Abstract
We are not aware of published cost-effectiveness studies addressing community transitional programs for HIV-infected jail detainees. To address this gap, data from 9 sites of EnhanceLink, a project that enrolled HIV-infected releasees from jails across the US, were examined. Figures on the number of clients served, cost of linkage services, number of linkages and 6-month sustained linkages to community HIV care, and number of clients achieving viral suppression were assessed for subjects released in the first quarter of 2010 (n = 543). The cost analysis included all costs that participating service agencies incurred. A cost-effectiveness analysis was conducted to estimate the new HIV cases averted by EnhanceLink and the cost per quality-adjusted life year saved by the program. The mean cost per linked client was $4,219; the mean cost per 6-month sustained linkage was $4,670; and the mean cost per client achieving viral suppression was $8,432. Compared to standard care, the cost per additional quality-adjusted life year saved was $72,285, suggesting that the EnhanceLink interventions were cost-effective from the societal perspective.
Introduction
In 2006, one-sixth of all HIV-infected persons in the US were incarcerated at least once [1]. Annually, 95 % of 150,000 HIV-infected persons leaving correctional institutions leave jails (short term facilities for those awaiting trial or serving brief sentences) [1]. Incarceration and subsequent community reentry often interrupts usual channels of healthcare, especially for short-term inmates [2]. The National HIV/AIDS Strategy advocates “unfettered access” to HIV care [3], as both a human right and a prevention strategy. Nonetheless, out of 377 Medline articles on HIV and incarceration in 2009, only 12 (3%) dealt exclusively with jails. HIV prevention strategies for jail releasees, and determining their cost-effectiveness, have received little attention in the prevention literature [4]. Cost-effective strategies for initiating or maintaining access to HIV care for those who move in and out of jails are needed.
A single city or county government runs the typical jail. Most receive little or no supplemental state or federal funding. Jails seldom have resources to expand services beyond what is critical to their core mission, promoting the safety of the public. By partnering with local health departments, jails can help their sister agencies attain health goals unachievable without cooperation (e.g., ensuring HIV-infected releasees link to care). The benefits realized by improved health outcomes, however, are often not viewed as part of justice reinvestment at the local level. Thus, while local jails may be the correct loci for public health interventions, and they may buy into the notion that collaborations constitute “good government”, the jails themselves seldom reap the benefits of such partnerships. The motivation to develop public health interventions in jail settings usually comes from informed leaders with a society-wide perspective. A demonstration that a particular intervention is cost effective and provides value to society as a whole could facilitate the allocation of funds to promote the health of the public in a correctional setting.
Although the full societal value of successfully linking HIV-positive detainees to medical care is unknown, there are key reasons to consider the cost and cost-effectiveness of such interventions. Successful and sustained linkage would be expected to improve the patient's health and quality of life. For patients prescribed ART, linkage would also be expected to reduce the likelihood that they would transmit HIV to sex partners. Specifically, a recent analysis suggests that linking patients to care, including ART, reduces the secondary HIV transmission rate [5]—which is defined as new cases per year per infected individual [6].
This study fills a void in the literature by analyzing the cost and cost-effectiveness of a program that links HIV-infected individuals who are released from jail settings to community-based HIV care. Data used in this analysis are from the Enhancing Linkages project (EnhanceLink) funded by the Health Resources and Services Administration (HRSA). As detailed previously [7], in 2007, HRSA released federal funds to assist select organizations with enhancing jail-based HIV testing and linkage of their positive clients to care. As a Special Project of National Significance, the ten site demonstration project was designed to develop several models of effective programming in a variety of settings—medium to large jails, in cities at different stages of the HIV epidemic, and in regions with varied drug use patterns.
Methods
A description of the varied services provided at each site has been previously published [7]. Clients began enrolling in the EnhanceLink program in 2008. Data for the present analysis reflect the first quarter of 2010, after the linkage programs were well-established at all study sites.
