Abstract
Objective:
To develop a cutaneous biomarker for Parkinson disease (PD).
Methods:
Twenty patients with PD and 14 age- and sex-matched control subjects underwent examinations, autonomic testing, and skin biopsies at the distal leg, distal thigh, and proximal thigh. α-Synuclein deposition and the density of intraepidermal, sudomotor, and pilomotor nerve fibers were measured. α-Synuclein deposition was normalized to nerve fiber density (the α-synuclein ratio). Results were compared with examination scores and autonomic function testing.
Results:
Patients with PD had a distal sensory and autonomic neuropathy characterized by loss of intraepidermal and pilomotor fibers (p < 0.05 vs controls, all sites) and morphologic changes to sudomotor nerve fibers. Patients with PD had greater α-synuclein deposition and higher α-synuclein ratios compared with controls within pilomotor nerves and sudomotor nerves (p < 0.01, all sites) but not sensory nerves. Higher α-synuclein ratios correlated with Hoehn and Yahr scores (r = 0.58–0.71, p < 0.01), with sympathetic adrenergic function (r = −0.40 to −0.66, p < 0.01), and with parasympathetic function (r = −0.66 to −0.77, p > 0.01).
Conclusions:
We conclude that α-synuclein deposition is increased in cutaneous sympathetic adrenergic and sympathetic cholinergic fibers but not sensory fibers of patients with PD. Higher α-synuclein deposition is associated with greater autonomic dysfunction and more advanced PD. These data suggest that measures of α-synuclein deposition in cutaneous autonomic nerves may be a useful biomarker in patients with PD.
Parkinson disease (PD) is a progressive neurodegenerative disorder defined by the clinical presentation of tremor, rigidity, bradykinesia, and postural instability. The pathologic hallmark of PD, the Lewy body, is found in the neuronal perikarya. Similar inclusions in neuronal cell processes are called Lewy neurites. α-Synuclein is the most prominent protein in Lewy bodies and Lewy neurites.1
Accumulating evidence suggests that α-synuclein deposition occurs early in the course of PD and may antedate the appearance of the clinical features of the disease.2 This has provided the rationale for the use of α-synuclein as a biomarker in PD.3
Skin punch biopsy could provide a simple means to measure α-synuclein deposition in the peripheral nervous system; however, preliminary studies used techniques optimized for CNS tissue, relied on small tissue volumes, and did not systematically study autonomic substructures, and therefore detected α-synuclein in only a minority of subjects with PD.4–8
We recently reported novel methods to study the cutaneous autonomic innervation in skin biopsies of patients with peripheral nerve disease.9,10 We hypothesized, based on the prominent autonomic manifestations of PD, that α-synuclein deposition would be elevated in cutaneous structures with autonomic innervation.
The aims of the study were to determine 1) whether α-synuclein was present in cutaneous sensory and autonomic nerves, 2) the relationship between cutaneous α-synuclein deposition and PD stage, and 3) the relationship between cutaneous α-synuclein deposition and measures of autonomic function.
METHODS
Subjects.
Twenty subjects with PD and 14 similarly age- and sex-matched healthy controls were prospectively recruited for the study. Subjects were recruited through the Movement Disorders Center at Beth Israel Deaconess Medical Center and through local support groups. Control subjects were recruited by local advertisement and were not family members of subjects with PD.
Standard protocol approvals, registrations, and patient consents.
The Beth Israel Deaconess Institutional Review Board approved the studies, and all subjects signed an informed consent.
Examination.
All subjects underwent a general history and detailed neurologic examination. PD severity was rated by the Hoehn and Yahr scale by physicians with expertise in movement disorders during the off stage after holding medications overnight.
Autonomic function testing.
Cardiovascular parasympathetic function testing (the heart rate response to deep respiration and the Valsalva maneuver) and cardiovascular sympathetic function (the blood pressure response to a Valsalva maneuver and tilt-table testing to 60° for 10 minutes) were performed in all subjects. Continuous ECG monitoring, continuous beat-to-beat blood pressure recordings, and manual blood pressure measurements every minute during tilt-table testing were recorded. Autonomic symptoms were quantified using the Boston Autonomic Symptom Questionnaires; symptoms ranged from 0 (none) to 10 (maximal).
Human skin biopsy and fixation.
Three-millimeter skin punch biopsies were obtained from the distal leg, distal thigh, and proximal thigh after local anesthesia with 2% lidocaine.11 Skin biopsy specimens were fixed in Zamboni solution for 18 hours and cryoprotected overnight (20% glycerol and 20% 0.4 M Sorensen buffer). Tissue blocks were cut by freezing microtome into 50-μm-thick sections. An average of 20 tissue sections were analyzed, but additional sections were analyzed in cases in which sweat glands or arrector pili muscles were not identified within the original sections.
Immunohistochemistry.
