Biomarkers Perv. 1999;8:770. [PubMed] [Google Scholar]
- 12.Elnakat H, Rantnam M. Adv Drug Deliv Rev. 2004;56:1067. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Kamen BA. Cancer Res. 1992;52:3396. [PubMed] [Google Scholar]
- 14.Ross JF, Wang H, Behm FG, Mathew P, Wu M, Booth R, Rantnam M. Cancer. 1999;85:348. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Pan XQ, Zheng X, Shi G, Wang H, Ratnam M, Lee RJ. Blood. 2002;100:594. doi: 10.1182/blood.v100.2.594. [DOI] [PubMed] [Google Scholar]
- 16.Sudimack J, Lee RJ. Adv Drug Deliv Rev. 2000;41:147. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Lee RJ. Adv Drug Delivery Rev. 2004;56:1193. doi: 10.1016/j.addr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Lee RJ, Low PS. Biochim Biophys Acta. 1995;1233:134. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- 19.Pan XQ, Lee RJ. Anticancer Res. 2005;25:343. [PubMed] [Google Scholar]
- 20.Zhao XB, Muthusamy N, Byrd JC, Lee RJ. J Pharm Sci. 2007;96:2424. doi: 10.1002/jps.20885. [DOI] [PubMed] [Google Scholar]
- 21.Xiang GY, Wu J, Lu YH, Liu ZL, Lee RJ. Int J Pharm. 2008;356:29. doi: 10.1016/j.ijpharm.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Liu Q, Lee RJ. Int J Pharm. 2006;316:148. doi: 10.1016/j.ijpharm.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Kempen HM. US Patents. 1988:4751219.
- 24.Guo W, Lee T, Sudimack J, Lee RJ. J Liposome Res. 2000;10:179. [Google Scholar]
- 25.Gosselin MA, Guo W, Lee RJ. Bioconjug Chem. 2002;13:1044. doi: 10.1021/bc025512c. [DOI] [PubMed] [Google Scholar]
- 26.Pan X, Lee RJ. Int J Pharm. 2007;336:276. doi: 10.1016/j.ijpharm.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, Feng SS, Kocherginsky N, Kostetski I. Int J Pharm. 2007;338:258. doi: 10.1016/j.ijpharm.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Feng SS. J Colloid Interface Sci. 2005;285:326. doi: 10.1016/j.jcis.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick FA, Wheeler R. Int Immunopharmacol. 2003;3:1699. doi: 10.1016/j.intimp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Bedu-Addo FK, Tang P, Xu Y, Huang L. Pharm Res. 1996;13:718. doi: 10.1023/a:1016043431778. [DOI] [PubMed] [Google Scholar]
- 31.Popova AV, Hincha DK. Biophys J. 2007;93:1204. doi: 10.1529/biophysj.107.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzi P, Mourtas S, Klepetsanis PG, Antimisiaris SG. Int J Pharm. 2007;333:167. doi: 10.1016/j.ijpharm.2006.09.059. [DOI] [PubMed] [Google Scholar]
- 33.Lee SC, Lee KE, Kim JJ, Lim SH. J Liposome Res. 2005;15:157. doi: 10.1080/08982100500364131. [DOI] [PubMed] [Google Scholar]
- 34.Kucerka N, Pencer J, Nieh MP, Katsaras J. Eur Phys J E Soft Matter. 2007;23:247. doi: 10.1140/epje/i2007-10202-8. [DOI] [PubMed] [Google Scholar]
- 35.Garbuzenko O, Zalipsky S, Qazen M, Barenholz Y. Langmuir. 2005;21:2560. doi: 10.1021/la0479105. [DOI] [PubMed] [Google Scholar]
- 36.Moribe K, Maruyama K, Iwatsuru M. Chem Pharm Bull (Tokyo) 1997;45:1683. doi: 10.1248/cpb.45.1683. [DOI] [PubMed] [Google Scholar]
- 37.Stevens PJ, Lee RJ. Anticancer Res. 2003;23:4927. [PubMed] [Google Scholar]
- 38.Stevens PJ, Sekido M, Lee RJ. Pharm Res. 2004;12:2153. doi: 10.1007/s11095-004-7667-5. [DOI] [PubMed] [Google Scholar]
- 39.Gabizon A, Martin F. Drugs. 1997;54(Suppl 4):15. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 40.Awasthi VD, Garcia D, Goins BA, Phillips WT. Int J Pharm. 2003;253:121. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- 41.Fang JY, Hung CF, Hwang TL, Huang YL. J Drug Target. 2005;13:19. doi: 10.1080/10611860400015977. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed M, Lukyanov AN, Torchilin V, Tournier H, Schneider AN, Goldberg SN. J Vase Interv Radiol. 2005;16:1365. doi: 10.1097/01.RVI.0000175324.63304.25. [DOI] [PubMed] [Google Scholar]
- 43.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. Clin Cancer Res. 2003;9:6551. [PubMed] [Google Scholar]
- 44.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Adv Drug Deliv Rev. 2004;56:1177. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Turk MJ, Waters DJ, Low PS. Cancer Lett. 2004;213:165. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]