Abstract
Objective:
To analyze the clinical findings, response to therapy, and outcomes of patients with cerebral vascular amyloid-β (Aβ) deposition with and without inflammatory vascular infiltration.
Methods:
We report 78 consecutive patients with cerebral vascular Aβ deposition examined at Mayo Clinic Rochester over 25 years (1987 through 2011). Specimens reviewed by a neuropathologist showed 40 with vascular Aβ peptide without inflammation (cerebral amyloid angiopathy [CAA]), 28 with granulomatous vasculitis (Aβ-related angiitis or ABRA), and 10 with perivascular CAA-related inflammation. We also matched findings in 118 consecutive patients with primary CNS vasculitis (PCNSV) without Aβ seen over 25 years (1983 through 2007).
Results:
Compared to the 40 with CAA, the 28 with ABRA were younger at diagnosis (p = 0.05), had less altered cognition (p = 0.02), fewer neurologic deficits (p = 0.02), and fewer intracranial hemorrhages (<0.001), but increased gadolinium leptomeningeal enhancement (p = 0.01) at presentation, and less mortality and disability at last follow-up (p < 0.001). Compared with PCNSV, the 28 patients with ABRA were older at diagnosis (p < 0.001), had a higher frequency of altered cognition (p = 0.05), seizures/spells (p = 0.006), gadolinium leptomeningeal enhancement (p < 0.001), and intracerebral hemorrhage (p = 0.02), lower frequency of hemiparesis (p = 0.01), visual symptoms (p = 0.04), and MRI evidence of cerebral infarction (p = 0.003), but higher CSF protein levels (p = 0.03). Results of treatment and outcomes in ABRA and PCNSV were similar.
Conclusions:
ABRA appears to represent a distinct subset of PCNSV.
Sporadic cerebral amyloid angiopathy (CAA) is characterized by deposition of amyloid-β (Aβ) in the media and adventitia of cortical and leptomeningeal vessels.1–5 This vascular amyloid is composed of the same 39- to 43-amino acid Aβ peptide observed in the neuritic plaques of Alzheimer disease (AD). Vascular amyloid deposition may lead to vessel fragility, rupture, and intracerebral hemorrhage.1,2 Lobar intracerebral hemorrhage is the most frequently recognized clinical manifestation of CAA. In a subset of patients with Aβ vascular deposition, vascular inflammation is also present. Such patients often present with findings of subacute cognitive decline, seizures, headaches, and T2-hyperintense lesions, and respond to immunodepressive treatment.6–12 Two pathologic subtypes have been described: one with a perivascular nondestructive inflammatory infiltration, so-called CAA-related inflammation (CAA-RI),9,10 and the second with a vasculitic transmural, often granulomatous, inflammatory infiltrate (Aβ-related angiitis or ABRA).11,12
Some have proposed that ABRA is more closely related to primary CNS vasculitis (PCNSV) than to CAA without inflammatory infiltrates.11,12 However, because of the lack of uniform histopathologic criteria, small numbers of patients in most previous series, and the paucity of comparisons to PCNSV and CAA, the spectrum of ABRA and its long-term outcome remain uncertain.
In this study, we reviewed all cases of pathologically diagnosed Aβ vascular deposition seen at the Mayo Clinic (Rochester, MN) from 1987 through 2011. Uniform histopathologic criteria were used to identify patients with vascular Aβ with and without inflammation. To explore the relationship between ABRA and PCNSV, we compared the clinical and radiographic findings, response to therapy, and outcome of patients with CAA and ABRA to patients with PCNSV identified at Mayo Clinic over a 25-year period (1983–2007).12–15
METHODS
Identification of the patients.
The study included 78 patients seen at Mayo Clinic Rochester from January 1987 through December 2011 who were diagnosed pathologically with CNS Aβ vascular deposition either on in-house specimens or on Mayo review of pathologic material obtained at a different institution before our examination of the patient. The 78 cases were originally diagnosed by a neuropathologist at Mayo Clinic. All available pathologic specimens (n = 62) were re-reviewed by one neuropathologist (C.G.) of which 37 were obtained by open biopsy, 4 stereotactically, and 21 from hematoma evacuation. The re-reviewed specimens were reassessed for extent and distribution of Aβ vascular deposition and inflammation and divided in CAA, CAA-RI, and ABRA. In this study, we use CAA to designate cases without any type of vascular inflammation. Twenty-eight patients had histologic evidence of ABRA, 40 CAA, and 10 CAA-RI (figure 1). We also included in this analysis data from our cohort of 131 consecutive patients with PCNSV seen at Mayo Clinic Rochester from January 1983 through December 2007.13,14 The identification of patients with PCNSV was described in previous studies.13,15 Of the total of 131 patients, 41 were diagnosed by CNS biopsy and the remainder by classic angiograms. All were followed. Thirteen of this group were diagnosed with ABRA, and for this study were combined with the amyloid group, leaving 118 patients with PCNSV without vascular amyloid. Patients with pathologic evidence of AD were excluded.