Fluorescent immunostaining was performed using our previously published methods for covisualizing total nerve fibers and sympathetic cholinergic, sympathetic adrenergic, or α-synuclein–positive nerve fibers.9 Complete details on the immunohistochemical methods are provided in appendix e-1 on the Neurology® Web site at www.neurology.org. Antibodies and reagents are listed in table e-1. The primary antibodies used to visualize total nerve fibers included protein gene product (PGP) 9.5, the sympathetic cholinergic marker vasoactive intestinal peptide, and the sympathetic adrenergic marker tyrosine hydroxylase. The antibody used to detect α-synuclein was polyclonal and recognized multiple binding sites from amino acids 111 to 131.12
Confocal imaging.
All stained sections were initially examined under a fluorescent microscope (Zeiss-Axioplan2; Carl Zeiss, Thornwood, NY), with areas of interest imaged by confocal microscopy (Zeiss LSM5 Pascal Exciter; Carl Zeiss). A series of images of optical sections was acquired at 3-μm intervals throughout the depth of the 50-μm section as a Z-stack (Lens Plan-Apochromat ×20/0.8; Carl Zeiss). All samples were deidentified and studied in a blinded manner.
Quantification of nerve fiber density.
Intraepidermal nerve fiber density.
Biopsies underwent blinded intraepidermal nerve fiber density counting with PGP 9.5–positive fibers and results were expressed as number of fibers crossing the dermal-epidermal junction per millimeter as previously described.13
Sweat gland nerve fiber density.
Sweat glands were imaged and nerve fiber densities quantified in a blinded manner using our previously described manual unbiased stereologic technique.10 The number of nerve fibers intersecting the circles within the area of interest was counted and reported as the percentage of circles with nerve fiber crossing.10
Pilomotor nerve fiber density.
Pilomotor muscles were imaged and nerve fiber densities were quantified in a blinded manner as previously published.14 The average number of nerve fibers intersecting 5 horizontal lines across the width of the pilomotor muscle in 3-μm-thick confocal images was reported in fibers/mm.14
α-Synuclein nerve fiber density ratio (α-synuclein ratio).
The deposition of α-synuclein within nerve fibers (immunostained by PGP 9.5) was confirmed by merged confocal images. The number of nerve fibers that contain α-synuclein are expressed as a percent of the total nerve density. This technique normalizes the deposition of α-synuclein to nerve density to account for the presence of a peripheral nerve degeneration.
Statistical analysis.
Statistical data analysis was performed using SPSS v17.0 (IBM, Chicago, IL). Based on our preliminary data, we estimated that 20 subjects with PD and 15 control subjects would provide >90% power to detect a difference in α-synuclein ratios between groups. Data are presented as mean ± SD. The primary outcome was the α-synuclein ratio. Differences in total nerve fiber density, nerve fiber subtypes (cholinergic and adrenergic), α-synuclein levels, and autonomic test results are reported. Continuous variables were measured against Hoehn and Yahr scores by Kruskal-Wallis tests, with Mann-Whitney U tests for post hoc analysis (with Bonferroni corrections). Correlations between tests are expressed as Pearson correlations. A 2-sided p value of 0.05 was used to define statistical significance for all data sets (with corrections for multiple comparisons).
RESULTS
Demographics.
Twenty subjects with PD (mean age 61 ± 10 years, body mass index 28.3 ± 4.7 kg/m2, mean duration of disease 4.2 ± 3.7 years, 4 women) participated in the study. The Hoehn and Yahr score was 2.6 ± 1.0 (range 1–4). Fourteen healthy controls (mean age 58 ± 6 years, body mass index 29.2 ± 3.1 kg/m2, 4 women) participated in the study. Demographic data were similar. Twelve of 20 subjects had been taking levodopa for an average of 1.4 ± 1.2 years.
Autonomic symptoms.
Patients with PD reported more postural lightheadedness (2.6 ± 2.2 vs 0.1 ± 0.2 controls, p < 0.05), constipation (3.3 ± 3.1 vs 0.2 ± 0.4 controls, p < 0.05), urinary frequency (4.8 ± 3.6 vs 1.1 ± 1.6 controls, p < 0.05), urinary urgency (4.5 ± 3.3 vs 1.5 ± 1.1 controls, p < 0.05), nocturia (5.7 ± 3.4 vs 2.1 ± 1.6 controls, p < 0.05), urinary incontinence (2.3 ± 2.1 vs 0.1 ± 0.2 controls, p < 0.05), erectile dysfunction (3.5 ± 2.7 vs 1.4 ± 1.6 controls, p < 0.05), and sweating dysfunction (3.9 ± 2.2 vs 1.0 ± 1.1 controls, p < 0.05).
Autonomic function tests.
