Abstract
Objective:
Migraine is associated with white matter hyperintensities (WMH) cross-sectionally, but its effect on WMH progression is uncertain.
Methods:
Participants in the Atherosclerosis Risk in Communities cohort study (n = 10,924) completed a standardized headache questionnaire between 1993 and 1995. A subset of participants (n = 1,028) received 2 MRIs 8 to 12 years apart: once at the time of headache ascertainment, and again from 2004 to 2006. WMH were quantified using both a visually graded score (0–9) and semiautomated volumetric analysis. Linear and logistic regression models adjusted for age, sex, and other vascular risk factors were constructed.
Results:
Individuals who had migraine without aura were cross-sectionally associated with an 87% greater odds of having a WMH score ≥3 than individuals without headache (adjusted odds ratio = 1.87; 95% confidence interval [CI]: 1.04, 3.37). Participants with migraine had an average of 2.65 cm3 more WMH than those without headache (95% CI: 0.06, 5.24). However, there was no significant difference in WMH progression over the study period between individuals with and without migraine (1.58 cm3 more progression for individuals with migraine compared to those without; 95% CI: −0.37, 3.53).
Conclusion:
Migraine is associated with WMH volume cross-sectionally but not with WMH progression over time. This suggests that the association between migraine and WMH is stable in older age and may be primarily attributable to changes occurring earlier in life, although further work is needed to confirm these findings.
Several epidemiologic studies, including 2 recent population-based studies,1,2 have demonstrated a cross-sectional association between migraine and white matter hyperintensities (WMH), a common MRI finding that is believed to represent chronic small-vessel cerebrovascular disease.3 However, these studies differ regarding the effect of sex and the presence of aura on the association between migraine and WMH. A follow-up of one of these studies found that migraine was associated with greater WMH progression, but that this was specific to women with supratentorial deep WMH.4
To further investigate the relationship between migraine and white matter disease, we analyzed data from the Atherosclerosis Risk in Communities (ARIC) brain MRI study. We hypothesized that a history of migraine would be associated with both baseline WMH volume and WMH progression over time.
METHODS
Study design and participants.
The ARIC study is an ongoing prospective cohort study designed to investigate the etiology of atherosclerosis and the variation in cardiovascular disease and risk factors by race and socioeconomic status.5 Participants were recruited from 4 locations: Forsyth County, NC; Jackson, MS; the northwestern suburbs of Minneapolis, MN; and Washington County, MD. A total of 15,792 individuals were enrolled at the first visit, which occurred between 1987 and 1989. During the first 2 years of visit 3 (1993–1995), participants from Jackson, MS, and Forsyth County, NC, who were 55 years or older were invited to undergo a brain MRI.6 Of the 2,891 participants who were initially screened, 2% of women and 6% of men were ineligible for safety reasons. Of those meeting eligibility criteria, 25% of women and 21% of men declined to participate, resulting in 1,949 visit-3 MRIs. These participants were invited for an ancillary study consisting of a second brain MRI, which took place between 2004 and 2006 (brain MRI visit). The time between MRIs ranged from 8 to 15 years (figure 1).
Figure 1. Atherosclerosis Risk in Communities MRI study timeline.
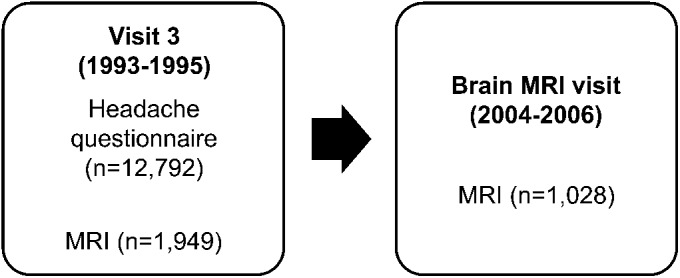
All participants who underwent MRI also completed the headache questionnaire.
Ascertainment of headache status.
All participants completed an in-person headache questionnaire at visit 3. Among subjects reporting a lifetime history of severe headaches, headache status was classified as one of the following: migraine with aura, migraine without aura, or nonmigraine headache. Participants were defined as having migraine if they advocated a lifetime history of headaches that 1) lasted 4 or more hours in duration; 2) were characterized as throbbing, pounding, or pulsating in nature, or that were unilateral in location; 3) were accompanied by nausea, vomiting, or sensitivity to light or sound; and 4) constituted one or more years with history of such headaches. Headaches lasting at least 4 hours but not meeting all of the other criteria are defined as nonmigraine headaches. Participants who denied ever having headaches lasting 4 hours or longer were defined as having “no severe headache”—also referred to in this report as being headache-free or without headache. A separate question about the occurrence of visual aura (i.e., spots, jagged lines, or heat waves in one or both eyes) was also included in the questionnaire.