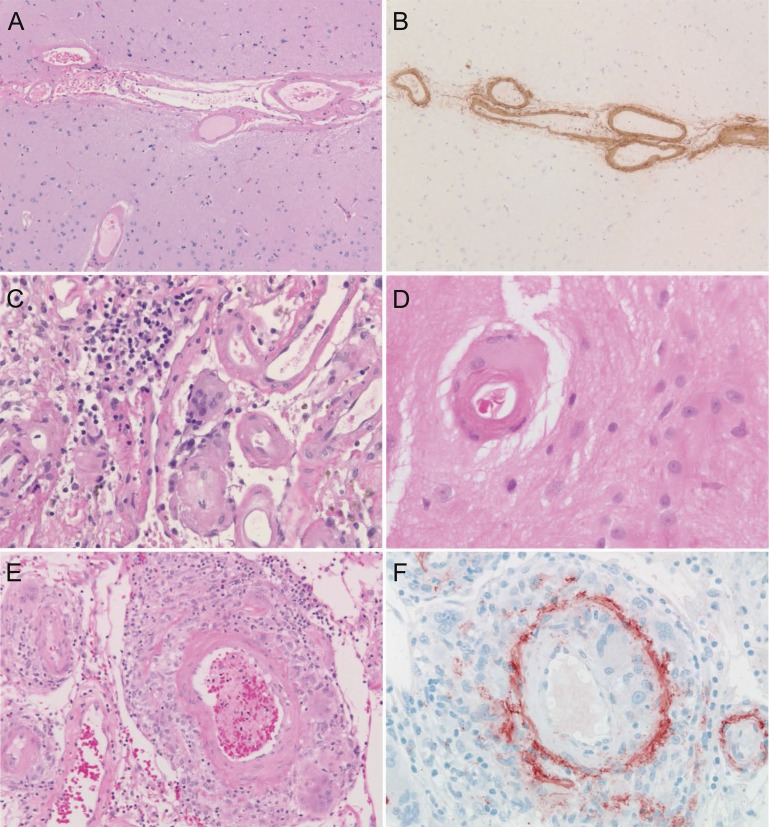
Figure 1. Pathologic findings related to cerebral vascular amyloid deposition.
Extensive vascular amyloid-β deposition without inflammation is present in the leptomeningeal and parenchymal vessels in this case of cerebral amyloid angiopathy (CAA) (A), as confirmed by immunohistochemistry (B), while mild perivascular inflammation often with giant cells surrounding leptomeningeal (C) and cortical (D) small vessels is characteristic of CAA-related inflammation, and transmural inflammation, often granulomatous and associated with vascular wall disruption, is typical of amyloid-β–related angiitis (E, F).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Mayo Clinic Institutional Review Board.
Clinical data collection.
The same data collection form used for PCNSV studies was completed for all present cases.13,15 It included comprehensive information about clinical manifestations at presentation and follow-up, other medical conditions, laboratory investigations, radiologic imaging, results of CNS biopsy or autopsy, treatment, relapses, follow-up functional status, and cause of death. To assess the effect of treatment, we used the treating physician's global opinion about the response to therapy. A neurologist examined all patients at the time of diagnosis and on subsequent visits including the last visit or death. Median follow-up was 11.3 months.
Relapse was defined as a recurrence of or increase in symptoms, or evidence of worsening of existing lesions and/or new lesions on subsequent MRI examinations while the patient received no medication or received a stable dosage of medication. Patients with relapse required an increase in therapy. The degree of disability at presentation and at the last visit was defined by a review of the detailed clinical data in the medical record and was categorized by using the modified Rankin Scale.16
Statistical analysis.
Numeric parameters were compared by using a 2-sided 2-sample t test or a Wilcoxon rank-sum test when the distributions were skewed. Comparisons of categorical variables were performed using the χ2 or Fisher exact test when cell counts were small. Survival was estimated with the Kaplan-Meier method. Cox proportional hazards modeling was performed for age-adjusted survival comparison. Significance was defined at p < 0.05. The statistical analysis was performed using SAS version 8 (SAS Institute, Cary, NC).
RESULTS
Demographic and clinical features.
