Abstract
Objective:
To investigate Epstein-Barr virus (EBV) oral shedding frequency and EBV genetic diversity in pediatric patients with multiple sclerosis (MS).
Methods:
This was a prospective case-control study. We used PCR-based assays to detect viral DNA in the monthly mouth swabs of 22 pediatric patients with MS and 77 age- and sex-matched healthy controls. EBV-positive samples were further analyzed for sequence variation in the EBV BCRF1 (ebvIL-10) gene using direct DNA sequencing methods, and in the EBV LMP1 gene by mass spectrometry.
Results:
Nineteen of the 22 (86.4%) children with MS were seropositive for remote EBV infection compared to 35 out of 77 (45.5%) healthy controls (p = 0.008). Baseline analysis of mouth swabs revealed a higher proportion of EBV-positive samples from EBV-seropositive patients with MS compared to EBV-seropositive healthy controls (52.6% vs 20%, p = 0.007). Longitudinal analysis of monthly swabs revealed average EBV detection rates of 50.6% in patients with MS and 20.4% in controls (p = 0.01). The oral shedding frequencies of Herpesviruses herpes simplex virus–1, cytomegalovirus, human herpesvirus (HHV)-6, and HHV-7 did not differ between groups. Changes in the predominant EBV genetic variants were detected more frequently in patients with MS; however, no specific EBV genetic variant was preferentially associated with MS.
Conclusion:
Children with MS demonstrate abnormally increased rates of EBV viral reactivation and a broader range of genetic variants, suggesting a selective impairment in their immunologic control of EBV.
Epstein-Barr virus (EBV) has strong epidemiologic associations and biological plausibility in multiple sclerosis (MS) pathophysiology.1 Serologic evidence of prior EBV infection is evident in 99% of adult-onset MS patients, exceeding the 95% seropositivity rate in the general adult populations living in the same temperate regions.2 The seroprevalence of EBV is also higher in children with MS (85%–99%) than region-, age- and sex-matched healthy children (42%–72%).3–5 Elevated EBV-specific antibody titers have been demonstrated in young adults sampled years before the onset of MS.6,7
EBV establishes lifelong persistence in memory B cells.8 Differentiation of B cells into plasma cells initiates EBV reactivation from latency resulting in the release of virions into the peripheral blood and oral mucosa.8 Further viral replication occurs in oropharyngeal epithelial cells and the virus is shed into the saliva.9 EBV reactivation is typically controlled by EBV-specific T-cell responses, which ensure the EBV replicative (lytic) cycles are brief.10 EBV gene products, such as viral interleukin (IL)-10 and latent membrane protein-1 (LMP-1), have been shown to exhibit immunomodulatory functions.11–13
Building on our prior demonstration of a strong association between remote EBV infection and pediatric MS,3,5 and on the concept that MS may be influenced by host immune–viral interactions, we compare EBV viral shedding in saliva and genetic variation of BCRF1 and LMP1 genes between EBV-seropositive children with MS and EBV-seropositive age-matched healthy children. We also analyze serologic evidence and viral shedding frequency for other common Herpesviruses.
METHODS
Study participants.
Children with relapsing-remitting MS (RRMS)14 were enrolled consecutively from the pediatric MS clinic at the Hospital for Sick Children, Toronto, Canada, from January 2009 through July 2011. The healthy control group comprised children with no known neurologic diseases or medical conditions recruited through community advertisement, frequency matched for age and sex with the MS participants.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Research Ethics Board at the Hospital for Sick Children, and written informed consent was obtained from the parents or guardians and from the participants.
Biological sample collection and processing.
Blood samples were collected from all participants at study entry and also at subsequent scheduled clinic visits for patients with MS. Blood specimens were processed at the Experimental Therapeutics Programs at the Montreal Neurological Institute (McGill University, Montreal, Canada) according to standard operating procedures. Aliquoted sera were stored at −80°C until analysis.
At enrollment, participants were taught oral swab collection techniques, which included swabbing the inner cheeks, the hard palate, and under the tongue, 3 to 5 times with a sterile nylon swab (Copan, Murrieta, CA). The swab was placed into a vial containing 1 mL of sterile saline solution and kept in the participants' home freezers until they were returned to our laboratory. Participants were instructed to collect mouth swabs once a month for 12 consecutive months.
For viral DNA isolation, the specimen tubes were vortexed for 30 seconds, and DNA was extracted using the QIAamp Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Extracted DNA was stored at −20°C until analyzed.
Serologic analyses.
