Abstract
Objective:
To determine the neurocognitive deficits associated with newly diagnosed untreated childhood absence epilepsy (CAE), develop a model describing the factorial structure of items measuring academic achievement and 3 neuropsychological constructs, and determine short-term differential neuropsychological effects on attention among ethosuximide, valproic acid, and lamotrigine.
Methods:
Subjects with newly diagnosed CAE entering a double-blind, randomized controlled clinical trial had neuropsychological testing including assessments of general intellectual functioning, attention, memory, executive function, and achievement. Attention was reassessed at the week 16–20 visit.
Results:
At study entry, 36% of the cohort exhibited attention deficits despite otherwise intact neurocognitive functioning. Structural equation modeling of baseline neuropsychological data revealed a direct sequential effect among attention, memory, executive function, and academic achievement. At the week 16–20 visit, attention deficits persisted even if seizure freedom was attained. More subjects receiving valproic acid (49%) had attention deficits than subjects receiving ethosuximide (32%) or lamotrigine (24%) (p = 0.0006). Parental assessment did not reliably detect attention deficits before or after treatment (p < 0.0001).
Conclusions:
Children with CAE have a high rate of pretreatment attentional deficits that persist despite seizure freedom. Rates are disproportionately higher for valproic acid treatment compared with ethosuximide or lamotrigine. Parents do not recognize these attentional deficits. These deficits present a threat to academic achievement. Vigilant cognitive and behavioral assessment of these children is warranted.
Classification of evidence:
This study provides Class I evidence that valproic acid is associated with more significant attentional dysfunction than ethosuximide or lamotrigine in children with newly diagnosed CAE.
Childhood absence epilepsy (CAE) is a common pediatric epilepsy syndrome affecting 10% to 17% of all children with epilepsy,1,2 with onset generally between 4 and 8 years of age. The classic clinical symptoms include frequent staring spells in an otherwise neurologically normal child and generalized 3-Hz spike-wave discharges on EEG.3 Although previously considered a “benign” epilepsy syndrome with easily controlled seizures that eventually remit, many of these children have significant cognitive and behavioral disability.4 Studies with small numbers of subjects have reported difficulties in the areas of visual attention and visuospatial skills,5,6 verbal learning and memory,7 and language abilities.8 The precise nature and severity of pretreatment cognitive impairments are unclear, and it is unknown whether antiepileptic drug (AED) therapy will improve or worsen the dysfunction.
A double-blind, randomized clinical trial (RCT) of 446 children with newly diagnosed CAE compared the efficacy/effectiveness of ethosuximide, lamotrigine, and valproic acid as initial monotherapy.9,10 Using this cohort of children with newly diagnosed untreated CAE, this study's primary hypothesis was that ethosuximide, valproic acid, and lamotrigine would exhibit differential short-term effects on attention. This study also examined the nature of attentional and cognitive deficits associated with newly diagnosed CAE, a model of the interaction among neuropsychological constructs (attention, memory, executive function, and achievement), parental ability to detect attentional deficits, and the differential effect of seizure control on attention.
METHODS
Subject population.
Details of the inclusion/exclusion criteria for the efficacy/effectiveness RCT were previously reported.9,10 Key inclusion criteria included clinical diagnosis of CAE, EEG demonstrating generalized 2.7- to 5-Hz spike waves with normal background and at least one burst lasting ≥3 seconds, and age 2.5 to 13 years at study entry. Key exclusion criteria included treatment for CAE with AEDs for >7 days before randomization and history of major psychiatric disease or autism spectrum disorder. The efficacy/effectiveness RCT's study cohort provided the baseline cognitive and attentional data for this study. This study's attention RCT cohort used the efficacy/effectiveness RCT cohort with 2 additional exclusion criteria: age 4 years or younger at study entry, and discontinuation on double-blind medication on or before the week-4 visit.
Standard protocol approvals, registrations, and patient consents.
The institutional review boards of all 32 sites approved the study. Written parental informed consent and, when appropriate, child assent was obtained from all subjects. The trial was conducted under US Food and Drug Administration–approved Investigational New Drug for the investigation of these AEDs in children with CAE. The study is listed at ClinicalTrials.gov, identifier NCT00088452.
Study design.