The demographics and clinical manifestations at diagnosis of the 4 patient groups are compared in table 1. Females and males were relatively equally represented in all groups. Patients with ABRA were younger and had a longer time from symptom onset to diagnosis than patients with CAA (p = 0.05 and p = 0.06). Altered cognition was the most common symptom at presentation in both groups, but the frequency was higher in the patients with CAA (p = 0.02). Persistent neurologic deficit at follow-up or stroke, and intracranial hemorrhage were also more frequent in those with CAA than in those with ABRA (p = 0.02 and p < 0.001, respectively). Other major medical disorders were similar in the 2 groups (data not reported).
Table 1.
Findings at diagnosis in 4 patient groups
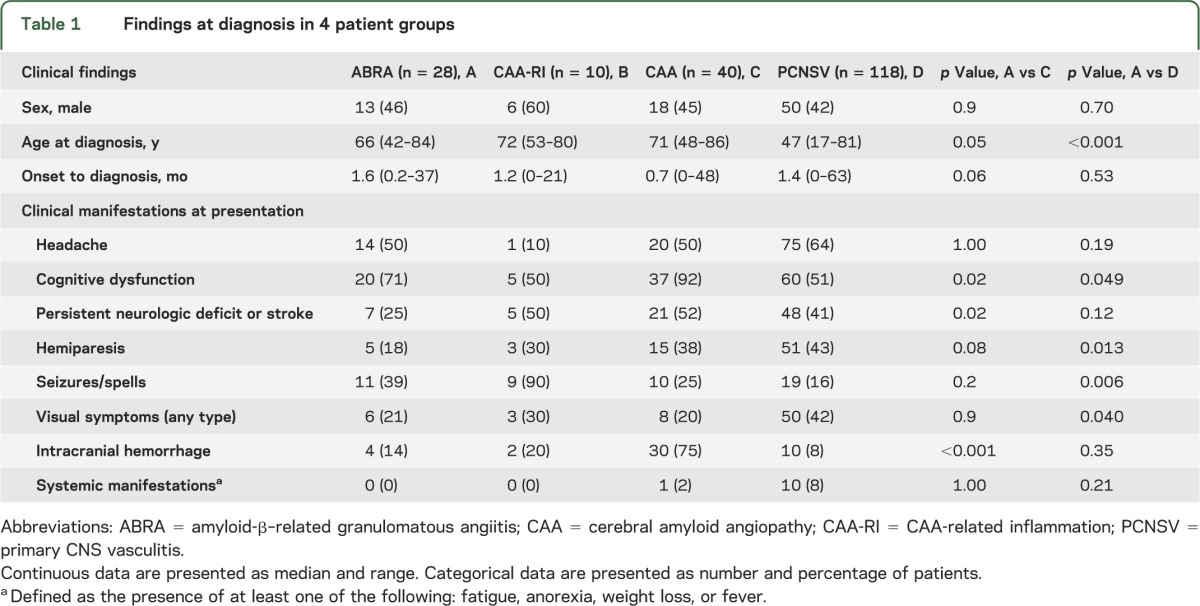
Table 1 also compares the clinical findings of the 28 patients with ABRA and 118 with PCNSV. The median age at diagnosis was lower in patients with PCNSV (p < 0.001), while the frequencies of altered cognition and seizures/spells at presentation were higher in patients with ABRA (p = 0.05 and p = 0.006, respectively). Hemiparesis and visual symptoms at presentation were less frequent in patients with ABRA (p = 0.01 and p = 0.04, respectively). Statistical analysis was not performed in CAA-RI because of small numbers.
Laboratory investigations.
The results of CSF examinations and erythrocyte sedimentation rates at diagnosis are noted in table 2. CSF findings were abnormal in 96% of the patients with ABRA who had lumbar punctures, in 80% with CAA, and in 80% with CAA-RI. Median CSF protein levels were higher in patients with ABRA than in PCNSV (103 mg/dL, range 41–522 mg/dL, vs 67 mg/dL, range 15–1034 mg/dL, respectively; p = 0.03). However, no differences in the frequency of patients with CSF abnormalities were observed in the 2 groups.
Table 2.