The EnhanceLink grantees were situated in the following ten cities: Atlanta, GA; Chester, PA; Chicago, IL; Cleveland, OH; Columbia, SC; New Haven, CT; New York, NY; Philadelphia, PA; Providence, RI; and Springfield, MA. Inclusion and exclusion criteria varied by site. For example, one chose to enroll only women; two excluded the seriously mentally ill. Resources from other funding streams could augment the budget of the transitional care programs. The program in New York, however, was excluded from the present analyses due to the magnitude of its budget, in which HRSA funds accounted for only one-seventh of expenditures, its incomparably large number of clients served, and the economies of scale realized at this site.
Sites provided case management services for HIV-infected jail detainees that began before release. Grantees were allowed to select how they allocated their resources related to pre- versus post-release services. Programs varied by emphasis placed on linking patients to psychiatric and substance abuse services.
Data Collection
Each quarter, sites submitted program level data to a coordinating center. Grantees used study-wide instruments to collect quantitative, individual level data on a subset of clients, all of whom consented to be followed for 6 months after leaving jail. Clients were free to disenroll at any time; the total time inside and outside of jail for which the typical client received services was about 6 months. In addition to data from face-to-face interviews, the programs sent the evaluation center clinical data pertaining to the management of HIV for clients on whom individual level data were collected.
Main Study Outcomes
Main study outcome indicators included: (1) the total number of clients served (“number of cases on the books”, inside and outside of jail) during the quarter of analysis; (2) the rate at which clients were successfully linked to medical care in the community setting; (3) the rate that clients demonstrated sustained linkages to medical care; and (4) the rate that those linked to medical care achieved an suppressed viral load through taking highly-active ART. Linkage was considered successful when the medical chart showed the client obtained a CD4 cell count in the 6 months following release. Linkage was considered sustained when the client had at least two CD4 counts in the 6 months post release. Rates of various degrees of linkage in outcomes (2), (3), and (4) were derived from the mean rates experienced by clients released during the last two quarters of 2009 through the first quarter of 2010 who consented to provide client-level data (n = 543). Because clients’ receipt of case management services averaged 6 months, mean rates from these releasees were considered representative of those from the clients “on the books” during the quarter of analysis.
Cost Analyses
The cost analyses were conducted from the provider perspective and included all costs incurred by the participating service agencies in the course of providing linkage services to HIV-positive jail detainees. Cost data collected during the first quarter of 2010 were adjusted for regional differences using the Consumer Price Index and are expressed in 2010 dollars.
Program cost information was derived from time and activity logs maintained by project staff and through comprehensive surveys completed by project coordinators. Specifically, project staff reported their activities on an Activity Time Sheet over a period of 5 different days of the week in 1 month. Staff time was categorized into five activities: arrangement for in-jail services; arrangement for community services; referrals to outside programs; follow-up; and miscellaneous (which included office work, travel, and downtime waiting for jail gates to be opened). The project coordinator survey inquired about the costs required to sustain program operations after the start-up phase. Costs were disaggregated both by when the costs were incurred (pre- or post-release) and by cost category (personnel/staff, materials, overhead).
Paralleling the main study outcomes, the main cost-effectiveness analyses focused on the means of the following economic efficiency indicators, weighted by the number of clients served at each site: (1) cost per client served; (2) cost per client successfully linked to medical care; (3) cost per sustained linkage; and (4) cost per client with suppressed viral load at 6 months. If viral load data were missing for a client, that client was considered unsuppressed.
HIV Prevention Cost-Effectiveness Analysis
The main cost analyses were supplemented by an HIV prevention cost-effectiveness analysis that estimated the cost per quality-adjusted life year (QALY) saved by the EnhanceLink program compared to standard of care. This analysis used an HIV transmission rate model [8] to estimate the number of secondary HIV infections prevented by the linkage program. The transmission rate for a particular group of HIV-infected persons is defined as the mean number of secondary infections per member of the group [6]. To illustrate, approximately 1,178,350 persons are currently living with HIV in the US [9] and an estimated 50,125 persons acquire HIV each year [10]. The overall annual transmission rate is therefore 50,125/1,178,350 = 4.3%. That is, on average, each person living with HIV (PLWH) in the US transmits the virus to about 0.043 previously-uninfected persons per year. The transmission rate for any particular subgroup of PLWH could be larger or smaller than this overall rate. For example, we calculated a transmission rate of γL = 0.0145 for PLWH who are sustainably linked to care, versus a rate of γU = 0.0393 for serostatus-aware PLWH who are not sustainably linked to care (the derivation of these rates is described below). These transmission rates suggest that, for each client who is sustainably linked to care, γU–γL = 0.0248 secondary HIV infections are prevented each year.
