Abstract
Relapsing symptoms post herpes simplex virus 1 (HSV) encephalitis (HSVE) usually occur a few weeks after viral therapy and represent either 1) a true viral relapse of HSVE (CSF PCR positive for HSV, new necrotic lesions on brain MRI, and response to acyclovir therapy) or 2) a disorder postulated to be immune-mediated (CSF negative for HSV, no new necrotic lesions, and no response to acyclovir).1,2 It has been suggested that this immune-mediated disorder may be related to NMDA receptor (NMDAR) antibodies,3 and we recently reported a child in whom relapsing symptoms post HSVE were the presentation of anti-NMDAR encephalitis.4 We report an adult with this disorder, demonstrate that synthesis of NMDAR antibodies began after HSVE, and show that relapsing symptoms were due to steroid-responsive anti-NMDAR encephalitis.
Relapsing symptoms post herpes simplex virus-1 (HSV) encephalitis (HSVE) usually occur a few weeks after viral therapy and represent either 1) a true viral relapse of HSVE (CSF PCR positive for HSV, new necrotic lesions on brain MRI, and response to acyclovir therapy) or 2) a disorder postulated to be immune-mediated (CSF negative for HSV, no new necrotic lesions, and no response to acyclovir).1,2 It has been suggested that this immune-mediated disorder may be related to NMDA receptor (NMDAR) antibodies,3 and we recently reported a child in whom relapsing symptoms post HSVE were the presentation of anti-NMDAR encephalitis.4 We report an adult with this disorder, demonstrate that synthesis of NMDAR antibodies began after HSVE, and show that relapsing symptoms were due to steroid-responsive anti-NMDAR encephalitis.
Case report.
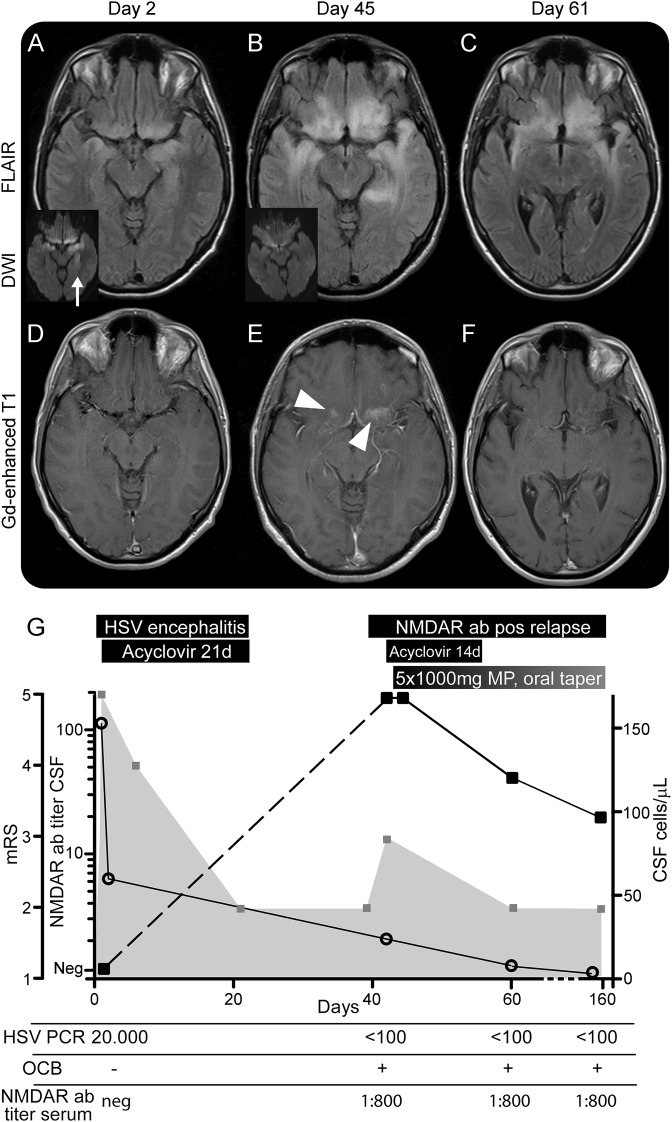
A 24-year-old man presented with a 24-hour history of confusion, delusional thoughts, and disorientation. His prior medical history was unremarkable. On examination, he was disoriented to time and place and showed severe anterograde amnesia, aphasia, and psychotic behavior. CSF analysis showed lymphocytic pleocytosis (153 leukocytes/µL), normal protein, glucose, and lactate concentrations, and no oligoclonal bands (OCB). HSV PCR in CSF was positive (20,000 copies/µL) and he was started on IV acyclovir 750 mg TID for 21 days. Initial brain MRI showed asymmetric bilateral increased T2 signal in the gyrus rectus and insular and hippocampal regions, with diffusion restriction but without contrast enhancement (figure, A and D). Frontotemporal slowing was observed on EEG (appendix e-1 on the Neurology® Web site at www.neurology.org). Immunoglobulin G (IgG) NMDAR antibodies in serum and CSF using 2 different techniques (brain immunohistochemistry and HEK cells expressing NR1) were negative5 (figure, G). The patient recovered slowly and was discharged to rehabilitation 3 weeks after symptom onset. He had residual retrograde and anterograde amnesia and altered executive functions but was able to carry out most of his activities of daily living (modified Rankin Scale score [mRS] 2).