WMH measurements.
Visit-3 brain MRIs (1993–1995) were performed with 1.5-tesla scanners, and contiguous axial images 5 mm thick were obtained and interpreted at the ARIC MRI Reading Center.7 WMH severity was graded on a 0 to 9 scale previously developed for the Cardiovascular Health Study (CHS).8 The total volume of deep and periventricular white matter abnormalities was compared with 8 reference images that successively increased from barely detectable (grade 1) to extensive and confluent (grade 8). Studies with no detectable white matter changes received a grade of 0, and those with changes worse than the grade-8 image received a grade of 9. Images were assessed without knowledge of participants' age, sex, race, previous imaging findings, or vascular risk factors. Agreement within one grade was 92% between readers and 94.5% within the same reader. Relaxed kappas were 0.81 and 0.93, respectively.
At the brain MRI visit (2004–2006), 1,028 participants underwent a second 1.5-tesla brain MRI, which was also interpreted at the ARIC MRI Reading Center. These images were analyzed using both the CHS grading system (which was also used for the first MRI at visit 3) and a new semiautomated volumetric analysis.9 Axial fluid-attenuated inversion recovery images were used to obtain brain and leukoaraiosis volumes. These images were segmented into voxels denoting normal brain, CSF, and leukoaraiosis using an automated algorithm based on signal intensity. The leukoaraiosis maps were manually edited to exclude infarcts and other lesions. The mean absolute error and test-retest coefficient of variation were 6.6% and 1.4%, respectively, for leukoaraiosis volume using this method. Total intracranial volume was manually measured from T1-weighted sagittal images, and WMH volumetric measurements were standardized to a total intracranial volume of 1,500 cm3.
Fully quantitative WMH measurements were not obtainable at visit 3. Therefore, WMH volumes at visit 3 were estimated from categorical WMH scores at visit 3 using a prediction equation (R2 = 0.80) previously used in ARIC.10 This equation was derived from a quadratic relationship between categorical WMH score (using the same CHS scale) and volumetric WMH measurements were obtained from the same scans at the brain MRI visit. Change in WMH volume was calculated by subtracting the estimated WMH volume at visit 3 (calculated from this equation) from the WMH volume at the brain MRI visit. One individual with an apparent decrease in WMH volume of >20 cm3 was labeled as missing, as this was likely attributable to measurement error.
Statistical analysis.
All statistical analyses were performed using STATA version 12 (College Station, TX). Because they were too rare to provide reliable estimates, all nonwhite, nonblack participants (n = 38) were excluded from the primary analysis, although results including their data are provided as supplemental tables. The association between migraine and categorical WMH score at visit 3 was examined using logistic regression adjusted for age, race/center, sex, diabetes, total serum cholesterol, blood pressure, use of antihypertensive medications, smoking, history of coronary heart disease, body mass index, alcohol consumption, and family income. A score of ≥3 was defined on the categorical scale (0–9) as having significant white matter disease. WMH volume at the brain MRI visit and the progression of white matter disease over time were analyzed using linear regression adjusted for the same covariates, with analyses of progression adjusted additionally for the number of years between MRIs. Migraine × sex interaction terms were used to test for effect modification by sex. All analyses were performed separately for all headache, all migraine headaches, migraine with aura, migraine without aura, nonmigraine headache with aura, and nonmigraine headache without aura, each compared in separate models with individuals without history of headache. The distribution of WMH volumes was somewhat right-skewed, but efforts to transform this into a normally distributed variable were of limited success and did not affect the overall results of the study. We also conducted sensitivity analyses in which individuals with very high WMH volumes were excluded, and this did not change the results of our analyses either. Covariates were selected based on a priori knowledge of the risk factors for white matter disease. Statistical significance was defined at the p = 0.05 level.
Standard protocol approvals, registrations, and patient consents.
Study protocols were approved by the institutional review boards of all participating institutions, and all participants provided informed consent.
RESULTS
Baseline clinical and demographic characteristics according to headache status at visit 3 are presented in table 1. The mean age of this cohort was 60 years, 23% were African American, and 56% were women. Among all participants, 3.3% had migraine with aura, 7.8% had migraine without aura, 10% had nonmigraine headaches, and 79% had no severe headaches. Within the brain MRI subcohort, 3.2% had migraine with aura, 5.8% had migraine without aura, 8.5% had nonmigraine headaches, and 83% had no severe headaches. Those with headaches reported having them for an average of 17 years, and 69% reported having no headaches in the past year.