All children had a baseline visit including a detailed medical history, physical and neurologic examination, a 1-hour video EEG, an age-specific battery of neuropsychological tests, as well as questionnaires on behavior and quality of life. Subjects were randomized in a 1:1:1 ratio to ethosuximide, lamotrigine, or valproic acid.9,10 The overall study design was a parallel, randomized, double-blinded study, with partial crossover to open label (at treatment failure only) with subsequent follow-up. This report describes only results from the initial double-blind portion and does not include results from the open-label partial crossover portion. The details of the efficacy/effectiveness RCT design were previously described.9,10 Subjects continued receiving double-blind study medication as long as they did not meet any treatment failure criteria.9,10
Neuropsychological testing.
Baseline neuropsychological evaluation utilized a battery of tests designed to assess overall intellectual ability, educational achievement, learning and memory, attention, and executive functions.11–21 Because subjects ranged from 2.5 to 13 years, 3 separate age-specific test batteries were used (table e-1 on the Neurology® Web site at www.neurology.org). Baseline neuropsychological testing was performed either before or within 7 days after study medication start. Parents completed the Child Behavior Checklist (CBCL)22,23 parental report. At the week 16–20 visit, subjects were administered only the attention test, Continuous Performance Test (CPT)-II or Kiddie (K)-CPT, described below, and the CBCL.
At each test site, the evaluations were performed under the supervision of a licensed psychologist. The order of test administration was the same at each site. The CPT-II or K-CPT was the first test administered. All paper tests were initially scored at the individual sites and then sent to central scoring. Blinded evaluators performed central scoring. The central scoring supervisor (D.M.) was not a treating provider and was blinded to treatment allocation. Results in this report reflect the central scoring for these tests.
Study hypothesis.
The primary hypothesis of the RCT was that there would be a differential effect on attention (as measured by CPT Confidence Index [CI] scores) among ethosuximide, lamotrigine, and valproic acid by the week 16–20 visit in children with newly diagnosed CAE.
Study outcomes and sample size.
This RCT's primary outcome was attention as measured by the CI of the CPT-II or K-CPT in study participants 4 years or older at randomization who continued in double-blind therapy past the week-4 visit. A CI ≥0.60 was considered evidence of clinically significant attention difficulties and corresponds to a 60% probability that the child has a clinical attention deficit disorder.9–11 Secondary study outcomes were omission and commission T-scores, each grouped as <60 (normal), 60–70, and >70. CI, omission T-score, and commission T-score outcomes were prespecified.
The sample size of 446 was designed to detect a 20% difference in freedom from failure rates among the 3 medications at the week 16–20 visit.9,10 This sample size could detect a 20% difference in attention dysfunction as measured by a CI of ≥0.60 in pairwise comparisons among treatment groups, with >80% power while using a Bonferroni correction for multiple comparisons.
Statistical analysis.
Baseline neurocognitive test results and their correlations with the CI were summarized. A confirmatory factor analysis model was used to assess the factorial structure of the neuropsychological constructs of attention, memory, and executive function as well as academic achievement based on the pretreatment tests administered to the subjects aged 6 years and older. Muthén's robust estimator MLR72 was applied for estimation of all the models.24 Different structural models were used to examine how neuropsychological factors affect academic achievement, and the most parsimonious model, with significance for all path coefficients, was used.
Baseline demographics and neuropsychological test results were compared among treatments within age groups (≥6 years, <6 years), using either an exact χ2 test or analysis of variance (ANOVA) (with treatment as one factor and age stratum as the other factor). Baseline executive function assessments were provided by the software as grouped percentile ranks; distribution of those rank groups between treatment groups was compared using the Kruskal-Wallis test.
Comparisons of CPT CI values between treatment groups were based on data using a dichotomous grouping approach (CI ≥0.6 vs CI <0.6). The primary outcome was the CI on the CPT at the week 16–20 visit or at any earlier discontinuation visit occurring after the week-4 visit. Fisher exact test for the pairwise comparisons between treatments groups was performed with a Bonferroni correction, as well as an overall exact χ2 test including all 3 treatments. Secondary analyses included an exact χ2 test for a change in grouping (improved from CI ≥0.6 to CI <0.6, stayed the same, or became worse). Secondary descriptive analyses explored changes within the subgroup with CI ≥0.60 at baseline. To examine gradients of attention change, CPT data were also analyzed as a continuous variable. ANOVAs compared the CI actual values between the 3 treatment groups at the week 16–20 visit and the changes from baseline to the week 16–20 visit; analysis of covariance adjusted for baseline attention values. Because of the potential impact of sex on attention,25 the above analyses were also performed controlling for sex using the Cochran-Mantel-Haenszel test along with the Breslow-Day test for homogeneity of odds ratios for categorical variables and analysis of covariance adding sex as a factor for continuous variables. Pearson correlations were calculated between the CI and the 2 attention scales on the CBCL, and a McNemar test assessed agreement between the CBCL and the CI, using CBCL >70 as a cutoff for grouped CBCL results.