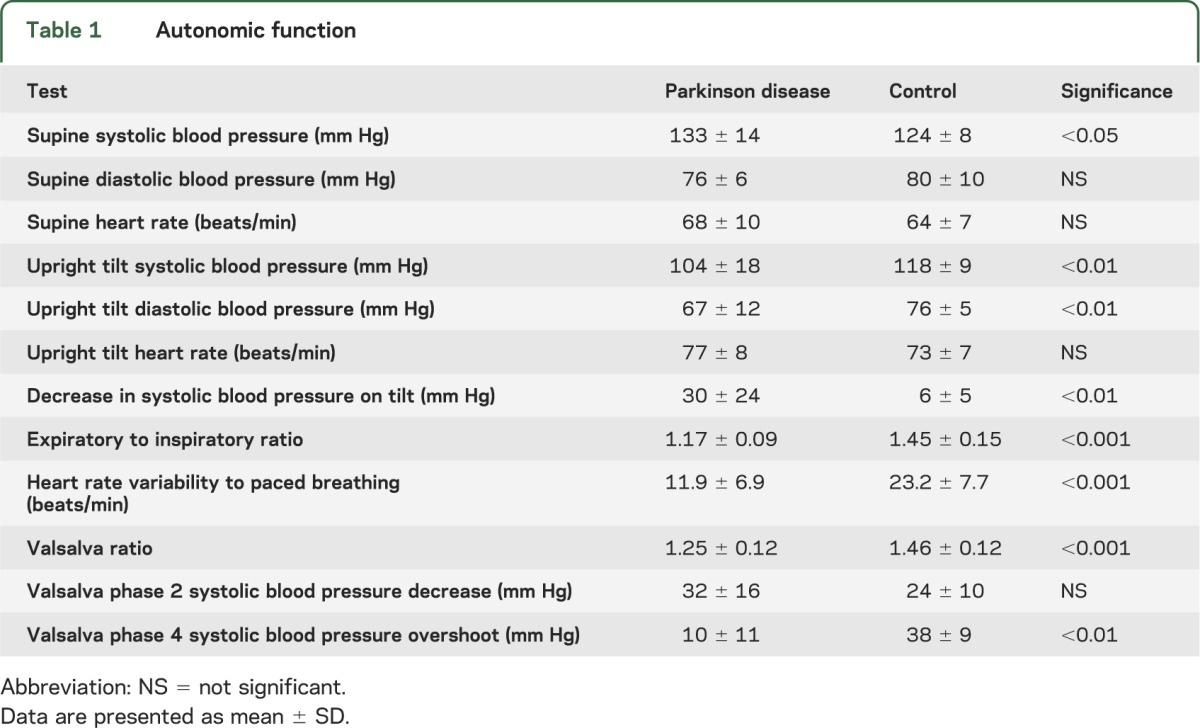
Individuals with PD had significantly reduced sympathetic and parasympathetic function compared with healthy control subjects. Full results of autonomic testing are reported in table 1.
Table 1.
Autonomic function
Sensory nerve fiber density.
Intraepidermal nerve fibers were reduced in individuals with PD at the distal leg (4.9 ± 3.6 vs 10.3 ± 3.0 fibers/mm control, p < 0.001), distal thigh (8.9 ± 4.2 vs 13.2 ± 3.8 fibers/mm control, p < 0.01), and proximal thigh (10.8 ± 4.0 vs 16.2 ± 6.6 fibers/mm control, p < 0.01). Ten of 20 patients with PD met criteria for small fiber neuropathy based on a reduced intraepidermal nerve fiber density.
Pilomotor nerve fiber density.
Pilomotor nerve fibers were reduced in individuals with PD at the distal leg (45.8 ± 15.9 vs 70.4 ± 26.5 fibers/mm control, p < 0.01), distal thigh (54.2 ± 18.0 vs 75.4 ± 20.3 fibers/mm control, p < 0.01), and proximal thigh (60.9 ± 19.3 vs 78.1 ± 16.3 fibers/mm control, p < 0.01) (figure 1, A and C). The reduction in pilomotor nerve fiber density in patients with PD appeared to be a selective loss of sympathetic adrenergic fibers at all sites.
Figure 1. Autonomic innervation and α-synuclein deposition.