CSF and MRI results in 4 patient groups
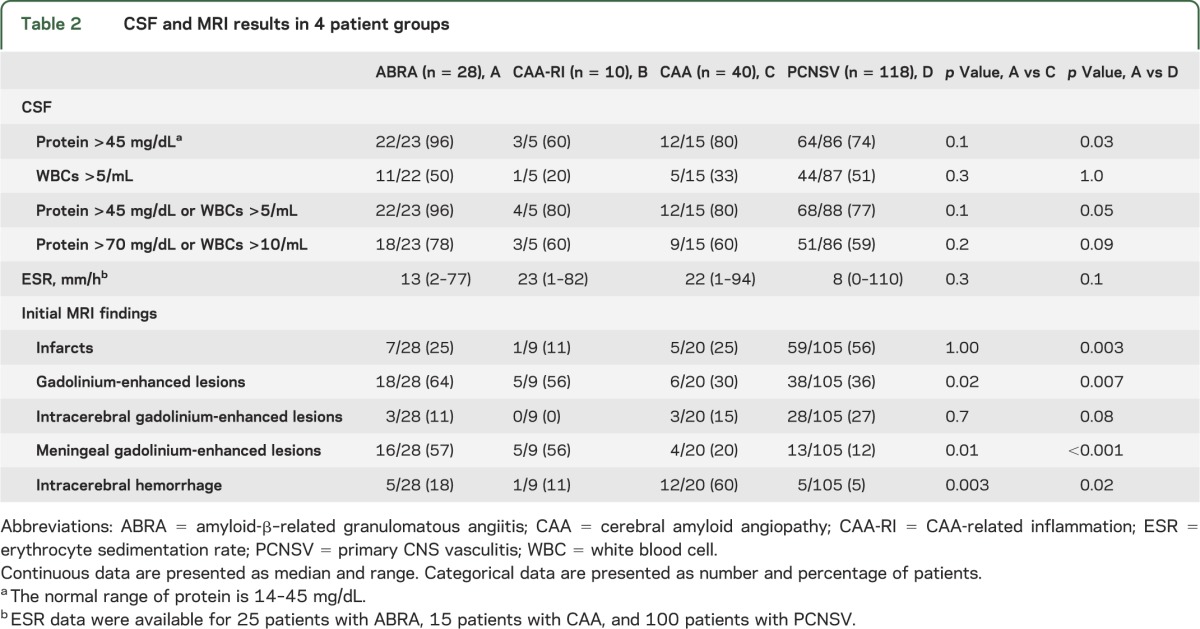
The median erythrocyte sedimentation rate was normal in all groups. It was normal in 88% of 25 patients with ABRA, in 53% of 15 patients with CAA, in 80% with CAA-RI, and in 81% of patients with PCNSV.
Radiologic imaging.
The MRI findings at presentation are reported in table 2. Gadolinium leptomeningeal enhancement was more frequent in patients with ABRA than CAA (p = 0.01), whereas intracerebral hemorrhage was more frequent in CAA (p = 0.003). Subarachnoid hemorrhage was observed in only one patient with CAA, and none in ABRA. Infarctions were the most common type of lesion observed in patients with PCNSV and were more frequent in patients with PCNSV compared to those with ABRA (p = 0.003). The patients with ABRA had a higher frequency of gadolinium leptomeningeal enhancement and intracerebral hemorrhage compared to those with PCNSV (p < 0.001 and p = 0.020, respectively). Subarachnoid hemorrhage was observed in only 3 patients with PCNSV. Gadolinium leptomeningeal enhancement was the most frequently observed radiographic finding in the 9 patients with CAA-RI.
The neuroimaging findings (brain CT and/or MRI) at the time of diagnosis were available for 56 of the 78 patients and were reviewed by a neuroradiologist (J.M.) who was blinded to the histologic diagnosis. The imaging of 2 patients was excluded because there were only postoperative imaging studies. Therefore, the neuroimaging findings of 22 patients with ABRA, 26 patients with CAA, and 6 patients with CAA-RI were analyzed (table e-1 on the Neurology® Web site at www.neurology.org). Leptomeningeal enhancement as the only lesion and leptomeningeal disease with underlying nonenhancing mass-like infiltrative white matter abnormalities were more frequently observed in patients with ABRA and CAA-RI compared to those with CAA (25% vs 3.8%, p = 0.05; and 35.7% vs 7.7%, p = 0.02, respectively). Lobar hemorrhage and vasogenic edema/mass effect were more frequent in the patients with CAA (61.5% vs 10.7%, p < 0.001; and 100% vs 78.6%, p = 0.02, respectively), while no differences were present in the 2 groups comparing the patients with nonenhancing mass-like infiltrative white matter abnormalities without overlying leptomeningeal enhancement (mimicking a low-grade glioma) (15.4% vs 14.3%; p = not significant) and the patients with foci of hemosiderin deposition (30.8% vs 46.4%, p = not significant).
Other abnormalities were observed in 8 patients. Two patients with ABRA had multiple cortical infarcts, and leptomeningeal disease overlying cortical infarcts (no white matter abnormalities), respectively. One patient with CAA-RI presented with multiple bilateral acute hemorrhages. Five patients with CAA had the following findings at diagnosis: one focal subarachnoid hemorrhage at the vertex; one multiple bilateral hemorrhages of varying ages; one enhancing intraparenchymal mass (similar to a high-grade glioma); one enhancing hemorrhagic mass crossing corpus callosum; and one multiple cortical infarcts and multiple intraparenchymal hemorrhages of different ages.