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC), StatXact version 8.0 (Cytel Inc., Cambridge, MA), and Mplus 5.273.26 A p value of <0.05 was considered significant, with a Bonferroni correction for the primary outcome analyses.
RESULTS
Patient population.
For baseline neuropsychological data analysis, all 446 eligible children enrolled in the efficacy/effectiveness trial9 were included. For the attention RCT analysis, the 393 children aged 4 years or older at study entry who continued in double-blind therapy past the week-4 visit were included as the intent-to-treat group.
Baseline cognitive functioning.
Performance rates for baseline tests ranged from 90% to 96% for all tests except the computerized Wisconsin Card Sorting Test (76%) for which some study sites had technical difficulties. The cohort demonstrated intellectual ability, educational achievement, learning, and verbal memory within the normal range with no differences across age strata (table 1). Visual memory results were within the lower part of the average range. The percentage of subjects achieving the >16th percentile for the number of trials to achieve success in the first category (63%) and number of categories successfully achieved (69%), both measures of executive function, is lower than would be expected of a healthy control group (84%).
Table 1.
Baseline neuropsychological test results of entire cohort (n = 446)
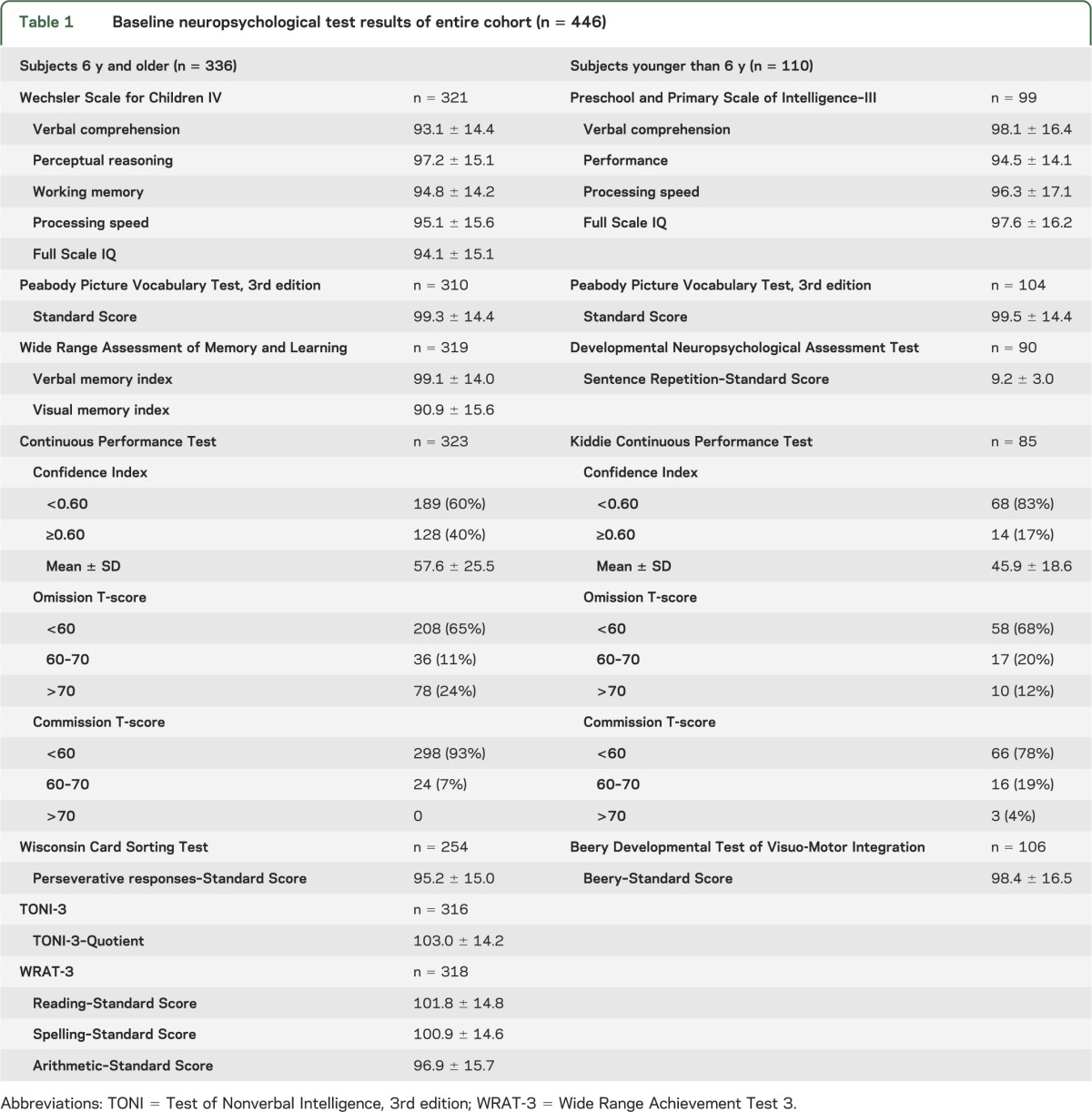
A total of 408 of 446 subjects (91%) completed baseline CPT evaluations (table 1). Overall, 36% had a CI of ≥0.60. Omission errors at baseline predominated over commission errors. Correlations between the CI and the other parameters in table 1 were low to moderate, with the largest correlation, r = −0.44, being between the CI and Full Scale IQ. Correlations were similar in the ≥6 and <6 years subgroups. No difference was noted in baseline results between subjects tested before study drug was started and those tested during the week after randomization.
Baseline confirmatory factor analysis and structural equation modeling.