All serologic determinations were performed blinded to clinical diagnosis in a licensed clinical microbiology laboratory at the Hospital for Sick Children. Immunoglobulin G (IgG) antibodies against EBV viral capsid (EBV-VCA), early antigen (EBV-EA), and nuclear antigen–1 (EBV-EBNA1) were measured using commercially available ELISA kits (DiaSorin, Stillwater, MN), per the manufacturer's instructions. An individual was classified as “remotely infected” if VCA IgG and EBNA IgG were detected, “recently infected” if VCA IgG and EA IgG (but not EBNA) were detected, and EBV naive if IgG against all 3 EBV antigens were absent.
Serum samples were analyzed for IgG antibodies against herpes simplex virus (HSV; Euroimmun, Lübeck, Germany), cytomegalovirus (CMV; Zeus Scientific, Raritan, NJ), varicella-zoster virus (Siemens Healthcare Diagnostics, Marburg, Germany), and human herpesvirus–6 (HHV-6; Abnova, Taiwan), according to manufacturer's instructions.
Detection of viral DNA in mouth swabs.
HHV was detected by PCR in DNA isolated from the mouth swabs of participants with serologic evidence of prior infection with HSV, EBV, CMV, HHV-6, or HHV-7.15,16 Detailed description of the PCR methods employed is provided in appendix e-1 and table e-1 on the Neurology® Web site at www.neurology.org.
Identification of EBV BCRF1 and LMP1 variants in mouth swabs.
DNA sequence analyses of the EBV BCRF1 and LMP1 genes were performed with DNA extracted from mouth swabs collected from all EBV-seropositive participants. Amplification and sequencing of the full-length EBV BCRF1 gene was performed as described in appendix e-1.
Seven LMP-1 variants have been previously identified based on unique amino acid substitutions compared with the B95-8 prototype sequence.17 To define the EBV LMP-1 variants present in the mouth swabs, a matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry approach, which allows for simultaneous detection of multiple viral genetic variants, was used.18 The detailed protocol is provided in appendix e-1.
DNA sequence analysis.
DNA sequence chromatograms were inspected and edited using FinchTV version 1.4.0 software (Geospiza Inc, Seattle, WA). DNA primers were designed using GeneRunner version 3.05 (Hastings Software Inc., Hastings, NY). Multiple DNA sequence alignments were performed using program CLUSTAL W19 with default settings.
Statistical analyses.
Sample size calculations were precluded by the paucity of data on EBV shedding. All pediatric patients with MS identified during the study period were included. Continuous variables were summarized as mean (standard deviation) or median (interquartile range) as appropriate. Categorical variables were described as frequency (%). Missing data were not imputed. The proportion of seropositive samples was compared between groups using χ2 or Fisher' exact tests. Continuous variables were compared between groups using Student t test. A p value < 0.05 was considered to be statistically significant.
RESULTS
Participants.
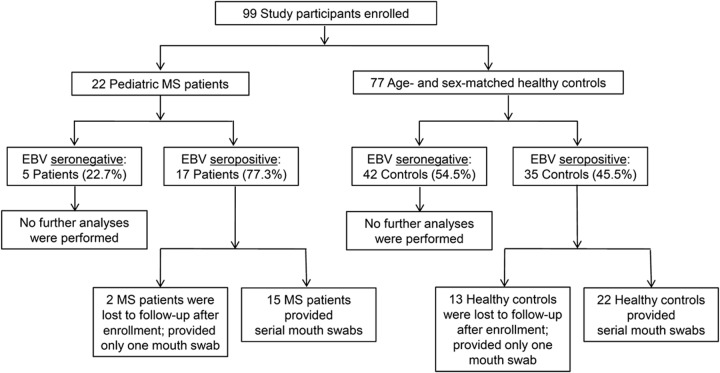
Twenty-two children aged 12–18 years (mean age 16.2 ± 1.7 years) with RRMS and 77 children with no known neurologic or autoimmune diseases (mean age 14.9 ± 2.5 years; range 8–18 years) were enrolled (figure 1). None of the participants had clinically apparent Herpesvirus infection during the study.
Figure 1. Flow diagram of participants recruited in the study.
A total of 99 children were enrolled in the study. Mouth swabs were collected from participants at the time of enrollment and every month thereafter. Subsequent analyses were carried out only on samples obtained from participants with confirmed serologic evidence of prior Epstein-Barr virus (EBV) infection. Participants from whom more than one mouth swab was collected were included in the longitudinal assessment of oral shedding of Herpesviruses, including EBV, cytomegalovirus, herpes simplex virus–1, human herpesvirus (HHV)–6, and HHV-7, by PCR analysis. MS = multiple sclerosis.
Baseline analyses.