Cerebral angiography was performed in 14 patients with ABRA, 4 with CAA-RI, and 14 with CAA. Changes characteristic of vasculitis were observed in 2 patients (14%) with ABRA and 1 patient (7%) with CAA.
Treatment and outcome.
The details of treatment are shown in table 3. Glucocorticoids alone were prescribed for 10 patients (36%) with ABRA and 5 patients (12%) with CAA, whereas immunodepressants and prednisone were used in 46% and 5% of the patients of the 2 groups, respectively. More patients with ABRA than CAA received treatment with one or both drugs (82% vs 17%, p < 0.001). Cyclophosphamide was the most frequently used immunodepressant. The total duration of prednisone and cyclophosphamide therapies was not different in the 2 groups. The frequency of relapsing disease was higher in the ABRA group (18% vs 5%), but the difference was not significant. At the end of the follow-up, the frequency of patients free of immunodepressant and/or prednisone was higher in patients with CAA (57% vs 17%, p = 0.04).
Table 3.
Treatment and outcome (Rankin score at last follow-up) in 4 patient groups
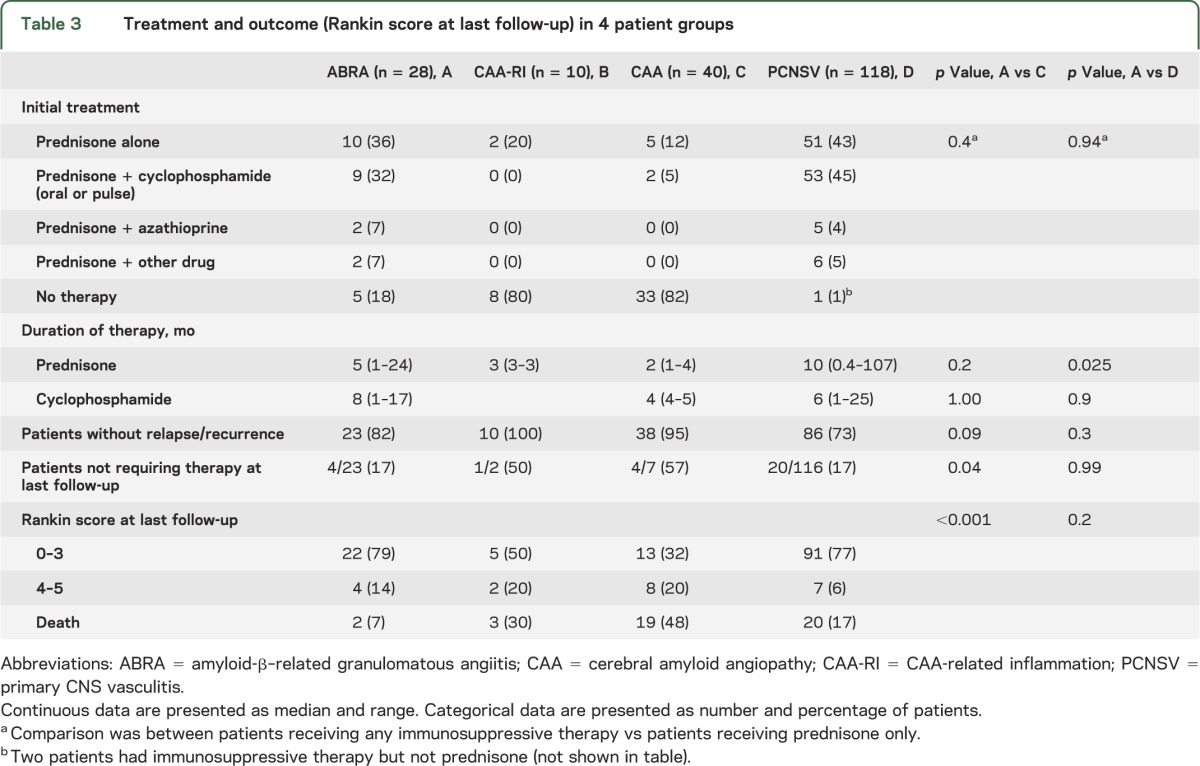
In patients with PCNSV, glucocorticoid therapy alone was prescribed for 51 patients (43%) and an immunodepressant (usually cyclophosphamide) was added in 45% more. These treatments were similar to ABRA. The duration of prednisone therapy in PCNSV (median: 10 months) was higher than in ABRA (median: 5 months) (p = 0.03), while the total duration of cyclophosphamide therapy was similar in the 2 groups (median: 6 vs 8 months). The frequencies of patients with PCNSV who had relapsing disease (27%) and the number free of immunodepressant and/or prednisone (17%) at last follow-up were similar to ABRA.