Factor analysis confirmed that the neuropsychological test variables loaded with the expected construct (figure 1). All factor loadings except one (commission T-score on Attention) were >0.40 and statistically significant. Factors of Memory, Executive Function, and Achievement were highly correlated with each other, and ranged from r = 0.65 to r = 0.77, while Attention was moderately correlated with other factors, and ranged from 0.31 to 0.39. The confirmatory factor analysis model established a good measurement model for further structural equation modeling.
Figure 1. Structural equation model for attention impact on achievement mediated by memory and executive function.

Factor loading: all prespecified CPT variables (Confidence Index, omission T-score, and commission T-score) had loadings on the Attention factor, with standardized factor loadings of 0.84 (SE = 0.061), 0.91 (SE = 0.064), and 0.26 (SE = 0.063), all at the p < 0.05 level. Both verbal memory and visual memory from the WRAML-2 assessment had significant loadings with Memory (0.70, SE = 0.053, and 0.63, SE = 0.052, respectively). WCST Perseverative response (0.447, SE = 0.064) and TONI-3 quotient (0.758, SE = 0.066) loaded with Executive Function; and Reading (0.685, SE = 0.039), Spelling (0.738, SE = 0.034), and Arithmetic (0.949, SE = 0.030) loaded with Achievement. Model characteristics: χ2 = 56.41, df = 30, p = 0.0025, Comparative Fit Index = 0.975; Tucker-Lewis coefficient = 0.963; RMSEA = 0.051 (90% confidence interval: 0.030, 0.072); p (RMSEA ≤0.05) = 0.43; standardized root mean square residual = 0.051. CPT = Continuous Performance Test; RMSEA = root mean square error of approximation; SE = standard error; WCST = Wisconsin Card Sorting Test; TONI-3 = Test of Nonverbal Intelligence, 3rd edition; WRAML-2 = Wide Range Assessment of Memory and Learning, 2nd edition; WRAT-3 = Wide Range Achievement Test 3.
In the structural equation modeling, the more parsimonious model was a sequential model (figure 1), in which Attention affected Memory (path coefficient 0.413, standard error [SE] = 0.072, p < 0.001); Memory affected Executive Function (0.853, SE = 0.098, p < 0.001); and Executive Function affected Achievement (0.814, SE = 0.052, p < 0.001). Although Memory and Attention did not have direct effects on Achievement, Memory affected Achievement through Executive Function (0.695, SE = 0.10, p < 0.001); and Attention affected Achievement through Memory and then Executive Function (0.287, SE = 0.081, p < 0.001). Overall, this model fit the data well.