Each prevented secondary infection saves society the lifetime medical care costs, T, associated with treating a case of HIV infection and also prevents the loss of Q quality-adjusted life years. The cost-effectiveness ratio associated with the EnhanceLink program—that is, the net cost per QALY saved by the program compared to standard of care—can be expressed as [(CE–CS)–(AE–AS)T]/(AE–AS)Q, where CE is the annual cost of the EnhanceLink program; CS is the cost of the standard of care; AE is the reduction, due to ART, in the total number of secondary HIV infections per year for EnhanceLink clients who were sustainably linked to care; and AS is the reduction in the total number of secondary HIV infections for the standard of care. For EnhanceLink, the reduction in the number of secondary infections can be estimated as AE = PE(γU–γL), where γL and γU, as above, are the annual HIV transmission rates for clients who are or are not sustainably linked to care, respectively, and PE is the number of EnhanceLink clients sustainably linked to care. Similarly, for the standard of care condition, AS = PS(γU–γL).
Because the EnhanceLink study did not include a standard of care comparison group, we used data from the Antiretroviral Treatment and Access Study (ARTAS) [11] which compared an intensive case management intervention (similar to the one evaluated in the EnhanceLink study) to a passive referral standard of care condition—to estimate the number of clients who would have been initially linked to care in the absence of a case management-based linkage program. In ARTAS, clients in the case management condition were 1.3 times more likely to be successfully linked to care than persons in the standard of care condition. We assumed that the same ratio would have been obtained in the present study had it included a standard of care condition. In addition to the base case ratio (1.3), we examined linkage ratios of 1.17 and 1.43 (10% smaller or larger) in the sensitivity analyses.
Our analyses hypothetically followed the cohort of persons sustainably linked to care by the EnhanceLink program over a period of 10 years to estimate the number of secondary infections prevented during each 6-month interval. Secondary infections were discounted at a 3% annual rate in the base-case analysis. Based on data from ARTAS, we assumed an 18% attrition rate (loss to care) in the second 6-month period among persons sustainably linked to medical care services. Thereafter, the attrition rate was reduced by 50% in each succeeding 6-month period. In the sensitivity analyses, the analytic time frame was varied from 5 to 15 years, the discount rate from 0 to 5%, the attrition rate from 13.5 to 22.5%, and the attrition rate reduction factor from 25 to 75%. All analyses assumed a medical care treatment cost of $10,000 per 6-month period [12, 13] for each client sustainably linked to care.
Lifetime HIV-related medical care costs (discounted at a 3% annual rate) were drawn from a published source [14] and inflated to 2010 dollars, yielding T = $482,512. The number of QALYs saved by preventing a secondary case of HIV infection, Q = 6.43[15], was also discounted at a 3% annual rate. In the base-case analysis we assumed that the passive-referral standard of care cost CS = $10 per client (equivalent to approximately 30 min of a case worker's time), regardless of whether or not the client was successfully linked to care; this value was varied from 0 to $20 in the sensitivity analyses. The cost per client for the EnhanceLink program, CE, was estimated from program records as described above.
Secondary transmission rates were derived using the methods described by Pinkerton [8] using updated parameter values. The full transmission rate model is specified by the following equations:
| (1) |
| (2) |
| (3) |
| (4) |
In these equations, I is the annual incidence of HIV infection in the US; N0 is the number of persons living with (presumably undiagnosed) acute HIV infection; N1 is the number of PLWH with non-acute infection who are unaware of their serostatus; N2 is the number of serostatus-aware PLWH with detectable viral load; N3 is the number of serostatus-aware PLWH with undetectable viral load; γk is the transmission rate for group Nk; and the μ(k, k + 1) terms are transmission risk reduction factors.
