TO THE EDITOR
Melanoma is a highly metastatic and therapeutically resistant cancer, whose incidence has more than tripled in the last decades (Smalley et al., 2010). Physiologically, melanocytes produce and store melanin pigments in the melanosomes, which are transported to the cell periphery and transferred to keratinocytes, a process that requires the tripartite complex Rab27a/melanophilin/myosin-Va (Hume and Seabra, 2011). Myosin-Va is an actin-based molecular motor that also serves a multitude of other functions, such as plasma membrane receptor recycling, exocytosis, association with nuclear speckles and the centrosome (see Woolner and Bement, 2010); interaction with PTEN, thereby modulating PI3K pathway (van Diepen et al., 2009), interaction with Bcl-xL, proposed to promote invasion of islet-tumor cells (Du et al., 2007); as biomarker of invasiveness for nonfunctioning pituitary adenomas (Galland et al., 2010). Moreover, myosin-Va was shown to be up-regulated by Snail to promote cancer cell invasion (Lan et al., 2010), and was postulated to control apoptosis by sequestering the pro-apoptotic protein Bmf, which is unleashed upon loss of cell attachment (Puthalakath et al., 2001).
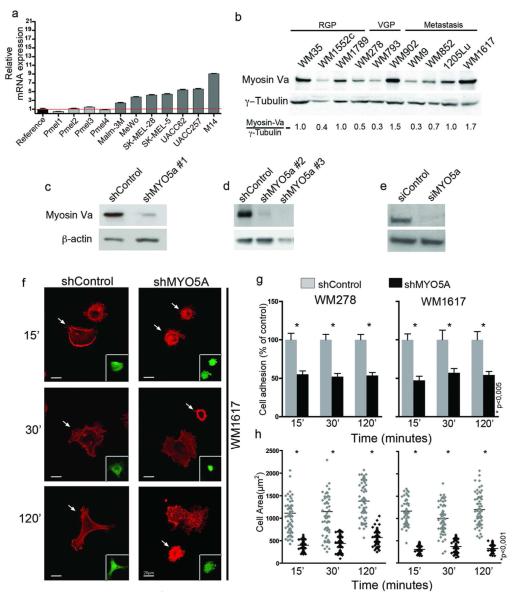
Up-regulation of MYO5A gene in melanoma and other cancer types was revealed in different microarray studies compiled here (Table S1; Figure S1). However, these data did not clarify whether MYO5A up-regulation was associated with melanocyte transformation or simply reflected tissue specificity since comparison was against normal skin and melanocytes are minor cells in the skin. Here, we extended this evidence by showing that MYO5A is up-regulated in a variety of melanoma cell lines in comparison with primary melanocytes (Figure 1a), as well as in metastatic cells in comparison to paired vertical growth phase cells (Figure 1b and S2), implicating myosin-Va in malignant transformation and/or melanoma progression. Interestingly, in this WM panel, myosin-Va expression correlated with that of the oncogenic transcription factor MITF (Sousa and Espreafico, 2008).
Figure 1. Myosin-Va is highly expressed in melanoma cells and its knockdown impairs cell adhesion and spreading on fibronectin-coated surface.
(a) Relative MYO5A mRNA expression detected by qPCR in melanocytes (pMel1 to 4) versus melanoma cells, using β-actin for normalization and mean of all melanocytes as reference value. (b) Western-blot of myosin-Va in a panel of melanoma cell lines of radial growth phase (RGP), vertical growth phase (VGP) and metastasis (M), including two genetic pairs (WM793 and 1205Lu; WM278 and WM1617). (c-e) Western-blots for myosin-Va in (c, d) WM1617 and (e) UACC-257. WM1617 cells were lysed 3 days after transduction with lentiviral vectors carrying shRNAs targeted to bacterial Lac-Z (shControl) or MYO5A (shMYO5A#1), or after stable selection with antibiotics for about 2-3 weeks in the case of shMYO5A#2-3 and respective shControl (Figure S3). UACC-257 cells were lysed 3 days after transfection with siRNA against myosin-Va or control. (f) Confocal images of F-actin stained cells adhered to fibronectin-coated coverslips for the indicated times. Arrows indicate transduced cells visualized by GFP expression (inserts). Scale bar = 20μm. (g) Cells allowed to adhere on fibronectin-coated surface for the indicated times were counted and data were plotted as mean ± SD from 3 independent experiments. (h) Cell spreading. Imaged as in (f) and the areas for 60 cells/time point were measured using ImageJ.