RCT attentional results.
Among the attentional RCT trial cohort (figure 2) there were no significant demographic or neuropsychological test result differences between treatment groups at baseline except for higher scores in the lamotrigine cohort on the spelling subtests of the Wide Range Achievement Test 3 (WRAT-3) (tables 2, 3, and e-2). The lamotrigine cohort had slightly better performance on the reading and arithmetic subtexts of the WRAT-3 and the Peabody Picture Vocabulary Test (table e-2). The baseline CI scores for the treatment groups (mean ± SD) were as follows: ethosuximide 0.56 ± 0.24; lamotrigine 0.52 ± 0.24; and valproic acid 0.58 ± 0.26 (overall ANOVA p = 0.16).
Figure 2. CONSORT diagram for attention randomized clinical trial.
CONSORT = Consolidated Standards of Reporting Trials; CPT = Continuous Performance Test.
Table 2.
Subject demographics for attention RCT by treatment group (n = 393)

Table 3.
Baseline and week 16–20 Continuous Performance Test results for attention RCT by treatment group

Primary outcome CPT data were available for 83% (326/393) of this RCT cohort (table 3). Overall, 49% of subjects on valproic acid had a CI of ≥0.60, compared with 32% of subjects on ethosuximide (p = 0.02) and 24% on lamotrigine (p = 0.0003), with no significant differences between the ethosuximide and lamotrigine cohorts. This differential valproic acid effect was detected regardless of the presence or absence of adverse events at the time of CPT testing. Among the subgroup of patients with CI ≥0.60 at baseline, only 26% (10/38) of those on valproic acid improved to CI <0.60, while 43% and 47% improved on ethosuximide and lamotrigine, respectively, with no differences based on seizure freedom status. Omission T-scores at the week 16–20 visit showed more subjects on valproic acid with omission T-scores >70 than on the 2 other treatments (p = 0.001), with no differences in commission T-scores (table 3).
At the week 16–20 visit, adjusting for age group and sex, subjects on valproic acid had higher (worse) mean CI than subjects on either ethosuximide or lamotrigine (table e-3, p < 0.0001). Similarly, subjects on valproic acid had higher (worse) omission T-scores compared with the 2 other treatments; commission T-scores were comparable among the 3 medications. Subjects in the valproic acid cohort had worsening CI scores from baseline to the week 16–20 visit whereas subjects in the ethosuximide and lamotrigine cohorts had improving CI scores (table e-3, p < 0.001).
Both in the overall RCT attention cohort and within treatment groups, there were no differences in CI scores between seizure-free subjects and those with ongoing seizures at the week 16–20 visit.
CPT and CBCL correlations.
In the RCT attentional cohort, correlations between attention problems as assessed by parental CBCL report (Attention Problems Scale, DSM-IV Attention Scale) and the CPT CI were 0.26–0.27 for both the baseline and week 16–20 visits. Among the subjects whose CI was ≥0.60, either at baseline or at week 16–20 visit, 73%–89% of parents assessed their child's attention problems on the CBCL subscales at less than the clinical cutoff of 70 (McNemar test, p < 0.0001 in all comparisons). Thus, the majority of parents did not identify an attention problem when an attention problem existed.
DISCUSSION
This study demonstrates the magnitude of attention problems in the CAE population, the impact of these deficits on academic achievement (despite otherwise apparent intact cognitive parameters), their persistence despite subsequent seizure freedom, the difficulty in their detection by parents, and the differential effects of AED therapy. Although some of these findings were suggested by previous smaller studies of mixed seizure populations including treated subjects,4,27,28 this constellation of findings in this large, well-characterized, prospective cohort highlights the subtle but profound nature of this attentional comorbidity.