The parameter values used to derive the transmission rates are listed in Table 1, together with the transmission rates themselves. Of note, the transmission rate model assumed that persons with undetectable viral load are incapable of transmitting HIV (γ3 = 0) [16, 17]. In the EnhanceLink study, 63% of clients sustainably linked to care had undetectable viral load. Consequently, the transmission rate for sustainably-linked clients can be estimated as γL = 0.37*γ2 + 0.63*γ3 = 0.0145. Because PLWH who are not sustainably linked to care can be presumed to have detectable viral load, the transmission rate for this group of PLWH is simply γU = γ2 = 0.0393.
Table 1.
HIV transmission rate model parameter values
| Model parameter | Parameter value |
|---|---|
| HIV incidence, I | 50,125 [10] |
| Persons living with HIV in the US, N = N0 + N1 + N2 + N3 | 1,178,350 [21] |
| Acutely-infected persons on any given day, N0 | 6,729a |
| Non-acutely infected PLWH who are unaware of their status, N1 | 229,671b |
| Serostatus-aware PLWH with detectable viral load, N2 | 613,475 [21] |
| Serostatus-aware PLWH with undetectable viral load, N3 | 328,475 [21] |
| Ratio of transmission rates for N0 and N1, μ(0,1) = γ1/γ0 | 0.1235 [22] |
| Ratio of transmission rates for N1 and N2, μ(1,2) = γ2/γ1 | 0.43 [23] |
| Ratio of transmission rates for N2 and N3, μ(2,3) = γ3/γ2 | 0 |
| Annual HIV transmission rate, acutely-infected PLWH, γ0 | 0.7409 |
| Annual HIV transmission rate, serostatus-unaware PLWH, γ1 | 0.0915 |
| Annual HIV transmission rate, aware PLWH with detectable VL, γ2 | 0.0393 |
Results
Across the nine study sites, the cost of providing linkages to medical care and social support services over 6 months ranged from 737 to $7,856 per client, with a mean per-client cost of $2,551, as shown in Table 2. Per-client costs were negatively-correlated with the number of clients served at each site (Spearman's rho = –0.83, p = 0.005), indicating economies of scale.
Table 2.
Cost of HIV primary care linkage programs at 9 US jail sites, January–March 2010, using confirmed measurements of linkage
| Site #1 | Site #2 | Site #3 | Site #4 | Site #5 | Site #6 | Site #7 | Site #8 | Site #9 | |
|---|---|---|---|---|---|---|---|---|---|
| Six-month program cost a | $91,238 | $129,490 | $134,804 | $135,108 | $141,540 | $154,066 | $184,878 | $196,394 | $217,444 |
| Cost of services | |||||||||
| Pre-release CMS | 11 % | 21 % | 38 % | 72 % | 10 % | 38 % | 90 % | 13 % | 19 % |
| Post-release CMS | 89 % | 79 % | 62 % | 28 % | 90 % | 62 % | 10 % | 87 % | 81 % |
| Distribution of costs | |||||||||
| % spent on personnel | 99 % | 67 % | 83 % | 64 % | 70 % | 85 % | 71 % | 89 % | 77 % |
| % spent on materials | 0 % | 10 % | 1 % | 12 % | 8 % | 3 % | 10 % | 5 % | 3 % |
| % spent on overhead | 1 % | 23 % | 16 % | 24 % | 22 % | 12 % | 19 % | 6 % | 20 % |
| Linkages to care | |||||||||
| Number of clientsb | 26 | 64 | 183 | 43 | 24 | 72 | 45 | 25 | 61 |
| % of clients medically linked (had CD4 assay) | 88 % | 39 % | 42 % | 60 % | 75 % | 77 % | 50 % | 72 % | 78 % |