Table 1.
Clinical and demographic characteristics of the ARIC study population by headache status at visit 3 (1993–1995)
In all of the white matter analyses, headache × sex interaction terms were nonsignificant; therefore, we present pooled results for men and women. At visit 3, the presence of any headache, migraine, or nonmigraine headache was not associated with a statistically significant increase in the odds of having a WMH score of ≥3 compared to individuals without any headache after adjustment for potential confounders (figure 2).
Figure 2. Adjusted odds ratios for having a categorical white matter hyperintensity score ≥3 at visit 3 (1993–1995) by headache status.
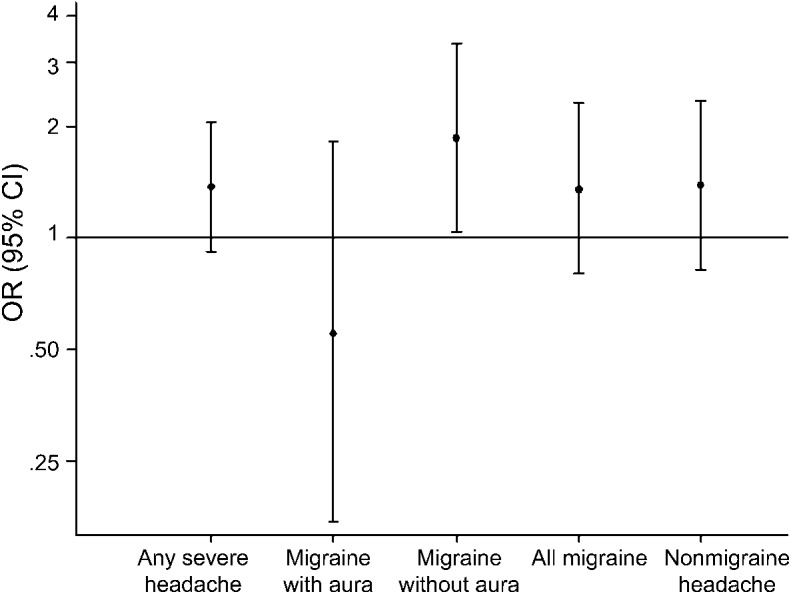
Each headache subgroup is compared with the “no severe headache” group as the referent. Dots represent the odds ratios (ORs) with bars representing the 95% confidence intervals (CIs).
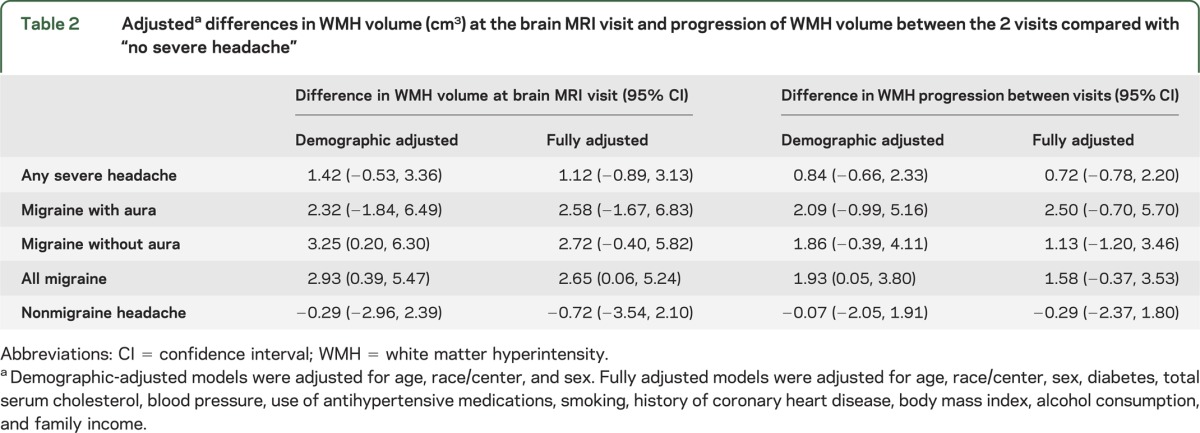
When migraine was subcategorized according to the presence of visual aura, migraine without aura was associated with a significantly increased risk of severe white matter disease (odds ratio [OR] = 1.87; 95% confidence interval [CI]: 1.04, 3.37), whereas migraine with aura was not (OR = 0.55; 95% CI: 0.17, 1.83). However, when migraine with and without aura were compared directly in a separate model, this difference was not statistically significant (OR = 0.53; 95% CI: 0.27, 1.01). At the brain MRI visit, individuals with migraine had an average of 2.65 cm3 more WMH than those without headache (95% CI: 0.06, 5.24), whereas nonmigraine headache was not associated with greater WMH volume (−0.77 cm3 WMH; 95% CI: −3.54, 2.10) (table 2). When migraine and nonmigraine headache were compared directly, this difference was not statistically significant (2.79 cm3 WMH; 95% CI: −0.75, 6.33). Compared with the reference group, migraine was associated with a significantly greater rate of progression in WMH between the 2 visits in the partially adjusted model but not in the fully adjusted model. Neither migraine with nor without aura was separately associated with greater WMH volume or progression. Results including all race groups are presented in tables e-1 and e-2 on the Neurology® Web site at www.neurology.org.