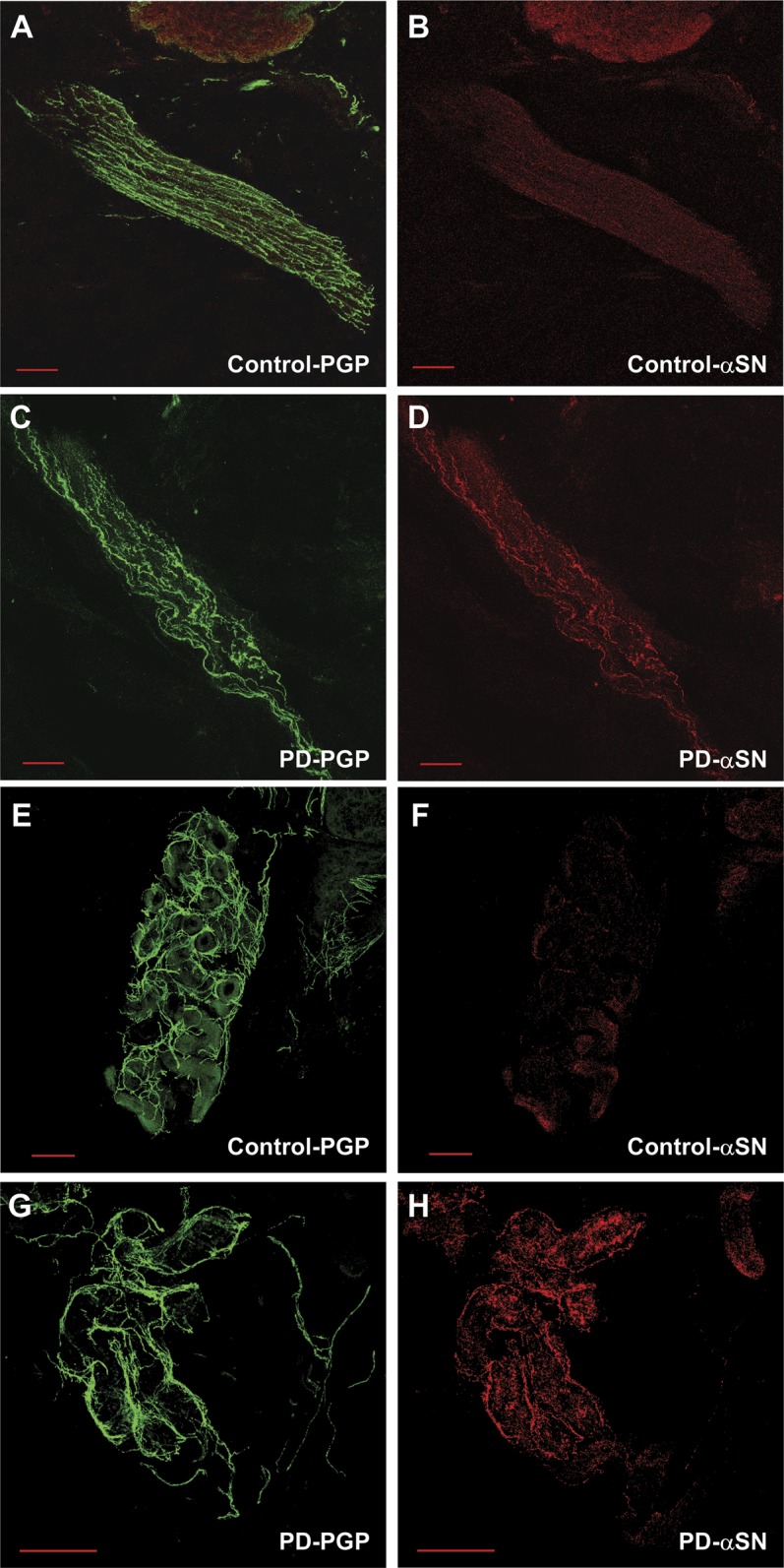
Sample images are shown for healthy control subjects (A, B, E, F) and subjects with PD (C, D, G, H). In (A) the pan-axonal marker PGP 9.5 in green reveals nerve fibers in a pilomotor muscle of a healthy subject. The fibers are linear with normal morphology. In (B), nerve fibers that contain α-synuclein (red) are shown for the corresponding pilomotor image (A). There is nonspecific α-synuclein staining in the hair follicle of the control subject and a small amount of α-synuclein deposition within the pilomotor nerve fibers. However, this small amount of α-synuclein deposition in a large number of fibers accumulates to a modest total amount of α-synuclein, but a low α-synuclein ratio. In (C), there is a reduction in the pilomotor nerve density in an individual with PD. Note the loss of linearity and thickened fibers. In contrast, (D), α-synuclein, shown in red, accumulates in pilomotor fibers of a patient with PD. The total amount of α-synuclein is not much greater than in the control subject (B), but the α-synuclein ratio is much higher. Sudomotor nerve fibers detected by the pan-axonal marker PGP 9.5 for a control subject (E) and a patient with PD (G). α-Synuclein is detected at low levels in the control with a low α-synuclein ratio (F), but is visible within the sudomotor fibers of a patient with PD and a high α-synuclein ratio (H). Scale bar = 100 μm. αSN = α-synuclein; PD = Parkinson disease; PGP = protein gene product 9.5.
Sweat gland nerve fiber density.
Sweat gland nerve fiber densities were similar in control subjects and individuals with PD at the distal leg (51.9% ± 14.3% vs 52.1% ± 7.1% control), distal thigh (54.5% ± 16.1% vs 57.2% ± 6.6% control), and proximal thigh (54.8% ± 15.4% vs 56.1% ± 6.8% control). Patients with PD had notable morphologic changes in sudomotor innervation, with thickened, segmented, and dystrophic-appearing nerve fibers visible in many tissue sections that were not seen in control subjects (figure 1, E and G).
α-Synuclein.
α-Synuclein was detected in the biopsy samples of all subjects. The α-synuclein was deposited within autonomic nerve fibers throughout the dermal layer and was not found within nociceptive sensory fibers (intraepidermal fibers). Greater deposition of α-synuclein and higher α-synuclein ratios were present in the nerve fibers surrounding sweat glands (p < 0.001, all sites) and innervating arrector pilorum muscles (p < 0.001, all sites) of patients with PD (figures 1 and 2).