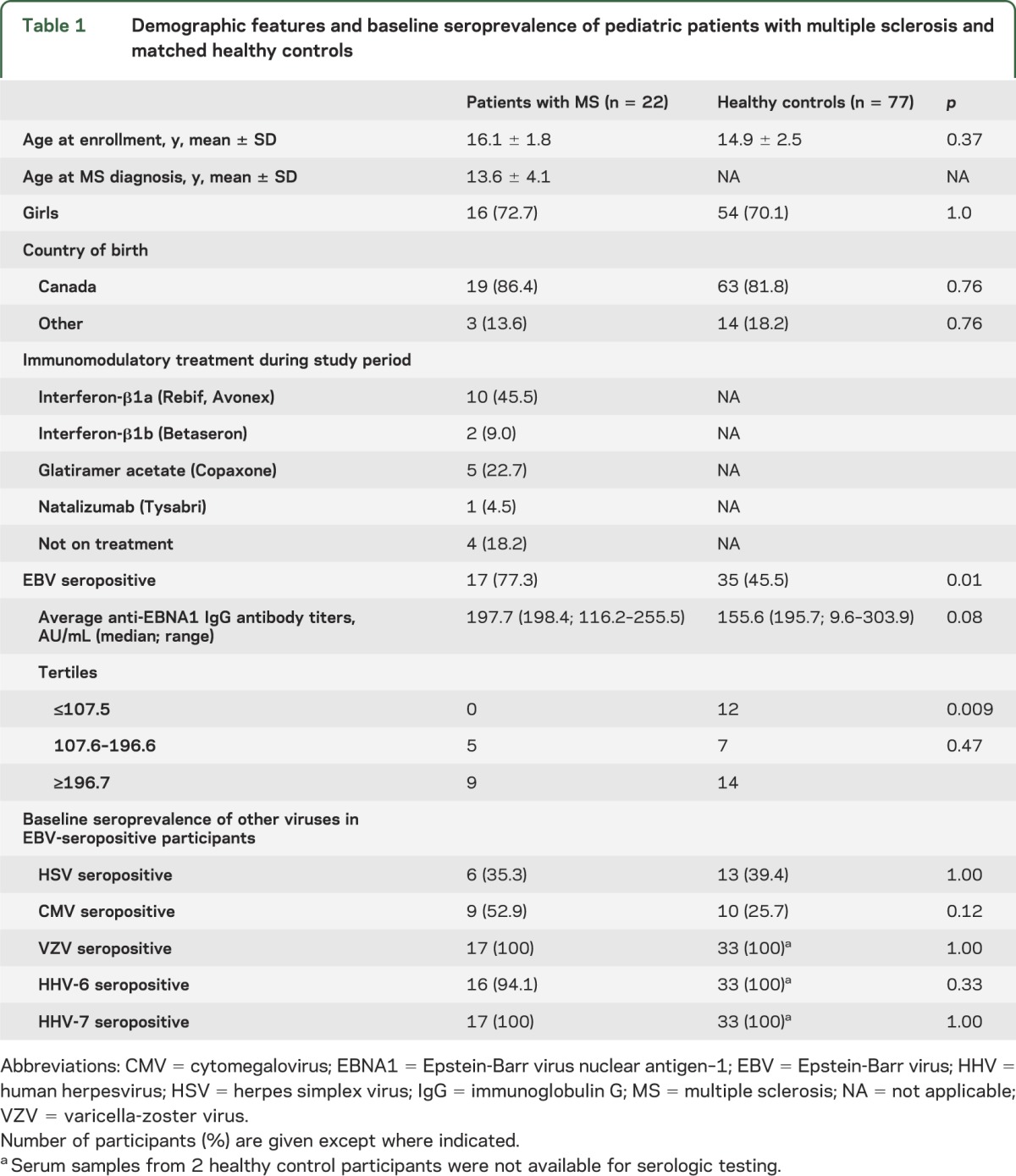
At study entry, 17 out of 22 (77.3%) patients with MS had serologic evidence for remote EBV infection compared to 35 out of 77 (45.5%) controls (p = 0.008). Fourteen EBV-positive patient serum samples and 33 EBV-positive control samples were available for quantitative evaluation of anti-EBNA1 antibody titers. There was a trend for increased average anti-EBNA1 IgG titers among children with MS compared to healthy controls (p = 0.08; figure e-1). None of the 14 patients with MS had anti-EBNA1 IgG titers in the lowest quartile, compared to 12 out of 33 (36.4%) healthy controls (p = 0.009; table 1). Seropositivity rates of HSV, CMV, HHV-6, and HHV-7 did not differ between children with MS and healthy children (table 1).
Table 1.