To investigate the role of myosin-Va in melanoma cells, we knocked down this protein using three different shRNAs (shMYO5A#1-3) carried by lentiviral vectors (Figure S3 and Qin et al., 2003) and an siRNA (siMYO5A). Once efficient knockdown was attained (Figures 1c-e), functional studies were performed. Upon adhesion to fibronectin-coated glass coverslips, MYO5A-depleted cells showed numerous small blebs on their surface and reduced lamellipodia/filopodia formation (Figure 1f), besides deficient adhesion (Figure 1g) and spreading (Figure 1h).
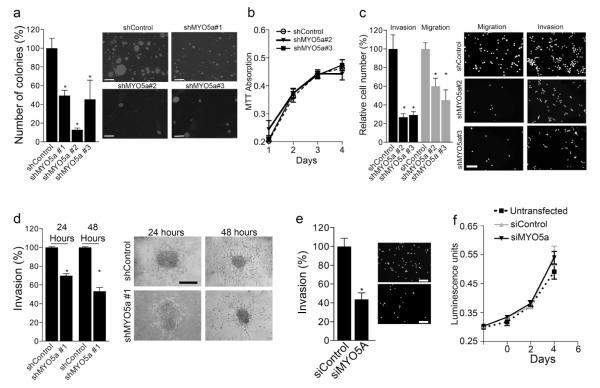
Next, we examined the role of myosin-Va in adhesion-independent growth. The ability to form colony in soft agar, as analyzed after 25-30 days of incubation, was at least 50% lower for MYO5A-depleted cells than controls, for the three different shRNAs used (Figure 2a). Proliferation rates under adherent conditions were determined by crystal violet staining for WM1617 (Figure 2b) or ATP measurements for UACC-257 (Figure 2f), and no differences were observed, in the time courses analyzed, between MYO5A-knockdown and control cells. Subsequently, we analyzed transwell migration and invasion and found rates 50 to 70% lower for shMYO5A#2/3-transfected WM1617 cells than controls (Figure 2c). Similar decrease in transwell invasion was observed for siMYO5A-transfected UACC-257 cells (Figure 2e). Next, we performed spheroid assays (as in Smalley and Herlyn, 2008) with shMYO5A#1-transduced cells. Compact spheroids with intact appearance were added to a tri-dimensional collagen gel and imaged after 24 and 48 hours of culture. Myosin-Va-depleted cells exhibited migration distances from spheroid margin to invasion front 50 to 60% shorter than controls (Figure 2d). Also, knockdown cells that migrated out of the spheroids looked smaller than controls after 48 hours, suggesting that myosin-Va-depleted cells differ in the sensitivity to microenvironment factors during migration in collagen matrix.
Figure 2. Ablation of myosin-Va inhibits colony formation, migration and invasiveness of metastatic melanoma cells without affecting cell proliferation.
(a-c) Lentiviral transduced WM1617 cells, using three independent shMYO5A or shControls, were used to assess: (a) Colony formation in soft-agar after 30 days of growth. Scale bar = 500μm; (b) Proliferation rates by absorbance measurement of crystal violet staining; (c) Migration in transwell and invasion in transwell-matrigel assay. Cells were kept in starvation conditions 24 hour prior the assay and were then allowed to migrate/invade for 24 hours. Scale bar = 50μm. (d) Migration in 3D collagen. After 24 or 48 hours of incubation - distance from spheroid edge to invasive front was measured and the data from three independent experiments were plotted as a percentage of control. Scale bar = 100μm. (e-f) Transwell invasion and proliferation rates of UACC257 cells transiently transfected with duplex siRNA targeted to MYO5A and irrelevant siRNA. Invasion assay was done as described in c, and proliferation rates were estimated based on ATP measurements. Data were plotted as mean ± SD from 3 independent experiments. Scale bar = 50μm.
The multifunctional character of myosin-Va makes us believe that this molecular motor, in addition to its role in cell adhesion/motility by promoting focal adhesion dynamics and filopodia/lamillipodia growth (supported by work in progress from our group, Nader et al.), may also perform a role in extracellular matrix proteolysis, mediating surface exposure and positioning of matrix metalloproteinases. Indeed, the alignment of metalloproteinases along the cytoskeleton seems to be a prerequisite for cell invasion in melanoma. Also, co-localization of metalloproteinases with myosin-Va (Sbai et. al., 2011) in astrocytes, and a role for RAB27A (Bobrie et al., 2012) in the release of metalloproteinase-9 to promote metastasis of mammary carcinoma cells have been shown. Moreover, evidence that RAB27A (Akavia et al., 2010) functions as a driver of cancer supports the hypothesis that, likewise, myosin-Va promotes malignancy by functioning in vesicular trafficking. Indeed, endocytosis and recycling of plasma membrane receptors require Rab GTPases and molecular motors with reflexes in adhesion dynamics, cell signaling and metabolism in many instances shown to drive oncogenic transformation and invasion (Mosesson et al., 2008). Furthermore, the relevance of our findings is supported by recent report demonstrating that the formation of filopodia is a critical step in the metastasis cascade (Shibue, et. al., 2013).