Figure 2. α-Synuclein ratio.
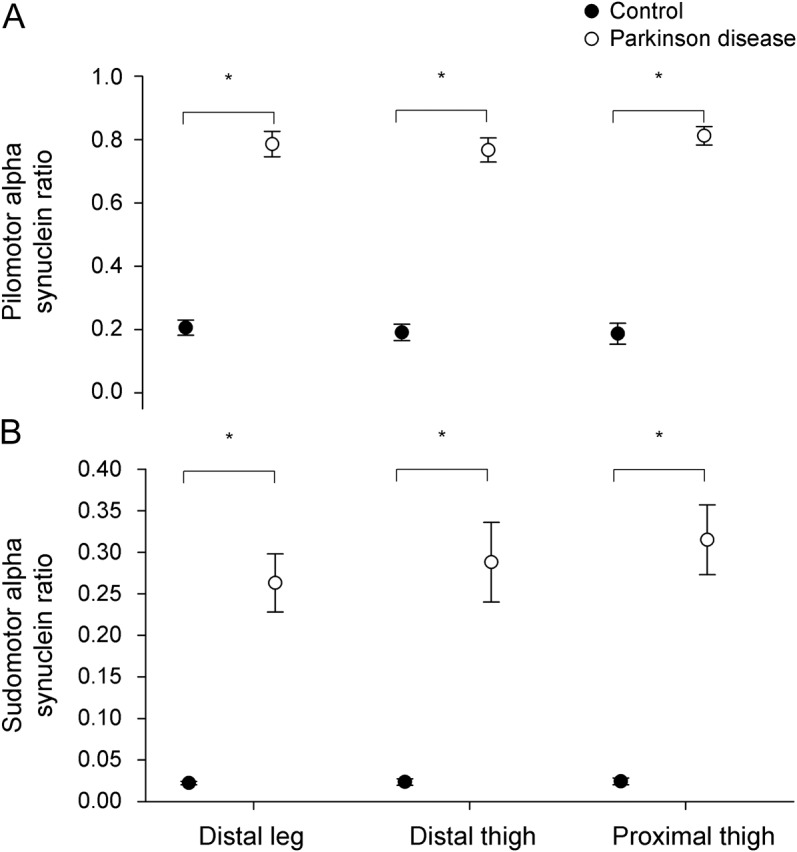
The pilomotor α-synuclein ratios (A) and sudomotor α-synuclein ratios (B) for healthy controls (black circles) and individuals with Parkinson disease (open circles) by biopsy site (distal leg, distal thigh, or proximal thigh). *p < 0.01 vs control subjects (paired t test with Bonferroni correction). Results shown are mean ± SEM.
Higher ratios of α-synuclein correlated with reduced pilomotor nerve fiber density at the distal leg (r = −0.53, p < 0.01), distal thigh (r = −0.48, p < 0.01), and proximal thigh (r = −0.61, p < 0.01). Higher ratios of α-synuclein correlated with reduced sudomotor nerve fiber density at the distal leg (r = −0.36, p < 0.05), distal thigh (r = −0.31, p < 0.05), and proximal thigh (r = −0.42, p < 0.05).
Relationship among α-synuclein deposition, examination, and autonomic function.
Higher α-synuclein ratios were associated with decreased heart rate variability to paced breathing, reduced heart rate response to a Valsalva maneuver, reduced blood pressure overshoot to a Valsalva maneuver, and a greater decrease in systolic blood pressure on upright tilt-table test (all p < 0.05). The correlations were significant across all autonomic function tests except the decrease in blood pressure during phase 2 of the Valsalva maneuver. Correlation coefficients are reported in table e-2.
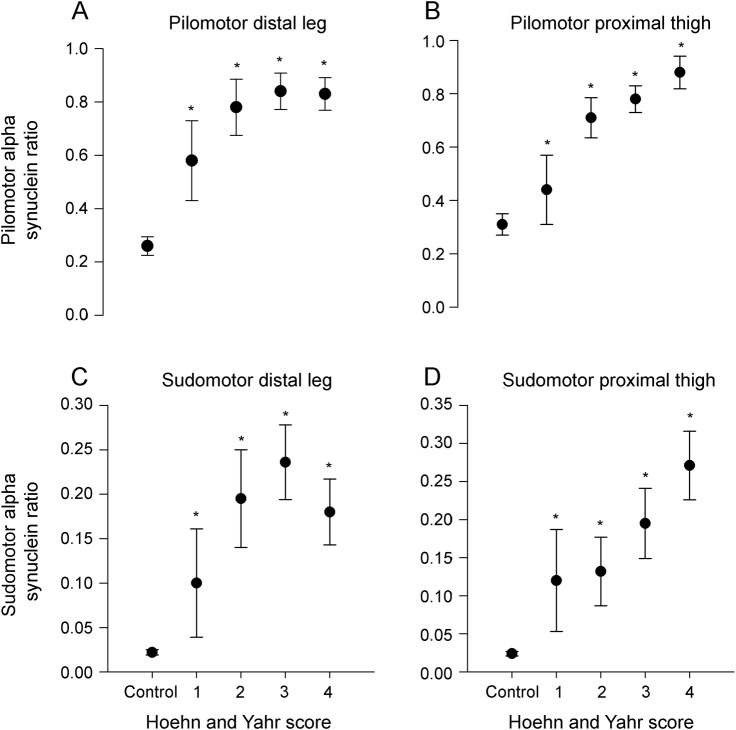
Higher Hoehn and Yahr scores had higher α-synuclein ratios (both pilomotor and sudomotor), and were higher than in healthy controls (p < 0.01 at all biopsy sites for sudomotor and pilomotor α-synuclein ratios). The results are shown in figure 3 and table e-2.