Despite average intellectual ability, 35% of children with newly diagnosed, untreated CAE demonstrated the presence of clinically significant attention problems, approximately 4-fold that observed in the general population.29,30 These data suggest that problems with attention are a potentially serious comorbidity of CAE, and appear to exist without a more global neurocognitive deficit being identified. The persistence of the attentional problems despite successful treatment that included not just clinical seizure freedom but absence of any burst of spike and wave lasting ≥3 seconds suggests that attentional problems are a core feature of CAE and not simply a result of the frequent absence seizures. This is consistent with a report of a high rate of cognitive and behavioral deficits in a smaller study of 56 young adults with a history of absence seizures.4
Our findings demonstrate a selective and robust pattern of deficits typically associated as an inattentive rather than hyperactive form of attention deficit. There were a disproportionate number of omission errors relative to commission errors, suggesting that children had a greater tendency to lose focus on the task rather than respond in an impulsive manner. A previous report of 75 children with idiopathic epilepsy (47% generalized, 84% on therapy) found a higher rate of attentional problems with the inattentive type being more common.27
The CBCL has been shown to discriminate between different levels of emotional dysregulation in attention deficit hyperactivity disorder (ADHD), but with greater success in those defined as having severe dysregulation.31 The sensitivity of the CBCL seems greatest when the behaviors are more extreme. This is consistent with our proposed explanation that the low rate of parental identification of attention problems was the result of an inability to observe the inattentive (subtle) behaviors that predominated in children with CAE.
While pretreatment academic/intellectual performance, memory, and language were within the average range, deficits in attention nevertheless affected cognitive performance. This was confirmed by findings from the structural equation modeling, whereby attention clearly had a direct effect on memory, which in turn exerted effects on both executive function and academic achievement. These findings indicate that despite ostensibly average neuropsychological functioning, attention deficits in children with CAE have an important impact on learning and achievement. These findings are consistent with a study of 52 children with epilepsy (42% with generalized epilepsy, 77% on therapy) that found neurobehavioral comorbidities at epilepsy onset were a major marker of abnormal cognitive development.28
Although children may become seizure-free with a normalized EEG, attention deficits persisted even with the use of the most efficacious medication. Prior studies have shown that AEDs in and of themselves affect cognition.32,33 In the current study, valproic acid affected attention more than either lamotrigine or ethosuximide. This highlights the importance of considering both cognitive and seizure outcome when evaluating optimal initial therapy.
Although the CPT has marginal utility in detecting behavioral characteristics of ADHD,34 the vast majority of studies have found the CPT to be an important and useful tool for not only demonstrating attention deficits in specific childhood disorders such as asthma35 and fetal alcohol syndrome,36 but also as a psychometric instrument for the detection of neural processing deficits in children with ADHD.37,38
Our current understanding suggests that in the majority of these children, seizures will remit in adolescence and they will enter adulthood seizure-free off medications.39,40 Less is known about what happens to the attentional difficulties and whether academic achievement worsens. A limitation of this study is its inability to address whether the presence of a baseline attention problem is associated with a worse long-term seizure prognosis. As we follow this cohort, we hope to be able to distinguish between medication effect and neurocognitive impairment specifically attributable to CAE. In the meantime, clinicians should be aware of the high rate of attentional problems in children with CAE even when seizures are fully controlled. Continued monitoring of cognitive performance is of particular significance in this population, and may provide important information regarding future treatment and educational planning.
Supplementary Material
GLOSSARY
- ADHD
attention deficit hyperactivity disorder
- AED
antiepileptic drug
- ANOVA
analysis of variance
- CAE
childhood absence epilepsy
- CBCL
Child Behavior Checklist
- CI
Confidence Index
- CPT
Continuous Performance Test
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- K-CPT
Kiddie Continuous Performance Test
- RCT
randomized clinical trial
- SE
standard error
- WRAT-3
Wide Range Achievement Test 3
Footnotes
Editorial, page 1564
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Drs. Masur, Shinnar, Cnaan, Wang, Hirtz, and Glauser along with Ms. Shinnar, Clark, and Weiss had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors and their individual contributions to the manuscript: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision: D. Masur, S. Shinnar, A. Cnaan, R.C. Shinnar, P. Clark, E.F. Weiss, D.G. Hirtz, T.A. Glauser; statistical analysis: A. Cnaan, J. Wang. Dr. Masur wrote the first draft of the manuscript.
STUDY FUNDING
Supported by the NIH (U01-NS045911and U01-NS045803).