Figure. MRI and clinical course of NMDA receptor antibody–positive clinical relapse after herpes simplex virus-1 encephalitis.
MRI at the time of herpes simplex virus-1 (HSV) encephalitis (HSVE) (day 2; A, D), at readmission (day 45 after HSVE; B, E), and at follow-up (day 20 after readmission, day 61 after HSVE; C, F). MRI at day 160 after HSVE was unchanged from day 61 (not shown). (A–C) Axial fluid-attenuated inversion recovery (FLAIR)–weighted image in hippocampal and frontobasal plane; small insets in A and B are diffusion-weighted images (DWI) showing restricted diffusion in affected hippocampal regions in A (arrow) but not B. (D–F) Gadolinium-enhanced T1-weighted images show regional enhancement in E (arrowheads). (G) Clinical course and CSF findings during HSVE and at relapse. Modified Rankin Scale score (mRS): gray boxes and gray plane, left y-axis. CSF immunoglobulin G (IgG) NMDA receptor (NMDAR) antibody titer: black boxes and black lines, left y-axis. IgG NMDAR antibodies at day 2 were not detectable. CSF leukocytes/µL: open circles, right y-axis. Ab = antibody; Gd = gadolinium; MP = methylprednisolone; neg = negative; OCB = oligoclonal bands; pos = positive.
Eighteen days after discharge (41 days post HSVE), the patient was readmitted with progressive mania, irritability, racing thoughts, and pressured speech suspected to be a relapse of HSVE. Attention span, concentration, and memory were markedly reduced compared to discharge. No movement disorders or other neurologic abnormalities were observed. He needed continuous help for his daily activities (mRS 3). CSF showed mild pleocytosis (24 leukocytes/µL), increased protein (855 mg/L), and OCB. EEG was unremarkable (appendix e-1). MRI showed bilateral expansion of preexisting fluid-attenuated inversion recovery (FLAIR) hyperintensities into adjacent mesiotemporal, temporopolar, and frontobasal areas with regional gadolinium enhancement (figure, B and E). HSV-1 PCR was negative in CSF, and there was an increase of IgG HSV antibody index consistent with prior HSVE. Another 14-day course of IV acyclovir was commenced. Extensive workup did not reveal any infection or other cause of the symptoms (appendix e-1). However, IgG NMDAR antibodies were detected in CSF (titer 1:160) and serum (IgG 1:800; figure, G) with specific antibody index of 24.78; no other neuronal antibodies were identified (appendix e-1). He was started on IV methylprednisolone 1,000 mg (day 48 post HSVE) for 5 days followed by oral tapering. Memory function improved within 1 week, and the patient was able to read newspapers and restructure his daily activities. He was discharged 20 days after relapse (61 days after symptom onset, mRS 2).