Demographic features and baseline seroprevalence of pediatric patients with multiple sclerosis and matched healthy controls
The presence of Herpesvirus DNA in these specimens was assessed in EBV-seropositive participants. Ten out of 17 patients (58.8%) had detectable EBV DNA in their mouth swabs compared to 7 out of 35 (20%) healthy controls (p = 0.007). EBV type 1 was detected more frequently than EBV type 2 in both the MS (n = 9, 90%) and control groups (n = 6, 85.7%). Concurrent shedding of EBV type 1 and type 2 was not detected.
To exclude the possibility that pediatric EBV-infected patients with MS have a more generalized failure of host control of viral infection, we also analyzed viral shedding of HHV-6 and HHV-7 in the oral mucosa. HHV-6 and HHV-7 have similar biological properties to EBV, namely established latency in lymphocytes, and periodic lytic reactivation in oropharyngeal epithelial cells, leading to shedding of virions in the saliva.20 We found 1 out of 17 (5.8%) patients with MS had detectable HHV-6 DNA in their mouth swabs compared to 8 out of 35 (22.9%) healthy children (p = 0.40). HHV-6 type B was detected in all HHV6-positive participants, with the exception of 1 healthy control in whom HHV-6 type A was detected. HHV-7 DNA was detected in 16 out of 17 patients with MS (94%) and 30 out of 35 healthy controls (86%; p = 0.22). None of the participants had detectable HSV or CMV DNA in their baseline mouth swab samples.
Longitudinal analyses.
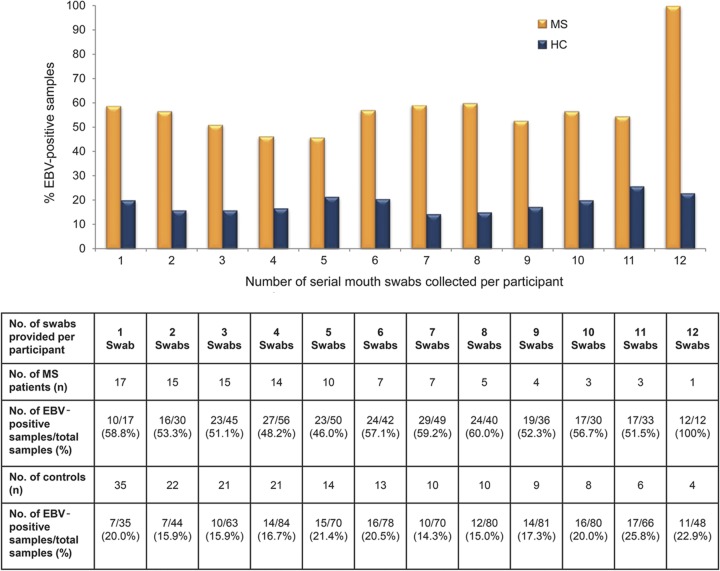
To evaluate the frequency of EBV shedding over an extended period of up to 1 year, serial mouth swabs were requested from all participants, although not all children were compliant with the monthly protocol. Fifteen EBV-seropositive patients with MS and 22 EBV-seropositive healthy controls provided more than one mouth swab over the 12-month study period. Ninety-nine mouth swabs were obtained from 15 patients with MS and 160 swabs were obtained from 22 healthy controls during the follow-up period. The average number of serial samples obtained per patient with MS was 5.6 (median 4, range 3–12), and was 6.3 (median 5, range 2–12) per healthy control.
EBV DNA was detected in the mouth swabs of all EBV-seropositive children with MS at least once during the study period, with an overall weighted average detection rate of 50.5% (51 EBV-positive samples out of 99 samples; range 20%–100%). In contrast, the weighted average EBV DNA detection rate among healthy controls was 19.4% (31 EBV-positive samples out of 160 samples; range 0%–83.3%; p = 0.01). Nine healthy controls (who provided 49 samples) did not shed EBV during the entire study period. Patients with MS demonstrated a consistently higher frequency of EBV detection in mouth swabs as compared to healthy controls, irrespective of the number of serial samples provided (figure 2).
Figure 2. Epstein-Barr virus (EBV) DNA positivity rates in serial mouth swabs obtained from EBV-seropositive pediatric patients with multiple sclerosis and matched healthy controls.
Serial mouth swabs were collected on a monthly basis for up to 1 year. The overall EBV DNA positivity rate in the serial samples obtained from patients with multiple sclerosis (MS) was consistently higher than in those obtained from healthy controls (HC), irrespective of the number of serial mouth swabs provided per participant.