Additionally, we cannot rule out the possibility that some of the effects observed could be due to an increase in the rates of apoptosis in the MYO5A knockdown cells. Although we have not observed alteration of viability after myosin-Va depletion in short term culturing under regular conditions, increase of apoptosis rates under adhesion blockage and poor recovery of frozen stocks were noted. In fact, recent independent findings reinforce participation of myosin-Va in the control of apoptosis. Bmf sequestration to the actin cytoskeleton, presumably in complex with myosin-Va/DLC2, promotes resistance to MEK-inhibitors (Van Brocklin et al., 2009). Accordingly, overexpression of myosin-Va tail fragments harboring the binding site for DLC2 leads to apoptosis in melanoma cells likely by disrupting Bmf and probably also Bim anchorage (Izidoro-Toledo and Borges et. al., 2013). Finally, miR-145, which is a transcriptional target of p53 and known to act as a tumor suppressor, was recently shown to target myosin-Va (Dynoodt et. al., 2012). Therefore, myosin-Va may integrate mechanisms that interconnect invasion/migration machinery and resistance to apoptosis. Interdependencies between these processes are reviewed in Alexander and Friedl (2012).
In summary, the data presented here show that myosin-Va promotes adhesion dynamics, anchorage-independent survival, migration and invasion in vitro. Therefore, up-regulation of myosin-Va during melanoma progression may be part of a general mechanism that promotes malignant properties.
Supplementary Material
Table S1: MYO5A is found overexpressed in different tumor types
Figure S1: Microarray expression of myosin-Va in normal skin versus melanoma. Expression analysis based on data published by Talantov et al. (2005) (Table S1). RNA isolated from 45 primary melanoma and 7 normal skin tissue specimens were analyzed.
Figure S2: Expression of myosin-Va in melanoma cell lines. Western-blot of myosin-Va in a panel of human melanoma cell lines represented by cell lines derived from primary tumor in the radial growth phase (RGP) or vertical growth phase (VGP), and metastasis, including paired lines (WM793 and 1205Lu; WM278 and WM1617). B16-F10 is a highly metastatic murine melanoma cell line that expresses wild-type myosin-Va. S91-6 is a poorly metastatic melanoma cell line derived from a mouse characterized by loss of myosin-Va expression in the melanocytic lineage due to insertion of an ecotropic murine leukemia virus in an intron of the MYO5A gene. Quantification was done using ImageJ software (available at http://rsb.info.nih.gov/ij/). M, molecular weight markers.
Figure S3: Lentiviral vector and oligonucleotide sequences used to generate the short hairpin RNA shMYO5a #1. Diagram of the lentiviral vector pFUG12 (top) used to express shMYO5a #1 or shControl. Note the presence of two independent promoters, driving the shRNA and EGFP expression. The shRNA sequences were cloned as described previously (Qin, 2003). The EGFP expression was used as a marker for transduction. The lentiviral particles containing a shControl, shMYO5a #2 and #3 were obtained commercially from Sigma Aldrich (SHCLNV-NM_000259, shM-488 and shM-489). shControl is a non-target shRNA that does not target to any human gene.
ACKNOWLEDGMENTS
We are thankful to Silmara Reis Banzi and Benedita Oliveira Souza for their technical assistance, as well as to the Laboratory of Confocal Microscopy of FMRP-USP. We are especially grateful to Dr Meenhard Herlyn (Wistar Institute, Philadelphia, PE, USA) for providing the WM melanoma cell lines and Dr. David Baltimore (Caltech, Pasadena, CA, USA) for providing lentiviral vectors used to make shMYO5A#1 and one of the control shRNAs. This work was supported by grants to EME from Fundação de Amparo á Pesquisa do Estado de São Paulo (FAPESP - #2009/50167-3) and CNPq (#401322/2005-0). CPA and MHM received fellowships from CAPES and CNPq. JFS, DMT, AR and CLSP received FAPESP fellowships and EME was awarded with CNPq research fellowship (311347/2011-8). DEF was supported by grants from NIH, the Adelson Medical Research Foundation, the Melanoma Research Alliance, the Doris Duke Medical Foundation, and the US-Israel Binational Science Foundation.
Footnotes
Conflict of interest The authors declare no conflict of interest.