Figure 3. α-Synuclein ratio by Hoehn and Yahr score.
Pilomotor α-synuclein ratios, plotted against Hoehn and Yahr score (1 = mild PD, 4 = severe PD), at the distal leg (A) and proximal thigh (B). Sudomotor α-synuclein ratios, plotted against Hoehn and Yahr score, at the distal leg (C) and proximal thigh (D). *p < 0.05 vs control subjects (Kruskal-Wallis tests, with Mann-Whitney U tests for post hoc analysis using Bonferroni corrections for multiple comparisons). Results shown are mean ± SEM. PD = Parkinson disease.
DISCUSSION
This study demonstrates the following: 1) α-synuclein is present within cutaneous autonomic nerve fibers of patients with PD and healthy controls, but α-synuclein deposition was significantly higher in patients compared with healthy controls; 2) the ratio of α-synuclein to PGP 9.5 in sudomotor and pilomotor nerves is significantly higher in patients with PD than in controls; 3) the α-synuclein ratio correlates with PD severity (measured by the Hoehn and Yahr score) and with measures of autonomic function in patients; and 4) patients with PD have a mild length-dependent sensory neuropathy that is not associated with α-synuclein deposition. Taken together, these data suggest that α-synuclein deposition in cutaneous autonomic nerve fibers may be a potential biomarker in PD.
A reliable biomarker could contribute to the diagnosis, disease modification, and treatment of PD by improving diagnostic accuracy and providing a diagnosis early in the course of the disease, ideally in the premotor state thereby facilitating interventions with neuroprotective and disease-modifying therapies.15,16
Autonomic nonmotor symptoms, which include cutaneous manifestations, occur frequently in PD. Thermoregulatory, cutaneous vasomotor, and sweating disturbances (both hypo- and hyperhidrosis) occur in almost two-thirds of patients with PD.17 Sudomotor system symptoms manifest at all stages of PD and subclinical sudomotor dysfunction may be present in the early stages of the disease.18 There is evidence of impaired cutaneous vasodilation capacity in PD19 and impaired vasoconstrictor function even in the early stages of PD.20 Our study is prompted by the presence of these cutaneous autonomic manifestations in PD, their progression throughout the disease, allied with the ease with which skin biopsy can be performed and repeated.
Measurement of α-synuclein in body fluids and tissues has been the basis for several investigations for potential biomarkers.4,7,21,22 There is growing pathologic and imaging evidence of peripheral autonomic and sensory nerve involvement even in the premotor stages of PD. Lewy bodies and Lewy neurites have been reported in Meissner and submucosal plexuses in postmortem specimens from asymptomatic individuals.23 α-Synuclein aggregates were found in surgical specimens of autonomic plexuses of asymptomatic adults,24 in the myenteric plexus of the esophagus, sympathetic ganglia, and vagus nerve of asymptomatic elderly,25 in the dorsal root ganglia,7,26 and in the cardiac sympathetic nerves of 90% of individuals with incidental Lewy body disease and 60% of individuals with PD.27 There is pathologic evidence that α-synuclein accumulation in distal autonomic axons in PD occurs early and may precede the accumulation in more proximal autonomic structures.27
α-Synuclein determination from colonic tissue is the best established in vivo biomarker in PD. Pathologic studies of surgical and autopsy specimens have documented Lewy body pathology at sites throughout the gastrointestinal tract from the esophagus to the rectum.7,23,28 The gastrointestinal symptoms experienced by patients with PD,29 which may antecede the appearance of the clinical manifestations of PD by at least 10 years,30 have provided the rationale for the in vivo assessment of α-synuclein from biopsy specimens obtained during endoscopy.31 However, colonoscopy and colon biopsies are invasive, require sedation and colonic preparation, and are performed typically only at scheduled times based on screening guidelines.
More recently, α-synuclein was visualized in submucosal neurites in all evaluable biopsies taken from the distal sigmoid colon during flexible sigmoidoscopy.21 This is a less invasive test that can be performed without prior preparation and is not dependent on cancer surveillance screening guidelines. However, discordant results have been reported.32 The discrepancy may be related to the specificity of the antibody used in the 2 studies. One study used an antibody that was not specific for phosphorylated α-synuclein21 whereas the other used an antibody specific for phosphorylated α-synuclein.32 This may have implications for the present study.