Modified Rankin disability scores were recorded at presentation and at last follow-up. The patients were grouped into 3 populations: those with Rankin score of 0 through 3 (good outcome), those with scores of 4 or 5 (poor outcome) and those with score 6 (death). Rankin scores at last follow-up are listed in table 3. The frequency of patients with good outcome at last follow-up was higher in patients with ABRA than in CAA, while the frequency of poor outcome and death was lower in ABRA (p < 0.001). The frequencies of the various outcomes in ABRA and in PCNSV were similar.
Figure 2 shows the survival curves of patients with ABRA, CAA, and PCNSV. Survival of the patients with CAA was reduced compared to patients with ABRA (p = 0.008). Significance remained after adjustment for age at diagnosis. The differences in survival between the patients who had ABRA and those with PCNSV were not statistically significant, either in the univariate analysis or after adjustment for age.
Figure 2. Survival curves of patients with ABRA, CAA, and PCNSV.
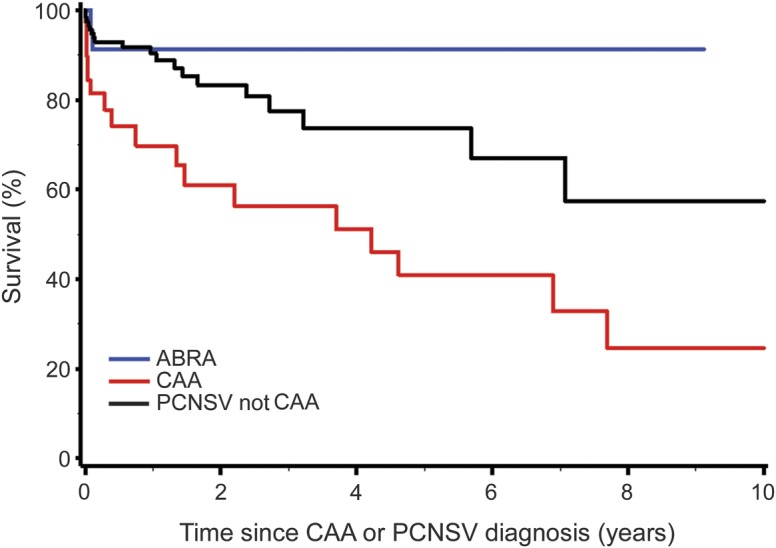
Survival of the patients with CAA was significantly reduced compared to patients with ABRA (p = 0.008). Significance remained after adjustment for age at diagnosis. The differences in survival between the patients who had ABRA and those with PCNSV were not statistically significant, univariately or after adjustment for age. ABRA = amyloid-β–related angiitis; CAA = cerebral amyloid angiopathy; PCNSV = primary CNS vasculitis.
DISCUSSION
In our cohort of patients with biopsy-proven amyloid angiopathy, evidence of vascular inflammation was found in half (49%). Two types of inflammatory response were observed, the first characterized by perivascular inflammation (CAA-RI), the second, more destructive with features of a true vasculitis, most frequently granulomatous (ABRA) in pattern. ABRA (36%) was more frequent than CAA-RI (13%). In a previous study, the presence of CAA-RI was reported in 7 (16.6%) of 42 consecutive patients with pathologically diagnosed CAA seen over 8 years at a large referral hospital.9 Although our and the previous series included patients with CAA similarly recruited, the previous study identified only patients with CAA-RI. In our series, 10 of 78 patients (13%) had CAA-RI similar to that observed by the previous study.9 However, considering the full spectrum of vascular inflammation (ABRA plus CAA-RI), the overall frequency was higher in our series. The reasons for the differences between the 2 series are uncertain.
The results showed a number of differences between ABRA and CAA: patients with ABRA had a lower median age at diagnosis, a lower frequency of altered cognition, less frequent neurologic deficit or stroke, and a lower rate of intracerebral hemorrhage. MRI evidence of gadolinium leptomeningeal enhancement was more frequent in patients with ABRA. Patients with ABRA had a more favorable response to treatment and outcome. It seems unlikely that the lower rate of treatment in patients with CAA influenced these results. The favorable response to treatment and better outcome in ABRA suggest that the vascular inflammation was a major influence in determining the disease manifestations, in comparison with the noninflammatory form of CAA, which is considered an untreatable condition. Neuroimaging also revealed differences between ABRA and CAA. Leptomeningeal changes with or without underlying nonenhancing infiltrative white matter abnormalities were more common in ABRA and CAA-RI, whereas lobar hemorrhage and vasogenic edema/mass effect were more frequently observed in patients with CAA.