DISCLOSURE
D. Masur is funded by NIH grants R01-NS043209 and 2U01-NS045911. He has also given expert testimony in medico-legal cases. S. Shinnar is funded by NIH grants NS R01-NS043209, 2U01-NS045911, NS 053998, NS 066929, U10NS077308, and UL1 RR025759. He serves on the Editorial Board of Pediatric Neurology, served on a DSMB for King Pharmaceuticals, has received personal compensation for serving on Scientific Advisory Boards for Questcor and Sunovion, for consulting for Eisai, Upsher-Smith, Questcor, and Neuronex, and speaker honoraria from Eisai, Questcor, and UCB. He has received royalties from Elsevier for coediting the book Febrile Seizures. He has also given expert testimony in medico-legal cases. A. Cnaan is funded by NIH grants 2U01-NS045911, UL1RR031988, P30HD040677, P50AR060836, R01AR061875, and R01HD058567, Department of Defense grant W81XWH-09-1-0592, and Department of Education grant H133B090001. She is also the statistician member of an Independent Data Monitoring Committee for GlaxoSmithKline. R. Shinnar is funded by NIH grants R01-NS043209 and 2U01-NS045911. She has received compensation for serving on advisory panels for Questcor Pharmaceuticals, Eisai, and Lundbeck and speaker honoraria from Cyberonics and Questcor. She also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medico-legal cases. P. Clark is funded by NIH grants 2U01-NS045911 and U10-NS077311. She has received consulting and speaking fees from Eisai. J. Wang is funded by NIH grants 2U01-NS045911, UL1RR031988, and P30HD040677. E. Weiss is funded by NIH grants R01-NS043209 and 2U01-NS045911. D. Hirtz has nothing to disclose. T. Glauser is funded by NIH grants 2U01-NS045911, U10-NS077311, R01-NS053998, R01-NS062756, R01-NS043209, R01-LM011124, and R01-NS065840. He has received consulting fees from Supernus, Sunovion, Eisai, UCB, Lundbeck, and Questcor and is on the speakers bureau of Eisai and Questcor. He also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medico-legal cases. He receives royalties from a patent license from AssureRx Health. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. How well can epilepsy syndromes be identified at diagnosis? A reassessment 2 years after initial diagnosis. Epilepsia 2000;41:1269–1275 [DOI] [PubMed] [Google Scholar]
- 2.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Reseau Observatoire Longitudinal de l' Epilepsie. Epilepsia 2001;42:464–475 [DOI] [PubMed] [Google Scholar]
- 3.Loiseau P, Duche B, Pedespan JM. Absence epilepsies. Epilepsia 1995;36:1182–1186 [DOI] [PubMed] [Google Scholar]
- 4.Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy: sometimes a wolf in sheeps' clothing. Arch Pediatr Adolesc Med 1997;151:152–158 [DOI] [PubMed] [Google Scholar]
- 5.Levav M, Mirsky AF, Herault J, Xiong L, Amir N, Andermann E. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. J Clin Exp Neuropsychol 2002;24:311–326 [DOI] [PubMed] [Google Scholar]
- 6.Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E. Neuropsychological assessment in children with absence epilepsy. Neurology 2001;56:1047–1051 [DOI] [PubMed] [Google Scholar]
- 7.Henkin Y, Sadeh M, Kivity S, Shabtai E, Kishon-Rabin L, Gadoth N. Cognitive function in idiopathic generalized epilepsy of childhood. Dev Med Child Neurol 2005;47:126–132 [DOI] [PubMed] [Google Scholar]
- 8.Vanasse CM, Beland R, Carmant L, Lassonde M. Impact of childhood epilepsy on reading and phonological processing abilities. Epilepsy Behav 2005;7:288–296 [DOI] [PubMed] [Google Scholar]
- 9.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med 2010;362:790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 2013;54:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conners C. Conners' Continuous Performance Test II: Technical Guide and Software Manual. North Tonawanda, NY: Multi-Health Systems; 2002 [Google Scholar]
- 12.Wechsler D. Wechsler Intelligence Scale for Children, 4th ed San Antonio: Psychological Corp.; 2003 [Google Scholar]
- 13.Brown LS, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence, 3rd ed Austin: Pro-Ed; 1997 [Google Scholar]
- 14.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test–III. Circle Pines, MN: American Guidance Service; 1997 [Google Scholar]
- 15.Sheslow D, Adams W. Wide Range Assessment of Memory and Learning. Wilmington: Wide Range; 2003 [Google Scholar]