REFERENCES
- Alexander S, Friedl P. Cancer invasion and resistance: interconnected processes of disease progression and therapy failure. Trends Mol Med. 2012;18:13–26. doi: 10.1016/j.molmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Akavia UD, Litvin O, Kim J, et al. An integrated approach to uncover drivers of cancer. Cell. 2010;143:1005–1017. doi: 10.1016/j.cell.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and - independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- Du YC, Lewis BC, Hanahan D, et al. Assessing tumor progression factors by somatic gene transfer into a mouse model: Bcl-xL promotes islet tumor cell invasion. PLoS Biology. 2007;5:e276. doi: 10.1371/journal.pbio.0050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynoodt P, Mestdagh P, Peer GV, et al. Identification of miR-145 as a key regulator of the pigmentary process. J Invest Dermatol. 2013;133(1):201–209. doi: 10.1038/jid.2012.266. [DOI] [PubMed] [Google Scholar]
- Galland F, Lacroix L, Saulnier P, et al. Differential gene expression profiles of invasive and non-invasive non-functioning pituitary adenomas based on microarray analysis. Endocrine-Related Cancer. 2010;17:361–371. doi: 10.1677/ERC-10-0018. [DOI] [PubMed] [Google Scholar]
- Hume AN, Seabra MC. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans. 2011;39:1191–1196. doi: 10.1042/BST0391191. [DOI] [PubMed] [Google Scholar]
- Izidoro-Toledo TC, Borges AC, Araújo DD, et al. A myosin-Va tail fragment sequesters dynein light chains leading to apoptosis in melanoma cells. Cell Death Dis. 2013;4:e547. doi: 10.1038/cddis.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Han H, Zuo H, et al. Upregulation of myosin Va by Snail is involved in cancer cell migration and metastasis. Int J Cancer. 2010;126:53–64. doi: 10.1002/ijc.24641. [DOI] [PubMed] [Google Scholar]
- Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Villunger A, O’Reilly LA, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbai O, Ould-Yahoui A, Ferhat L, et al. Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia. 2010;58:344–366. doi: 10.1002/glia.20927. [DOI] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Inan MF, et al. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012;2(8):706–721. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Lioni M, Noma, et al. In vitro three-dimensional tumor microenvironment models for anticancer drug discovery. Expert Opinion on Drug Discovery. 2008;3(1):1–10. doi: 10.1517/17460441.3.1.1. [DOI] [PubMed] [Google Scholar]
- Sousa JF, Espreafico EM. Suppression subtractive hybridization profiles of radial growth phase and metastatic melanoma cell lines reveal novel potential targets. BMC Cancer. 2008;8:19, 1–18. doi: 10.1186/1471-2407-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brocklin MW, Verhaegen M, Soengas MS, Holmen SL. Mitogen-activated protein kinase inhibition induces translocation of Bmf to promote apoptosis in melanoma. Cancer Res. 2009;69:1985–1994. doi: 10.1158/0008-5472.CAN-08-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diepen MT, Parsons M, Downes CP, et al. Myosin V controls PTEN function and neuronal cell size. Nat Cell Biol. 2009;11:1191–1196. doi: 10.1038/ncb1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolner S, Bement WM. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009;19:245–252. doi: 10.1016/j.tcb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: MYO5A is found overexpressed in different tumor types
Figure S1: Microarray expression of myosin-Va in normal skin versus melanoma. Expression analysis based on data published by Talantov et al. (2005) (Table S1). RNA isolated from 45 primary melanoma and 7 normal skin tissue specimens were analyzed.
Figure S2: Expression of myosin-Va in melanoma cell lines. Western-blot of myosin-Va in a panel of human melanoma cell lines represented by cell lines derived from primary tumor in the radial growth phase (RGP) or vertical growth phase (VGP), and metastasis, including paired lines (WM793 and 1205Lu; WM278 and WM1617). B16-F10 is a highly metastatic murine melanoma cell line that expresses wild-type myosin-Va. S91-6 is a poorly metastatic melanoma cell line derived from a mouse characterized by loss of myosin-Va expression in the melanocytic lineage due to insertion of an ecotropic murine leukemia virus in an intron of the MYO5A gene. Quantification was done using ImageJ software (available at http://rsb.info.nih.gov/ij/). M, molecular weight markers.
Figure S3: Lentiviral vector and oligonucleotide sequences used to generate the short hairpin RNA shMYO5a #1. Diagram of the lentiviral vector pFUG12 (top) used to express shMYO5a #1 or shControl. Note the presence of two independent promoters, driving the shRNA and EGFP expression. The shRNA sequences were cloned as described previously (Qin, 2003). The EGFP expression was used as a marker for transduction. The lentiviral particles containing a shControl, shMYO5a #2 and #3 were obtained commercially from Sigma Aldrich (SHCLNV-NM_000259, shM-488 and shM-489). shControl is a non-target shRNA that does not target to any human gene.