At a follow-up 119 days after relapse (160 days post HSVE), symptoms had further improved, CSF cell count and protein concentration had normalized, and OCB were still detectable. MRI showed improvement of FLAIR changes but temporal atrophy was evident (figure, C and F). The IgG NMDAR antibody titer in CSF had decreased to 1:20, and was unchanged in serum (figure, G).
Discussion.
We prospectively identified an adult case of NMDAR antibody–associated relapse post HSVE. The absence of NMDAR antibodies in serum and CSF within 48 hours after onset of HSVE and their appearance at relapse suggest an infectious trigger of anti-NMDAR encephalitis. A coincidental development of both disorders is unlikely because of the temporal association, low incidence of HSVE (1-3/1,000,000/year), and earlier observations of NMDAR antibody–positive HSVE relapse.4 The oligosymptomatic presentation of our patient could be due to early diagnosis and treatment given our awareness of this possible complication.4 Clinical improvement started after initiating steroids, and the NMDAR titers in CSF decreased in parallel. The MRI findings observed at relapse are within the spectrum of HSVE-related changes and did not represent new necrotic lesions. Whether this postinfectious entity is caused by mechanisms of mimicry or breakdown of immunologic tolerance towards the NMDAR expressed by damaged neurons in an inflamed environment is unknown. Research is needed to address this issue. This case also illustrates the necessity of testing for NMDAR antibodies in post-HSVE relapses in adults.
Supplementary Material
Footnotes
Supplemental data at www.neurology.org
Author contributions: Frank Leypoldt contributed by study design, collecting the data, analysis and interpretation of the data, and drafting and revising the manuscript. Maarten J. Titulaer contributed by analysis or interpretation of the data and revising the manuscript. Esther Aguilar contributed by analysis of data. Janine Walter contributed by collecting data and revising the manuscript. Marlene Bönstrup contributed by collecting data and revising the manuscript. Stefanie Havemeister contributed by collecting data and revising the manuscript. Bianca Teegen contributed by collecting data and revising the manuscript. Marc Lütgehetmann contributed by collecting data and revising the manuscript. Michael Rosenkranz contributed by interpretation of the data and revising the manuscript. Tim Magnus contributed by revising the manuscript. Josep Dalmau contributed by study design, analysis or interpretation of the data, and drafting and revising the manuscript.
Study funding: Supported by the Forschungsförderungsfonds Hamburg Eppendorf Exzellenzjahr, NIH RO1NS077851, Fundació la Marató TV3, and Fondo de Investigaciones Sanitarias (FIS, PI11/01780) (to J.D.) and an ErasmusMC fellowship (to M.T.).
Disclosure: F. Leypoldt has received speaker honoraria from Grifols and scientific funding from Euroimmun, Lübeck, Germany. M. Titulaer has previously received a clinical research fellowship by the Dutch Cancer Society (KWF Kankerbestrijding). E. Aguilar, J. Walter, M. Bönstrup, and S. Havemeister report no disclosures. B. Teegen discloses being an employee of the Institute for Experimental Immunology, affiliated to Euroimmun AG. M. Lütgehetmann has received speaker honoraria from Roche. M. Rosenkranz and T. Magnus report no disclosures. J. Dalmau has received a research grant from Euroimmun and receives royalties from patents for the use of Ma2 and NMDAR as autoantibody tests. Go to Neurology.org for full disclosures.
References
- 1.De Tiège X, Rozenberg F, Portes Des V, et al. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology 2003;61:241–243 [DOI] [PubMed] [Google Scholar]
- 2.Sköldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 2006;253:163–170 [DOI] [PubMed] [Google Scholar]
- 3.Prüss H, Finke C, Höltje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72:902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armangué T, Titulaer MJ, Málaga I, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 2013;162:850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.