| % with sustained medical linkages (≥2 CD4 tests) | 88 % | 31 % | 37 % | 60 % | 63 % | 62 % | 50 % | 72 % | 73 % |
| % of clients linked with undetectable viral load | 75 % | 31 % | 21 % | 35 % | 44 % | 35 % | 31 % | 17 % | 46 % |
| Per-client costs | |||||||||
| Cost per client | $3,509 | $2,023 | $737 | $3,142 | $5,898 | $2,140 | $4,108 | $7,856 | $3,565 |
| Cost per linked client | $4,010 | $5,203 | $1,750 | $5,237 | $7,863 | $2,782 | $8,217 | $10,877 | $4,548 |
| Cost per client with sustained linkage | $4,010 | $6,622 | $1,999 | $5,237 | $9,436 | $3,477 | $8,217 | $10,877 | $4,885 |
| Cost per client with undetectable viral load | $4,679 | $6,622 | $3,499 | $8,977 | $13,480 | $6,182 | $13,147 | $47,135 | $7,758 |
CMS case management services
Total 6-month program costs were calculated from first quarter 2010 costs and are expressed in 2010 dollars, geographically-adjusted using the Consumer Price Index
Number of clients over 6 month period (estimated as the number of clients in the first quarter of 2010)
All but two sites spent more on post-release case management services (CMS) than on pre-release CMS. Overall, approximately two-thirds (63%) of total expenditures were for post-release CMS. Personnel costs accounted for an average of 79% of total program costs across sites.
The percentage of clients initially linked to medical care services ranged from 39 to 88% across sites, with a weighted mean of 57%. Fifty-two percent of clients were sustainably linked to care. More than half (63%) of clients with sustained medical care linkages had undetectable viral load. The mean cost per linked client was $4,219; the mean cost per sustainably-linked client was $4,670. The mean cost per client with undetectable viral load was $8,432.
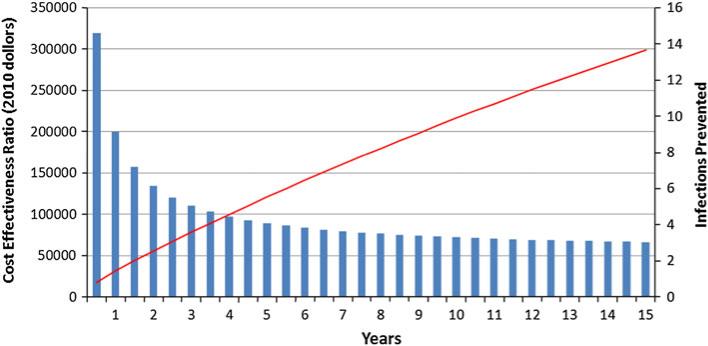
A total of 543 clients participated in the EnhanceLink study at the 9 sites included in the cost-effectiveness analysis, at a total cost of $692,478 per quarter, or $1,384,962 over 6 months. Figure 1 displays the cumulative number of infections prevented by the EnhanceLink program compared to the standard of care, together with the corresponding incremental cost-effectiveness ratios, over the 10-year modeling period. As shown in the figure, a total of 9.9 infections would have been prevented at the end of 10 years, yielding an incremental cost-effectiveness ratio of $72,285 per additional QALY saved. Because this ratio is less than $100,000, EnhanceLink can be considered cost-effective over this time period [18].
Fig. 1.
Cost-effectiveness of the EnhanceLink program and the number of prevented infections over time (See color version of figure on line)
The cost-effectiveness ratio was most sensitive to the time frame of the analysis, as shown in Table 3. Specifically, the EnhanceLink program would not be cost-effective (i.e., the cost-effectiveness ratio would exceed $100,000 per QALY saved) if the time frame of the analysis were restricted to 3.5 years. The EnhanceLink program remained cost-effective for all other parameter values considered in the sensitivity analyses.
Table 3.