Differences were also noted between ABRA and PCNSV. Patients with ABRA were older at diagnosis, had an increased frequency of altered cognition and seizures/spells at presentation, and had a reduced frequency of hemiparesis and visual symptoms. CSF protein levels were significantly higher in patients with ABRA. Cerebral infarctions were less frequent in patients with ABRA, whereas gadolinium leptomeningeal enhancement and intracerebral hemorrhage were more common. The response to treatment, duration of treatment, and outcomes were similar. The finding that ABRA was observed in 13 of 41 patients (31.7%) with biopsy-proven vasculitis in the PCNSV cohort suggests that ABRA may represent almost one-third of biopsy-proven PCNSV. The data together indicate that ABRA forms a definable subset of PCNSV characterized by older age, high frequency of cognitive dysfunction and seizures/spells, increased spinal fluid protein levels, high frequency of enhancing leptomeningeal lesions, and favorable response to glucocorticoids alone or in combination with cyclophosphamide.17
Our findings support the earlier studies,9,11 which also suggest that ABRA and CAA-RI more closely resemble PCNSV than CAA. It is possible that the perivascular form (CAA-RI) and ABRA are part of the same pathologic spectrum, but the evidence is not definitive. In our study, the number of patients identified with CAA-RI was too small to make meaningful comparisons. Also, as observed here and in previous studies, some patients with CAA may have clinical and radiologic features similar to those observed in ABRA and CAA-RI, but without pathologic evidence of inflammation.18,19 Segmental inflammatory involvement of cerebral vessels causing false-negative biopsy results could explain such findings.
Recently, autoantibodies against Aβ 1-40 and 1-42 forms of amyloid have been found to be increased in the spinal fluid of 2 patients, one diagnosed with ABRA and one with CAA-RI.20,21 A local intrathecal production of these antibodies was demonstrated. These antibodies decreased to normal levels after 3 months of steroid treatment paralleling the patient's clinical and radiologic improvements. These data suggest that the inflammation associated with ABRA and CAA-RI may result from an immune response directly triggered by cerebrovascular Aβ deposits. It has also been noted that active or passive immunotherapeutic approaches aimed at reducing the cerebral Aβ burden in AD may cause meningoencephalitis or vasogenic edema producing clinical, radiographic, and pathologic similarities to ABRA and CAA-RI.22,23 Imaging abnormalities suggestive of vasogenic edema/mass effect were observed in 22 of 28 (79%) of our patients with ABRA or CAA-RI. Vasogenic edema is also part of imaging abnormalities associated with bapineuzumab therapy, a humanized monoclonal antibody against Aβ.23 The similarities between these 2 syndromes raise the question as to whether immunotherapy-related vasogenic edema represents a treatment-induced counterpart to spontaneous inflammatory ABRA and CAA-RI. Furthermore, spontaneous inflammatory CAA-RI inflammation and immunotherapy-related conditions share a common genetic association with the APOE ε4 allele.10,23 These data raise the possibility that the E4 isoform of apolipoprotein E may have a role in promoting an inflammatory response to CAA in both conditions.
Strengths of our study include the relatively large number of unselected, consecutive patients with sporadic CAA defined by uniform pathologic criteria, the extensive clinical data available, and follow-up information. Limitations include the retrospective nature of the study, incomplete datasets, possible referral bias of cases, sampling error of the biopsy specimen because of the patchy nature of the inflammatory response, and lack of tissue diagnosis in the majority of comparison cases with PCNSV even though the angiograms were highly suggestive of vasculitis and long-term follow-up tended to confirm the diagnosis. Another potential limitation is that the majority of cases of CAA were diagnosed during hematoma evacuation. Other cases of CAA with small hematomas not evacuated or in unresectable regions of the brain may not have been diagnosed.
Our findings confirm earlier reports9–12 suggesting that ABRA represents a definable subset of PCNSV and that the vascular inflammation in ABRA more than Aβ deposition alone has a major influence in determining disease manifestations. Further study is needed to determine whether ABRA and CAA-RI are the same or separate conditions. An immune response directed against Aβ may represent a common disease mechanism shared by ABRA and in complications of therapy for AD. The recently observed presence of anti-Aβ antibodies in the CSF of patients with ABRA and CAA-RI supports this linkage and points to a possible diagnostic marker for these conditions. Furthermore, a better understanding of the molecular mechanisms associated with the pathogenesis of ABRA should provide opportunities to improve not only its treatment, but also to develop more effective immunotherapy for AD.