Other studies investigating α-synuclein deposition within cutaneous autonomic nerve fibers have not been as successful as the present study. This may be explained by methodologic differences.4,5 Our skin biopsies were fixed in Zamboni solution for only 18 hours because we find loss of immunostaining after 24 hours. Other studies have used fixation in 4% paraformaldehyde for 48 hours, a process frequently used in CNS immunohistochemistry, but not in peripheral nerves where prolonged fixation causes rapid degradation of nerve fibers and loss of immunofixation.13 We also used 50-μm-thick frozen sections and we examined 10 to 20 sections per skin biopsy (for a total analysis thickness of 500–1,000 μm). Other studies have measured small numbers of 4- to 6-μm-thick sections, thereby potentially missing α-synuclein deposition completely if arrector pili muscles or sweat glands were not present within the sample. In addition, all of our tissue sections were double-stained for α-synuclein and another neuronal marker (such as PGP 9.5, tyrosine hydroxylase, or vasoactive intestinal peptide), with 3-µm confocal images compiled as a Z-stack to confirm that α-synuclein is present within nerve fibers as successfully performed by other investigators in the skin of patients with pure autonomic failure.33
In contrast to prior postmortem7 and live human skin biopsy reports,8 our study showed high sensitivity for the detection of α-synuclein. This may be, in part, related to our systematic analysis of autonomic structures and our use of an antibody that was not specific for phosphorylated α-synuclein. A recent report also observed increased detection of α-synuclein in the colonic submucosa in early untreated PD using a nonspecific antibody.21
Consistent with prior reports that document the presence of a distal small fiber and autonomic axonopathy in individuals with PD,34 a peripheral neuropathy (defined by reduced intraepidermal nerve fiber density on skin biopsy) was present in half of our subjects. The mechanism underlying this neuropathy is not known. Several case reports and series have proposed that the neuropathy is related to levodopa, administered orally35 or intestinally,36 and that the neuropathy is due to vitamin deficiency (particularly vitamins B12 and B6).35,37 However, a peripheral neuropathy may be present in some subjects without prior levodopa exposure.38 We did not find evidence of α-synuclein accumulation within small sensory fibers in the skin, suggesting that the sensory neuropathy is not related to α-synuclein deposition in these distal nerve fibers. Alternately, the distal sensory neuropathy may be due to α-synuclein deposition at a more proximal site. The presence of a distal autonomic neuropathy attenuated the sensitivity of measuring absolute α-synuclein deposition within the skin. To circumvent this problem, we normalized our results relative to the density of autonomic innervation; the α-synuclein ratio is the ratio of the sudomotor and pilomotor nerve fibers that are positive for α-synuclein to those that are PGP-positive. This index of α-synuclein deposition supports a relationship between α-synuclein deposition and 1) PD disease severity, 2) pilomotor and sudomotor nerve fiber density, and 3) autonomic function.
There are several limitations to this study. Most of the studied subjects were in the mid and late stages of the disease. The study therefore does not address the question as to how early α-synuclein deposition occurs in PD. Future studies should include subjects in the earlier stages of the disease and subjects with other synucleinopathies, particularly given the expected differential deposition of α-synuclein in peripheral tissues in different neurodegenerative conditions.27 We do not have vitamin B6, vitamin B12, methylmalonic acid, or homocysteine levels in individual subjects. This also should be included in future studies. There is a strong and unmet need for a biomarker for PD. Despite these limitations, assessment of α-synuclein deposition within the skin has the potential to provide a safe, accessible, and repeatable biomarker.
Supplementary Material
GLOSSARY
- PD
Parkinson disease
- PGP
protein gene product
Footnotes
Editorial, page 1568
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Wang was involved in data collection, data analysis, data interpretation, and manuscript revision. Dr. Gibbons was involved in study design, data collection, data analysis, data interpretation, statistical analysis, and writing the manuscript. Mr. Lafo was involved in data collection, data analysis, data interpretation, and manuscript revision. Dr. Freeman was involved in study design, data analysis, data interpretation, and manuscript revision.
STUDY FUNDING
Supported by NIH National Institute of Neurological Disorders and Stroke K23NS020509 (C.H.G.), the Langer Family Foundation (R.F.), and the RJG Foundation (C.H.G.).