HIV prevention cost-effectiveness analysis: results of sensitivity analyses
| Parameter | Base-case Value | More favorable scenario |
Less favorable scenario |
||
|---|---|---|---|---|---|
| Value | ICERa | Value | ICERa | ||
| Time frame (years) | 10 | 15 | $66,300 | 5 | $89,444 |
| Linkage ratio | 1.3 | 1.43 | $67,239 | 1.17 | $85,048 |
| Attrition rate | 18 % | 13.5 % | $70,465 | 22.5 % | $74,332 |
| Attrition rate reduction factor | 50 % | 75 % | $70,250 | 25 % | $78,048 |
| Per-client cost of standard of care | $10 | $20 | $72,200 | $0 | $72,370 |
Incremental cost-effectiveness ratio (additional cost per additional quality-adjusted life year saved by the EnhanceLink program relative to the standard of care). Base-case ICER = $72,285
Discussion
Our study sites linked 64.6% of HIV-infected releasees to community HIV care, at an average cost of $2,551 per client served, using activities that the AIDS service organizations believed were appropriate to the unique contexts of their respective locations. For example, a site in a community with a strong safety net of services devoted most of its activity to the jail setting; those situated in cities where the referral network is less developed allocated more services to the post-release phase. Using the assumption that case management was as least as effective as published data on linkage, we demonstrated that, project-wide, HIV linkage services for jail releasees were cost-effective to society.
A major limitation of this study was its reliance on data from a previous randomized, controlled trial of case management, ARTAS, to show the added benefit in linkage with services versus the scenario of no services, where presumably a minority of persons would still link to medical care. The ideal data would come from a randomized trial of intense case management intervention in jail. Another limitation was the possibility of ascertainment bias. Our analyses used the most conservative estimates of linkage rates, ones verified by medical chart reviews. Thus, unconfirmed client self-reported linkages, which were likely true instances of linkage, were not considered as linkages in the analysis. Finally, although the EnhanceLink cost analysis was conducted from the payer perspective, a societal perspective was adopted for the cost-effectiveness analyses. The calculated cost-effectiveness ratio therefore overstates the true cost-effectiveness of the program by an unknown amount.
In August 2011, CDC unveiled a new approach for reducing HIV infections in the US: High Impact HIV Prevention. The strategy involves using “combinations of scientifically proven, cost-effective and scalable” prevention interventions [19]. Given this new evidence suggesting that case management to help HIV-infected persons transition from jail back to community care is cost-effective, transition services should continue to be studied. Effective interventions should be adopted nationwide as a standard part of local health departments’ prevention packages. Future analysis will need to assess where transitional case management fits into the mix of prevention interventions. Programs for routine HIV testing, syringe exchange, treatment of substance abuse, and housing all play a role in reducing new cases of HIV. Future studies could explore whether a combination of interventions, one layered upon another, would be incrementally cost-effective. Factorial, randomized controlled trials could simultaneously measure the effectiveness of several interventions, alone and in combination [20].
For now, we offer evidence that, once established, interventions to enhance linkage of jail releasees to community HIV care appear to be cost-effective from the societal perspective. Although linking clients who are released from jail settings to community-based HIV care is resource intensive, it may be a judicious intervention to ensure releasees have unfettered access to HIV care. Programs to promote linkage for HIV-infected jail releasees merit more study, which will likely justify sustained funding.
Acknowledgments
This study was supported, in part, by HRSA SPNS Cooperative Agreement U90HA07632; CFAR Grant P30 AI050409 from National Institute of Allergy and Infectious Disease (AS); and Center Grant P30-MH52776 from the National Institute of Mental Health (SP).
Contributor Information
Anne C. Spaulding, Rollins School of Public Health, Emory University, 1518 Clifton Road, Atlanta, GA 30322, USA aspauld@emory.edu
Steven D. Pinkerton, Department of Psychiatry and Behavioral Medicine, Center for AIDS Intervention Research, Medical College of Wisconsin, 2071 N Summit Ave, Milwaukee, WI, USA
Hillary Superak, Rollins School of Public Health, Emory University, 1518 Clifton Road, Atlanta, GA 30322, USA.
Marc J. Cunningham, Rollins School of Public Health, Emory University, 1518 Clifton Road, Atlanta, GA 30322, USA
Stephen Resch, Harvard School of Public Health Center for Health Decision Science, 718 Huntington Ave, Boston, MA, USA.