Table 2.
Adjusteda differences in WMH volume (cm3) at the brain MRI visit and progression of WMH volume between the 2 visits compared with “no severe headache”
DISCUSSION
In this large population-based cohort study, we show that migraine is associated with WMH cross-sectionally but not with the progression of white matter disease over time.
Our findings are consistent with prior studies that have shown a cross-sectional association between migraine and WMH in the general population.1,2 Although statistically significant, this association is small in magnitude, and the extent to which it affects clinically relevant outcomes such as cognitive function is unclear but likely to be limited. At visit 3, migraine with aura was not significantly associated with WMH, whereas migraine without aura was. This discrepancy was likely due to chance, as the difference in effect sizes was not statistically significant, and the 2 subtypes of migraine were associated with similar increases in WMH at the brain MRI visit. The fact that the presence of aura was determined using a single question also limits our ability to distinguish between these 2 subtypes and makes these results difficult to interpret.
The lack of an association between migraine and WMH progression in this study contrasts with recently published 9-year follow-up data from the CAMERA (Cerebral Abnormalities in Migraine, an Epidemiological Risk Analysis) study, which found that migraine was associated with significantly greater WMH progression among women.4 This finding appears to be driven by the virtual absence of WMH among controls at the first study visit, which reflects the fact that CAMERA featured a younger, healthier population that likely experienced more active headaches than in ARIC. Together, these studies suggest that the association between migraine and WMH is primarily attributable to changes occurring earlier in life, and that this effect is diluted as cardiovascular risk factors become more prevalent in the population. The effect of medications on WMH are unknown, but if the treatment of headaches, especially prophylactically, can be considered to be disease-modifying as some have suggested,11 then treatment at earlier ages is likely to have the greatest benefit for white matter disease. However, any effect of migraine prophylaxis on WMH is likely to be confounded by the pleiotropic effects of these medications. For example, β-blockers and calcium-channel blockers are often used for migraine prophylaxis but also lower blood pressure, which has been shown to slow WMH progression.12
However, one must be cautious in concluding that migraine does not affect WMH progression. Progression was calculated using 2 values, each with some degree of uncertainty. Furthermore, WMH volume at visit 3 was indirectly calculated using categorical measurements, making the analysis prone to measurement error. It is also worth noting that the estimates for migraine, migraine with aura, and migraine without aura were all in a positive direction, and that with a larger sample size, they have the potential to become statistically significant. Finally, the amount of WMH progression in our study was relatively small because of the limited duration of follow-up and the relatively young age of participants at the time of the second brain MRI. Longer studies allow for more accumulation of WMH and may make a difference easier to detect. Finally, it is also possible that as a result of type II error, we failed to find an association that is truly present.
The strengths of this study are that it is a large, biracial, population-based cohort with standardized headache ascertainment and white matter measurements. However, there are several limitations. First, despite the large sample size at visit 3, our study was limited by the size of the brain MRI subcohort, which limited our ability to detect differences in the progression of white matter disease over time. White matter analyses were also limited by the joint consideration of deep and periventricular WMH. Although most studies have found an association between migraine and total WMH burden, this association is specific to deep white matter lesions. Had we been able to separate the two, our estimates, especially for WMH progression, may have been more precise. Another limitation was that headache ascertainment was retrospective (i.e., participants were asked if they ever had a history of headaches) and was not based on the full International Classification of Headache Disorders–II criteria. Because migraine headaches decrease in frequency and severity with increasing age,13 especially among women, many of those who were classified as having headache formerly had headaches but were not actively experiencing them at the time of the study. As a result, we are unable to comment on the specific relationship between active migraine and white matter disease. Furthermore, the lifetime prevalence of migraine in our study was lower than that reported by other authors,14 so it is also possible that some former migraineurs forgot their history of headaches and were misclassified as having no history of severe headache. In general, the inclusion of migraineurs in the headache-free group would be expected to bias results toward the null, especially if individuals with greater white matter disease were cognitively impaired and therefore less likely to recall their history of headaches. Alternatively, if those with greater white matter disease experienced more frequent and severe headaches and were therefore more apt to recall their headache history, results would be biased in the opposite direction.