EBV type 1 was shed more often than EBV type 2 in both patients with MS (44 out of 51 EBV-positive samples; 85%) and healthy children (28 out of 31 EBV-positive samples; 90%), although one patient with MS (MS-02) and one healthy control (C-02) consistently shed only EBV type 2 throughout the study period. Temporal changes in the predominant EBV subtypes were observed in 2 patients with MS (MS-01 and MS-08), but in none of the healthy controls.
Figure e-2 summarizes the HHV-6 and HHV-7 shedding frequencies in EBV-seropositive MS and healthy control participants. The shedding frequencies of HHV-6 and HHV-7 did not differ significantly between patients with MS and controls. No HSV or CMV DNA was detectable in any of the serial samples analyzed.
Type I interferons (IFNs), including IFN-β, are important for the control of viral infections. To assess the effect of IFN-β on human Herpesvirus oral shedding, we compared HHV oral shedding frequencies in patients with MS who were undergoing IFN-β therapy (n = 7; 49 serial samples) to patients who were on other immunomodulatory therapies or not on any treatment (n = 8; 50 serial samples) during the study period (table 1). The overall average EBV shedding frequency among patients with MS treated with IFN-β (43.0%) did not differ from the patients with MS who were not on IFN-β treatment (51.0% p = 0.54). There was no difference in the average HHV-6 shedding frequencies between patients on IFN-β treatment (17.2%) and patients not on IFN-β treatment (16.7%; p = 0.97). HHV-7 shedding frequency also did not differ between the IFN-β–treated (74.2%) and non-IFN-β–treated (91.6%; p = 0.18) groups.
EBV BCRF1 (ebvIL-10) and LMP-1 genetic variants in children with MS.
Comparison of the full-length BCRF1 (ebvIL-10) gene to the EBV prototype strain B95-8 identified a total of 6 distinct DNA sequences from B95-8 (figure e-3A). Three amino acid substitutions were identified (Arg3Gln, Val6Met, Gly23Ser), all of which were located within the signal peptide of ebvIL-10 (amino acid residues 1–25), while 4 silent nucleotide changes (a9787g, g9848a, c9980a, c10020t) were present in the mature ebvIL-10 protein. The most common single nucleotide base change, c9980a, was present in 4 of the 6 BCRF1 sequence variants, 2 of which also carried at least 1 of the 3 amino acid substitutions. We did not identify any MS-specific nucleotide polymorphism within the BCRF1 (ebvIL-10 sequence). Five out of 15 (33%) patients with MS and 1 out of 14 (7%) healthy controls were infected with more than 1 EBV BCRF1 genetic variant.
Distinct LMP-1 variants can be distinguished by amino acid signature patterns at the carboxy terminus.17 Using a MALDI-TOF mass spectrometry approach,18 we identified 4 LMP-1 variants in the mouth swabs of patients with MS and controls (figure e-3B). Two patients with MS, but none of the controls, had more than 1 LMP-1 variant detected in serial samples.
DISCUSSION
Our primary objective was to explore whether pediatric patients with MS differ from healthy children in relation to EBV-specific biology. First, we confirm a higher seroprevalence for remote EBV exposure, but not for other common Herpesviruses in pediatric patients with MS relative to healthy children.3,5 We also demonstrate a difference in the distribution of anti-EBNA1 titers, with healthy children being more likely to have low titers. Furthermore, we show for the first time that patients with pediatric-onset MS are more likely to have detectable EBV DNA in their saliva compared to EBV-seropositive healthy children, and that this is consistently observed on serial evaluations. The increased frequency of EBV shedding in children with MS does not reflect a more generalized impairment in control of chronic viral infection, as demonstrated by the similar shedding frequencies of HHV-6 and HHV-7 in EBV-seropositive patients with MS and healthy children. Prior reports of a lack of difference in EBV DNA load in the plasma and peripheral blood mononuclear cells of patients with MS and that of healthy controls also argue against a generalized impairment of immune control of EBV infection in patients with MS.21
The antiviral nature of IFN-β prompted us to evaluate whether treatment of patients with MS influenced our findings. We observe no difference in EBV, HHV-6, or HHV-7 shedding frequencies among patients with MS treated with IFN-β as compared to patients with MS not exposed to IFN-β. However, given the small sample size, this finding should be interpreted cautiously.