Supplementary Material
GLOSSARY
- Aβ
amyloid-β
- ABRA
amyloid-β–related angiitis
- AD
Alzheimer disease
- CAA
cerebral amyloid angiopathy
- CAA-RI
cerebral amyloid angiopathy–related inflammation
- PCNSV
primary CNS vasculitis
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Carlo Salvarani: study design, acquisition of the data, interpretation of the data, drafting/revising the manuscript for content. Dr. Gene G. Hunder: study design, interpretation of the data, drafting/revising the manuscript for content. Dr. Jonathan M. Morris: acquisition of the data, interpretation of the data, revising the manuscript for content. Dr. Robert D. Brown, Jr.: study design, interpretation of the data, revising the manuscript for content. Teresa Christianson: statistical analysis, revising the manuscript for content. Dr. Caterina Giannini: study design, acquisition of the data, interpretation of the data, revising the manuscript for content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Greenberg S. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology 1998;51:690–694 [DOI] [PubMed] [Google Scholar]
- 2.Vinters HV. Cerebral amyloid angiopathy: a critical review. Stroke 1987;18:311–324 [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM, Vonsattel JPG, Stakes JW, Gruber M, Finklestein SP. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology 1993;43:2073–2079 [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke 2004;35(suppl 1):2616–2619 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SM, Vonsattel JPG. Diagnosis of cerebral amyloid angiopathy: sensitivity and specificity of cortical biopsy. Stroke 1997;28:1418–1422 [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg L, Geddes J, Valentine A. Amyloid angiopathy and granulomatous angiitis of the central nervous system: a case responding to corticosteroid treatment. J Neurol 1988;235:438–440 [DOI] [PubMed] [Google Scholar]
- 7.Fountain NB, Eberhard D. Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy: report of two cases and review of the literature. Neurology 1996;46:190–197 [DOI] [PubMed] [Google Scholar]
- 8.Schwab P, Lidov HGW, Schwartz RB, Anderson RJ. Cerebral amyloid angiopathy associated with primary angiitis of the central nervous system: report of 2 cases and review of the literature. Arthritis Rheum 2003;49:421–427 [DOI] [PubMed] [Google Scholar]
- 9.Eng JA, Frosch MP, Choi K, Rebeck GW, Greenberg SM. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol 2004;55:250–256 [DOI] [PubMed] [Google Scholar]
- 10.Kinnecom C, Lev MH, Wendell L, et al. Course of cerebral amyloid angiopathy-related inflammation. Neurology 2007;68:1411–1416 [DOI] [PubMed] [Google Scholar]
- 11.Scolding NJ, Joseph F, Kirby PA, et al. Aβ-related angiitis: primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 2005;128:500–515 [DOI] [PubMed] [Google Scholar]
- 12.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis: comparison of patients with and without cerebral amyloid angiopathy. Rheumatology 2008;47:1671–1677 [DOI] [PubMed] [Google Scholar]
- 13.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis presenting with intracranial hemorrhage. Arthritis Rheum 2011;63:3598–3606 [DOI] [PubMed] [Google Scholar]
- 14.Giannini C, Salvarani C, Hunder G, Brown RD. Primary central nervous system vasculitis: pathology and mechanisms. Acta Neuropathol 2012;123:759–772 [DOI] [PubMed] [Google Scholar]
- 15.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann Neurol 2007;62:442–451 [DOI] [PubMed] [Google Scholar]
- 16.Sulter G, Steen C, De Keyser J. Use of the Barthel Index and modified Rankin Scale in acute stroke trials. Stroke 1999;30:1538–1541 [DOI] [PubMed] [Google Scholar]
- 17.Salvarani C, Brown RD, Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet 2012;380:767–777 [DOI] [PubMed] [Google Scholar]
- 18.Silbert PL, Bartleson JD, Miller GM, Parisi JE, Goldman MS, Meyer FB. Cortical petechial hemorrhage, leukoencephalopathy, and subacute dementia associated with seizures due to cerebral amyloid angiopathy. Mayo Clin Proc 1995;70:477–480 [DOI] [PubMed] [Google Scholar]
- 19.Oh U, Gupta R, Krakauer JW, Khandji AG, Chin SS, Elkind MS. Reversible leukoencephalopathy associated with cerebral amyloid angiopathy. Neurology 2004;62:494–497 [DOI] [PubMed] [Google Scholar]
- 20.DiFrancesco JC, Brioschi M, Brighina L, et al. Anti-Aβ autoantibodies in the CSF of a patient with CAA-related inflammation: a case report. Neurology 2011;76:842–844 [DOI] [PubMed] [Google Scholar]
- 21.Hermann DM, Keyvani K, van de Nes J, et al. Brain-reactive β-amyloid antibodies in primary CNS angiitis with cerebral amyloid angiopathy. Neurology 2011;77:503–505 [DOI] [PubMed] [Google Scholar]
- 22.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46–54 [DOI] [PubMed] [Google Scholar]
- 23.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 2012;11:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.