Alison O. Jordan, New York City Department of Health and Mental Hygiene, Transitional Health Care Coordination, New York, NY, USA
Zhou Yang, Rollins School of Public Health, Emory University, 1518 Clifton Road, Atlanta, GA 30322, USA.
References
- 1.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of, and releasees from, US correctional facilities, 2006 declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. doi:10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westergaard RP, Kirk GD, Richesson DR, Galai N, Mehta SH. Incarceration predicts virologic failure for HIV-infected injection drug users receiving antiretroviral therapy. Clin Infect Dis. 2011;53(7):725–31. doi: 10.1093/cid/cir491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [3 Dec 2010];National HIV/AIDS strategy for the United States. 2010 http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 4.Shrestha RK, Sansom SL, Richardson-Moore A, et al. Costs of voluntary rapid HIV testing and counseling in jails in 4 states advancing HIV prevention demonstration project 2003–2006. Sex Transm Dis. 2009;36(Suppl 2):S5–8. doi: 10.1097/olq.0b013e318148b69f. [DOI] [PubMed] [Google Scholar]
- 5.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr: JAIDS. 2005;39(4):446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 6.Holtgrave DR, Hall HI, Rhodes PH, Wolitski RJ. Updated annual HIV transmission rates in the United States, 1977–2006 (Letter to the editor). J Acquir Immune Defic Syndr. 2009;50:236–8. doi: 10.1097/qai.0b013e31819001be. [DOI] [PubMed] [Google Scholar]
- 7.Draine J, Ahuja D, Altice FL, et al. Strategies to enhance linkages between care for HIV/AIDS in jail and community settings. AIDS Care. 2011;23(3):366–77. doi: 10.1080/09540121.2010.507738. [DOI] [PubMed] [Google Scholar]
- 8.Pinkerton S. HIV Transmission rate modeling: a primer, review, and extension. AIDS Behav. 2012;16(4):791–6. doi: 10.1007/s10461-011-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention HIV surveillance—United States, 1981–2008. MMWR. 2011;60:689–93. [PubMed] [Google Scholar]
- 10.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–31. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson AB, Farnham PG, Dean HD, Ekwueme DU, del Rio C, Kamimoto L. The economic burden of HIV in the US in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences. JAIDS. 2006;43(4):451–7. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]
- 13.Gebo KA, Fleishman JA, Conviser R, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24(17):2705–15. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44(11):990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 15.Farnham PG, Sansom SL, Hutchinson AB. How much should we pay for a new HIV diagnosis? A mathematical model of HIV screening in US clinical settings. Méd Decis Mak. 2012;32(3):459–69. doi: 10.1177/0272989X11431609. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 18.Centers for disease control and prevention [15 Jun 2012];HIV cost-effectiveness. 2012 http://www.cdc.gov/hiv/topics/preventionporgrams/ce/index.htm.
- 19.CDC'S new high-impact approach to HIV prevention funding for health departments advancing the national HIV/AIDS strategy. [5 Sept 2011];Fact sheet. 2011 http://www.cdc.gov/hiv/topics/funding/PS12-1201/resources/factsheet/pdf/foa-partner.pdf.
- 20.NIH, office of AIDS research [6 Mar 2010];Translating research from bench to bedside to community. 2010 http://www.oar.nih.gov/strategicplan/fy2011/pdf/FY2011ByPassPlan_08_Plan.pdf in FY Trans-NIH Plan for HIV-related research, available at: http://www.oar.nih.gov/strategicplan/fy2011/index.asp.
- 21.Cohen SM, van Handel MM, Branson BM, et al. Vital Signs: HIV prevention through care and treatment—United States. MMWR. 2011;60(47):1618–23. [PubMed] [Google Scholar]
- 22.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute phase HIV transmission? AIDS. 2007;21:1625–29. doi: 10.1097/QAD.0b013e32826fb6a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they infected with the virus in the USA. AIDS. 2006 Jun 26;20(10):1447–50. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]