There is very limited data on EBV oral shedding in children. Previous cross-sectional studies have reported that EBV was detectable in the saliva of 38% of 93 healthy EBV-seropositive children (age range, 0–6 years) in Japan,22 and in 50% of healthy pediatric controls in Germany (n = 14; age range, 1.5–15.8 years),23 compared to our finding of 50% detection rate in patients with MS and 20% detection rate in healthy controls. In healthy individuals, the level of EBV oral shedding does not appear to be correlated with the level of latently infected memory B cells,9 but is most likely to be governed by the efficacy of host immune responses. It was recently suggested that diminished functional capacity of EBV-specific CD8+ T cells rather than decrease in the number of EBV-specific CD8+ T cells might facilitate the reactivation of EBV in healthy individuals.24 Deficits in interferon-γ secretion and IL-2 production by human leukocyte antigen (HLA)-B7–restricted EBV-specific CD8+ T cells have recently been reported in patients with MS.21
Impaired host control of EBV lytic cycle is suggested by our finding of higher shedding frequency, but also by the observation that children with MS shed a broader range of EBV variants over time compared to controls. Coinfection with EBV type 1 and type 2 was recently shown to be higher in adult patients with MS (n = 75, 90% coinfected) compared to healthy controls (n = 186; 37%; p < 0.001).25 Our inability to identify MS-associated genetic variations within the BCRF1 (encoding ebvIL-10) and LMP1 genes in pediatric patients with MS is consistent with findings in studies in which EBV genetic variation (in the EBNA1, BRRF1, LMP2A, BHRF1, and LMP1 genes specifically) was evaluated in adult patients with MS, without detection of specific EBV mutations in the MS cohort.26,27 However, further research may be warranted given evidence that EBV genetic variations may influence cellular immune responses towards EBV.28 Of interest, ebvIL-10 is a viral homolog of hIL-10. Structural differences between hIL-10 and ebvIL-10 result in different binding affinities to IL-10 receptors, and altered signaling properties.12 ebvIL-10 modulates hIL-10 activities in vitro and may downregulate the production of hIL-10,11,12 though the in vivo functional consequences of ebvIL-10 secretion on human IL-10–mediated responses have not been elucidated. Reduced serum hIL-10 levels have been reported in patients with MS compared to healthy controls.29,30 Earlier studies showed that amino acid substitutions within the hIL-10 signal peptide resulted in decreased protein secretion.31 Given the sequence similarity between hIL-10 and ebvIL-10, changes in the signal peptide of ebvIL-10 could also have an effect on ebvIL-10 protein translocation or signal peptide production. Furthermore, the HLA-B27–restricted epitope (RRLVVTLQC; amino acid positions 3-11) is located within the signal peptide sequence of BCRF1,32 and changes in the epitope sequence, such as the 2 amino acid substitutions that we identified (Arg3Gln, Val6Met), may impact recognition of EBV-infected cells by cytotoxic T cells. EBV LMP-1 is another example of an EBV-encoded immunomodulatory protein. LMP-1 functionally mimics the CD40 receptor that is critical for B-cell activation and proliferation.13 Unlike CD40, LMP-1 is constitutively activated independent of ligand binding, resulting in enhanced B-cell activation.13 It has been proposed that LMP-1 may work together with ebvIL-10 to facilitate EBV replication and reactivation.12 Variations within LMP1 may result in different biological and signaling properties. For example, several LMP-1 variants demonstrate enhanced NF-κB signaling, which modulates gene expressions mediating the innate and adaptive immune responses in vitro.33
As we were unable to obtain blood specimens from our study participants on a monthly basis, a limitation of our study was that we could not assess the relationship between viral DNA in the mouth swabs and in peripheral blood. Detection of EBV DNA in saliva is positively correlated with detection in peripheral blood during active replication34; however, the prevalence of EBV strains may be different in these 2 sites,35 and may reflect selective tissue tropism of viral strains.36 With increasing availability of next-generation sequencing, whole-genome analysis of EBV strains isolated from patients with MS will likely provide greater insights into whether genetic variation in the EBV genome is associated with MS, or whether the potential biological impact of EBV in MS is independent of EBV genetics but perhaps related to host immune responses to EBV.
We show that children with MS are more likely than healthy children to experience frequent reactivation of EBV but not of other latent Herpesviruses, suggestive of selective impairment in the immunologic control of EBV lytic cycle in the oral mucosa. Further study of EBV-relevant immune cell responses in pediatric patients with MS appears warranted.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Lilia Rabinovich (Hospital for Sick Children, Toronto) for technical assistance in serologic analyses and Carolynn Darrell and Suzanne McGovern (Hospital for Sick Children, Toronto) for their help with the recruitment of healthy control participants; members of the Experimental Therapeutics Program at the Montreal Neurological Institute and Hospital for their help with sample handling; and all participants and their families involved in this study.