DISCLOSURE
N. Wang, C. Gibbons, and J. Lafo report no disclosures. R. Freeman has received personal compensation for serving on scientific advisory boards of Abbott. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol 2009;8:1150–1157 [DOI] [PubMed] [Google Scholar]
- 2.Navarro-Otano J, Gelpi E, Mestres CA, et al. Alpha-synuclein aggregates in epicardial fat tissue in living subjects without parkinsonism. Parkinsonism Relat Disord 2013;19:27–31 [DOI] [PubMed] [Google Scholar]
- 3.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx 2004;1:189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michell AW, Luheshi LM, Barker RA. Skin and platelet alpha-synuclein as peripheral biomarkers of Parkinson's disease. Neurosci Lett 2005;381:294–298 [DOI] [PubMed] [Google Scholar]
- 5.Ikemura M, Saito Y, Sengoku R, et al. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol 2008;67:945–953 [DOI] [PubMed] [Google Scholar]
- 6.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 2008;116:277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010;119:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miki Y, Tomiyama M, Ueno T, et al. Clinical availability of skin biopsy in the diagnosis of Parkinson's disease. Neurosci Lett 2010;469:357–359 [DOI] [PubMed] [Google Scholar]
- 9.Wang N, Gibbons CH, Freeman R. Novel immunohistochemical techniques using discrete signal amplification systems for human cutaneous peripheral nerve fiber imaging. J Histochem Cytochem 2011;59:382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology 2009;72:1479–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbons CH, Griffin JW, Polydefkis M, et al. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology 2006;66:256–258 [DOI] [PubMed] [Google Scholar]
- 12.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature 1997;388:839–840 [DOI] [PubMed] [Google Scholar]
- 13.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–909 [DOI] [PubMed] [Google Scholar]
- 14.Gibbons CH, Wang N, Freeman R. Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Ann Neurol 2010;68:888–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson's disease. Mov Disord 2012;27:54–60 [DOI] [PubMed] [Google Scholar]
- 16.Cersosimo MG, Benarroch EE. Autonomic involvement in Parkinson's disease: pathology, pathophysiology, clinical features and possible peripheral biomarkers. J Neurol Sci 2012;313:57–63 [DOI] [PubMed] [Google Scholar]
- 17.Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A, Quinn N. Sweating dysfunction in Parkinson's disease. Mov Disord 2003;18:1459–1463 [DOI] [PubMed] [Google Scholar]
- 18.Kihara M, Kihara Y, Tukamoto T, et al. Assessment of sudomotor dysfunction in early Parkinson's disease. Eur Neurol 1993;33:363–365 [DOI] [PubMed] [Google Scholar]
- 19.De Marinis M, Stocchi F, Testa SR, De Pandis F, Agnoli A. Alterations of thermoregulation in Parkinson's disease. Funct Neurol 1991;6:279–283 [PubMed] [Google Scholar]
- 20.Shindo K, Iida H, Watanabe H, Ohta E, Nagasaka T, Shiozawa Z. Sympathetic sudomotor and vasoconstrictive neural function in patients with Parkinson's disease. Parkinsonism Relat Disord 2008;14:548–552 [DOI] [PubMed] [Google Scholar]
- 21.Shannon KM, Keshavarzian A, Mutlu E, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson's disease. Mov Disord 2012;27:709–715 [DOI] [PubMed] [Google Scholar]
- 22.Shi M, Bradner J, Hancock AM, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol 2011;69:570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 2006;396:67–72 [DOI] [PubMed] [Google Scholar]
- 24.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, et al. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders? A cohort study. Neurology 2007;68:2012–2018 [DOI] [PubMed] [Google Scholar]
- 25.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 2006;32:284–295 [DOI] [PubMed] [Google Scholar]
- 26.Giasson BI, Duda JE, Forman MS, Lee VM, Trojanowski JQ. Prominent perikaryal expression of alpha- and beta-synuclein in neurons of dorsal root ganglion and in medullary neurons. Exp Neurol 2001;172:354–362 [DOI] [PubMed] [Google Scholar]
- 27.Orimo S, Uchihara T, Nakamura A, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain 2008;131:642–650 [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 1990;79:581–583 [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Parkinsonism Relat Disord 2011;17:10–15 [DOI] [PubMed] [Google Scholar]
- 30.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001;57:456–462 [DOI] [PubMed] [Google Scholar]
- 31.Lebouvier T, Neunlist M, Bruley des Varannes S, et al. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS One 2010;5:e12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pouclet H, Lebouvier T, Coron E, et al. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson's disease. Neurobiol Dis 2012;45:305–309 [DOI] [PubMed] [Google Scholar]
- 33.Shishido T, Ikemura M, Obi T, et al. Alpha-synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology 2010;74:608–610 [DOI] [PubMed] [Google Scholar]
- 34.Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 2008;131:1903–1911 [DOI] [PubMed] [Google Scholar]
- 35.Toth C, Breithaupt K, Ge S, et al. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol 2010;68:28–36 [DOI] [PubMed] [Google Scholar]
- 36.Urban PP, Wellach I, Faiss S, et al. Subacute axonal neuropathy in Parkinson's disease with cobalamin and vitamin B6 deficiency under duodopa therapy. Mov Disord 2010;25:1748–1752 [DOI] [PubMed] [Google Scholar]
- 37.Santos-Garcia D, Macias M, Llaneza M, Grande M, Fuente-Fernandez R. Serum vitamin B(12) and folate levels in Parkinson's disease patients treated with duodenal levodopa infusion. Mov Disord 2011;26:558–559 [DOI] [PubMed] [Google Scholar]
- 38.Nolano M, Provitera V, Lanzillo B, Santoro L. Neuropathy in idiopathic Parkinson disease: an iatrogenic problem? Ann Neurol 2011;69:427–428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.