We also chose to use individuals without a history of severe headache as our primary reference group. Although we suspect that most individuals with migraine would have had at least one headache lasting 4 hours or longer, it is possible that we may have missed some cases of migraine lasting less than 4 hours, especially if they were treated. If this was the case, however, we believe it would lead to a dilution of the true effect size, given the presence of patients with migraine in the headache-free group, and therefore would underestimate an association between migraine and WMH. A similar effect would be seen if some of the individuals with nonmigraine headache with aura (a condition that is rare but has been described) actually had migraine headache—this would lead to a dilution of the true effect size for the same reason. It is also possible, but unlikely, that individuals could have developed migraines between the 2 visits, resulting in misclassification. In addition, we lacked information on age at the time of headaches. Our analysis treats headaches during childhood and headaches during older age as equivalent, but it is possible that one may be more or less harmful than the other. Finally, our ability to control for medications, especially those used to treat migraine, was limited. Further work on the association between migraine and WMH progression, including the mechanism by which it occurs, is needed.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and participants of the ARIC study for their important contributions.
GLOSSARY
- ARIC
Atherosclerosis Risk in Communities
- CHS
Cardiovascular Health Study
- CI
confidence interval
- OR
odds ratio
- WMH
white matter hyperintensity
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Ali G. Hamedani conceived of the study, analyzed data, and wrote the manuscript. Kathryn M. Rose and B. Lee Peterlin provided input during study inception, consulted on migraine data, and critically reviewed the manuscript. Thomas H. Mosley, Laura H. Coker, Clifford R. Jack, David S. Knopman, and Alvaro Alonso critically reviewed the manuscript. Rebecca F. Gottesman provided input during study inception, provided input on data analysis, and critically reviewed the manuscript.
STUDY FUNDING
Supported by NHLBI contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The Atherosclerosis Risk in Communities Study is conducted as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data collection is supported by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01-HL70825.
DISCLOSURE
A. Hamedani: Dr. Hamedani's parents are employees of GlaxoSmithKline. Dr. Hamedani was funded by the Johns Hopkins Predoctoral Clinical Research Training Program (NIH TL1 training grant) and completed this work as part of a master's thesis in epidemiology. K. Rose: In 2001, Dr. Rose was a paid consultant for GlaxoSmithKline. These funds did not support the research presented in this report. B. Lee Peterlin: Dr. Peterlin is supported by a mentored patient-oriented career research award (K23 10896737) from the National Institute of Neurological Disorders and Stroke and receives grant support from GlaxoSmithKline and Luitpold Pharmaceuticals for investigator-initiated study proposals unrelated to the current study. She is also an associate editor for the journal Headache. T. Mosley, L. Coker, and C. Jack report no disclosures. D. Knopman: Dr. Knopman serves as deputy editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals, performed consulting activities for TauRx Pharmaceuticals, and receives research support from the NIH. A. Alonso reports no disclosures. R. Gottesman: Dr. Gottesman is funded by the NIH (NIA) with an R01 grant on an unrelated topic. She is on the Neurology® editorial board. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA 2004;291:427–434 [DOI] [PubMed] [Google Scholar]
- 2.Kurth T, Mohamed S, Maillard P, et al. Headache, migraine, and structural brain lesions and function: population based epidemiology of vascular ageing-MRI study. BMJ 2011;342:c7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 1996;27:1274–1282 [DOI] [PubMed] [Google Scholar]
- 4.Palm-Meinders IH, Koppen H, Terwindt GM, et al. Structural brain changes in migraine. JAMA 2012;308:1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 6.Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities Study. Neurology 2005;64:2056–2062 [DOI] [PubMed] [Google Scholar]
- 7.Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke 1996;27:2262–2270 [DOI] [PubMed] [Google Scholar]
- 8.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke 1994;25:318–327 [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, O'Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging 2001;14:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottesman RF, Coresh J, Catellier DJ, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) Study. Stroke 2010;41:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loder E, Biondi D. Disease modification in migraine: a concept that has come of age? Headache 2003;43:135–143 [DOI] [PubMed] [Google Scholar]
- 12.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005;112:1644–1650 [DOI] [PubMed] [Google Scholar]
- 13.Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R. Prognosis of migraine and tension-type headache: a population-based follow-up study. Neurology 2005;65:580–585 [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Wood C, Reed ML, Roy J, Lipton RB; AMPP Advisory Group Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008;28:1170–1178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