GLOSSARY
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- EBV-EA
Epstein-Barr virus early antigen
- EBV-EBNA1
Epstein-Barr virus nuclear antigen–1
- EBV-VCA
Epstein-Barr virus viral capsid
- HHV
human herpesvirus
- HLA
human leukocyte antigen
- HSV
herpes simplex virus
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- LMP-1
latent membrane protein–1
- MALDI-TOF
matrix-assisted laser desorption/ionization–time of flight
- MS
multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Carmen Yea conducted the experiments, analyzed and interpreted the data, performed statistical analyses, and wrote the manuscript. Raymond Tellier participated in the design of the study, analysis of data, and provided critical revisions of the manuscript. Garrett Westmacott and Patrick Chong generated and analyzed mass spectrometry data. Ruth Ann Marrie performed statistical analyses and provided critical revisions of the manuscript. Amit Bar-Or participated in the design of the study, interpretation of data, and provided critical revisions of the manuscript. Brenda Banwell participated in the design of the study, interpretation of data, and provided critical revisions of the manuscript.
STUDY FUNDING
Supported by the Multiple Sclerosis Society of Canada Scientific Research Foundation.
DISCLOSURE
C. Yea reports no disclosures. R. Tellier has received research grants from the Canadian Institutes of Health Research, The Ministry of Health and Long Term Care of Ontario, the Canadian Liver Foundation, and the Michael Smith Foundation for Health Research. P. Chong has received funding from the Genomics R&D Initiative through federal government of Canada. G. Westmacott has received funding from the Genomics R&D Initiative through federal government of Canada. R. Marrie receives research funding from Canadian Institutes of Health Research, Public Health Agency of Canada, Manitoba Health Research Council, Health Sciences Centre Foundation, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, and Rx & D Health Research Foundation, and has conducted clinical trials funded by Sanofi-Aventis. A. Bar-Or receives research funding from Canadian Institutes of Health Research, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, and National Institutes of Health. He has received speaker, consulting fees, and/or research support from Amplimmune, Aventis, Bayhill Therapeutics, Berlex/Bayer, Biogen Idec, BioMS, Diogenix, Eli-Lilly, EMD Serono, Genentech, Genzyme-Sanofi, GSK, Guthy Jackson Greater Good Foundation, Medimmune, Mitsubishi Pharma, Novartis, Ono Pharmacia, Receptos, Roche, Teva Neuroscience, and Wyeth. B. Banwell receives research funding from Canadian Institutes of Health Research, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, and the Dairy Farmers of Ontario. Dr. Banwell has received Speaker's Honoraria from Biogen-Idec. Dr. Banwell has provided non-remunerated consultant input to safety advisory boards for Biogen-Idec, Novartis, and Eli-Lilly. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol 2010;9:727–739 [DOI] [PubMed] [Google Scholar]
- 2.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis: part I: the role of infection. Ann Neurol 2007;61:288–299 [DOI] [PubMed] [Google Scholar]
- 3.Alotaibi S, Kennedy J, Tellier R, Stephens D, Banwell B. Epstein-Barr virus in pediatric multiple sclerosis. JAMA 2004;291:1875–1879 [DOI] [PubMed] [Google Scholar]
- 4.Pohl D, Krone B, Rostasy K, et al. High seroprevalence of Epstein-Barr virus in children with multiple sclerosis. Neurology 2006;67:2063–2065 [DOI] [PubMed] [Google Scholar]
- 5.Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol 2007;6:773–781 [DOI] [PubMed] [Google Scholar]
- 6.Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 2005;293:2496–2500 [DOI] [PubMed] [Google Scholar]
- 7.DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch Neurol 2006;63:839–844 [DOI] [PubMed] [Google Scholar]
- 8.Knipe DM, Howley PM, Griffin DE, et al. Fields Virology, 5th ed Philadelphia: Lippincott Williams & Wilkins; 2007:2603–2654 [Google Scholar]
- 9.Hadinoto V, Shapiro M, Sun CC, Thorley-Lawson DA. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog 2009;5:e1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000;343:481–492 [DOI] [PubMed] [Google Scholar]
- 11.Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog 2012;8:e1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon SI, Jones BC, Logsdon NJ, Harris BD, Kuruganti S, Walter MR. Epstein-Barr virus IL-10 engages IL-10R1 by a two-step mechanism leading to altered signaling properties. J Biol Chem 2012;287:26586–26595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol Rev 2010;237:226–248 [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846 [DOI] [PubMed] [Google Scholar]