- 16.Wilkinson G. The Wide Range Achievement Test, 3rd ed Wilmington: Jastak; 1993 [Google Scholar]
- 17.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993 [Google Scholar]
- 18.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence–Revised. San Antonio: The Psychological Corporation; 1989 [Google Scholar]
- 19.Korkman M, Kirk U, Kemp S. NEPSY-II: A Developmental Neuropsychological Assessment Manual. San Antonio: Psychological Corporation; 1998 [Google Scholar]
- 20.Korkman M, Kirk U, Kemp S. NEPSY-II: Administration Manual. San Antonio: Psychological Corporation; 2005 [Google Scholar]
- 21.Beery K, Buktenica N, Beery N. Beery-Buktenica Developmental Test of Visual-Motor Integration, 5th ed Cleveland: Modern Curriculum Press; 1997 [Google Scholar]
- 22.Achenbach T, Rescorla L. Manual for ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2000 [Google Scholar]
- 23.Achenbach T, Rescorla L. Manual for ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2001 [Google Scholar]
- 24.Muthén B. Mplus Technical Appendices. Los Angeles: Muthén & Muthén; 1998–2004 [Google Scholar]
- 25.Hasson R, Fine JG. Gender differences among children with ADHD on Continuous Performance Tests: a meta-analytic review. J Atten Disord 2012;16:190–198 [DOI] [PubMed] [Google Scholar]
- 26.Muthén L, Muthén B. Mplus User's Guide. Los Angeles: Muthén & Muthén; 1998–2008 [Google Scholar]
- 27.Hermann B, Jones J, Dabbs K, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain 2007;130:3135–3148 [DOI] [PubMed] [Google Scholar]
- 28.Hermann BP, Jones JE, Sheth R, et al. Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia 2008;49:1847–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polanczyk G, Rohde LA. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry 2007;20:386–392 [DOI] [PubMed] [Google Scholar]
- 30.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007;164:942–948 [DOI] [PubMed] [Google Scholar]
- 31.Biederman J, Petty CR, Day H, et al. Severity of the aggression/anxiety-depression/attention child behavior checklist profile discriminates between different levels of deficits in emotional regulation in youth with attention-deficit hyperactivity disorder. J Dev Behav Pediatr 2012;33:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masur D, Shinnar S. The neuropsychology of childhood seizure disorders. In: Segalowitz SJ, Rapin I. editors. Handbook of Neuropsychology. Amsterdam: Elsevier; 1992:457–470 [Google Scholar]
- 33.Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev 2007;17:413–425 [DOI] [PubMed] [Google Scholar]
- 34.Edwards MC, Gardner ES, Chelonis JJ, Schulz EG, Flake RA, Diaz PF. Estimates of the validity and utility of the Conners' Continuous Performance Test in the assessment of inattentive and/or hyperactive-impulsive behaviors in children. J Abnorm Child Psychol 2007;35:393–404 [DOI] [PubMed] [Google Scholar]
- 35.Annett RD, Bender BG, Gordon M. Relating children's attentional capabilities to intelligence, memory, and academic achievement: a test of construct specificity in children with asthma. Child Neuropsychol 2007;13:64–85 [DOI] [PubMed] [Google Scholar]
- 36.Kooistra L, Crawford S, Gibbard B, Ramage B, Kaplan BJ. Differentiating attention deficits in children with fetal alcohol spectrum disorder or attention-deficit-hyperactivity disorder. Dev Med Child Neurol 2010;52:205–211 [DOI] [PubMed] [Google Scholar]
- 37.Doehnert M, Brandeis D, Imhof K, Drechsler R, Steinhausen HC. Mapping attention-deficit/hyperactivity disorder from childhood to adolescence: no neurophysiologic evidence for a developmental lag of attention but some for inhibition. Biol Psychiatry 2010;67:608–616 [DOI] [PubMed] [Google Scholar]
- 38.Killory BD, Bai X, Negishi M, et al. Impaired attention and network connectivity in childhood absence epilepsy. Neuroimage 2011;56:2209–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouma PA, Westendorp RG, van Dijk JG, Peters AC, Brouwer OF. The outcome of absence epilepsy: a meta-analysis. Neurology 1996;47:802–808 [DOI] [PubMed] [Google Scholar]
- 40.Trinka E, Baumgartner S, Unterberger I, et al. Long-term prognosis for childhood and juvenile absence epilepsy. J Neurol 2004;251:1235–1241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