- 15.Johnson G, Nelson S, Petric M, Tellier R. Comprehensive PCR-based assay for detection and species identification of human herpesviruses. J Clin Microbiol 2000;38:3274–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks JM, Croom-Carter DS, Leese AM, Tierney RJ, Habeshaw G, Rickinson AB. Cytotoxic T-lymphocyte responses to a polymorphic Epstein-Barr virus epitope identify healthy carriers with coresident viral strains. J Virol 2000;74:1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards RH, Seillier-Moiseiwitsch F, Raab-Traub N. Signature amino acid changes in latent membrane protein 1 distinguish Epstein-Barr virus strains. Virology 1999;261:79–95 [DOI] [PubMed] [Google Scholar]
- 18.Ayers M, Siu K, Roberts E, Garvin AM, Tellier R. Characterization of hepatitis C virus quasispecies by matrix-assisted laser desorption ionization-time of flight (mass spectrometry) mutation detection. J Clin Microbiol 2002;40:3455–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994;22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward KN. The natural history and laboratory diagnosis of human herpesvirus-6 and -7 infections in the immunocompetent. J Clin Virol 2005;32:183–193 [DOI] [PubMed] [Google Scholar]
- 21.Jilek S, Schluep M, Harari A, et al. HLA-B7-restricted EBV-specific CD8+ T cells are dysregulated in multiple sclerosis. J Immunol 2012;188:4671–4680 [DOI] [PubMed] [Google Scholar]
- 22.Ikuta K, Satoh Y, Hoshikawa Y, Sairenji T. Detection of Epstein-Barr virus in salivas and throat washings in healthy adults and children. Microbes Infect 2000;2:115–120 [DOI] [PubMed] [Google Scholar]
- 23.Hug M, Dorner M, Fröhlich FZ, et al. Pediatric Epstein-Barr virus carriers with or without tonsillar enlargement may substantially contribute to spreading of the virus. J Infect Dis 2010;15:1192–1199 [DOI] [PubMed] [Google Scholar]
- 24.Vogl BA, Fagin U, Nerbas L, Schlenke P, Lamprecht P, Jabs WJ. Longitudinal analysis of frequency and reactivity of Epstein-Barr virus-specific T lymphocytes and their association with intermittent viral reactivation. J Med Virol 2012;84:119–131 [DOI] [PubMed] [Google Scholar]
- 25.Santón A, Cristóbal E, Aparicio M, Royuela A, Villar LM, Alvarez-Cermeño JC. High frequency of co-infection by Epstein-Barr virus types 1 and 2 in patients with multiple sclerosis. Mult Scler 2011;17:1295–1300 [DOI] [PubMed] [Google Scholar]
- 26.Brennan RM, Burrows JM, Bell MJ, et al. Strains of Epstein-Barr virus infecting multiple sclerosis patients. Mult Scler 2010;16:643–651 [DOI] [PubMed] [Google Scholar]
- 27.Simon KC, Yang X, Munger KL, Ascherio A. EBNA1 and LMP1 variants in multiple sclerosis cases and controls. Acta Neurol Scand 2011;124:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzellos S, Farrell PJ. Epstein-Barr virus sequence variation: biology and disease. Pathogens 2012;1:156–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salmaggi A, Dufour A, Eoli M, et al. Low serum interleukin-10 levels in multiple sclerosis: further evidence for decreased systemic immunosuppression? J Neurol 1996;243:13–17 [DOI] [PubMed] [Google Scholar]
- 30.Bar-Or A, Fawaz L, Fan B, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010;67:452–461 [DOI] [PubMed] [Google Scholar]
- 31.Whittington HA, Freeburn RW, Godinho SI, Egan J, Haider Y, Millar AB. Analysis of an IL-10 polymorphism in idiopathic pulmonary fibrosis. Genes Immun 2003;4:258–264 [DOI] [PubMed] [Google Scholar]
- 32.Kanai K, Satoh Y, Yamanaka H, et al. The vIL-10 gene of the Epstein-Barr virus (EBV) is conserved in a stable manner except for a few point mutations in various EBV isolates. Virus Genes 2007;35:563–569 [DOI] [PubMed] [Google Scholar]
- 33.Mainou BA, Raab-Traub N. LMP1 strain variants: biological and molecular properties. J Virol 2006;80:6458–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ling PD, Lednicky JA, Keitel WA, et al. The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: a 14-month longitudinal study. J Infect Dis 2003;187:1571–1580 [DOI] [PubMed] [Google Scholar]
- 35.Sitki-Green D, Covington M, Raab-Traub N. Compartmentalization and transmission of multiple Epstein-Barr virus strain in asymptomatic carriers. J Virol 2003;77:1840–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huynh GT, Adler FR. Alternating host cell tropism shapes the persistence, evolution and coexistence of Epstein-Barr virus infections in human. Bull Math Biol 2011;73:1754–1773 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.