Abstract
Objective:
To examine the efficacy of cognitive speed of processing training (SOPT) among individuals with Parkinson disease (PD). Moderators of SOPT were also examined.
Methods:
Eighty-seven adults, 40 years of age or older, with a diagnosis of idiopathic PD in Hoehn & Yahr stages 1–3 and on a stable medication regimen were randomized to either 20 hours of self-administered SOPT (using InSight software) or a no-contact control condition. Participants were assessed at baseline and after 3 months of training (or an equivalent delay). The primary outcome measure was useful field of view test (UFOV) performance, and secondary outcomes included cognitive self-perceptions and depressive symptoms.
Results:
Results indicated that participants randomized to SOPT experienced significantly greater improvements on UFOV performance relative to controls, Wilks λ = 0.938, F 1,72 = 4.79, p = 0.032, partial η2 = 0.062. Findings indicated no significant effect of training on secondary outcomes, Wilks λ = 0.987, F2,70 < 1, p = 0.637, partial η2 = 0.013.
Conclusions:
Patients with mild to moderate stage PD can self-administer SOPT and improve their cognitive speed of processing, as indexed by UFOV (a robust predictor of driving performance in aging and PD). Further research should establish if persons with PD experience longitudinal benefits of such training and if improvements translate to benefits in functional activities such as driving.
Classification of evidence:
This study provides Class III evidence that SOPT improves UFOV performance among persons in the mild to moderate stages of PD.
Cognitive dysfunction in Parkinson disease (PD)1 is associated with poorer prognosis and reduced quality of life (e.g., loss of independence due to driving impairments2). Reduced speed of processing3,4 and poor visual attention (as indexed by useful field of view [UFOV])5 are important aspects of cognitive decline in PD. UFOV difficulties in PD predict impaired driving performance6–8 and are associated with higher risk of driving cessation.2 Previous studies in aging have demonstrated that cognitive speed of processing training (SOPT) enhances UFOV performance,9–12 and transfers to improved performance of instrumental activities of daily living, including driving mobility and safety.10,13–15 Few clinical trials have examined nonpharmacologic cognitive interventions among persons with PD16,17 despite evidence that such techniques enhance cognition among older adults. We present the first study to utilize SOPT among persons with PD.
The primary study aim was to examine the efficacy of SOPT among individuals with PD. We hypothesized that individuals with PD randomized to SOPT would experience enhanced UFOV performance relative to a control group. We further hypothesized that such benefits would transfer to improved cognitive self-perceptions and depressive symptoms.
A secondary aim was to explore moderators of the impact of SOPT. Factors that may influence the cognitive capacities of persons with PD or SOPT responsiveness such as age at onset, disease duration and severity,18,19 education, depression,18–20 use of dopaminergic treatment,21–23 mental status,10 visual function, and health ratings were examined as moderators.
METHODS
Participants.
Ninety-three adults were recruited from neurology clinics and screened for enrollment. Inclusion criteria were age 40 years or older, a clinical diagnosis of idiopathic PD, Hoehn & Yahr stages 1–3, a stable medication regimen (no expected changes in next 6 months), and availability over the next 6 months. Subjects with unpredictable or severe motor fluctuations or dyskinesias were ineligible. Further inclusion criteria were a Mini-Mental State Examination (MMSE) score ≥24, no known diagnosis of dementia, and adequate visual acuity to potentially benefit from training (≥20/80). These criteria were based on prior studies of SOPT.10
The sex and race of participants screened for enrollment were 36% female and 100% Caucasian. Of the 93 participants who were screened, 6 were ineligible: 1 due to anticipated medication changes, 2 due to inadequate vision, 1 due to low MMSE score, 1 due to a dementia diagnosis, and 1 due to not having idiopathic PD. The 87 eligible participants (table) ranged in age from 48 to 85 years (mean = 68.85, SD = 8.13) and had an average education of 15.36 years (SD = 2.48). Binocular visual acuity ranged from −0.16 to 0.60 LogMAR, which is equivalent to Snellen scores of 20/13 to 20/80, while MMSE scores ranged from 24 to 30. Three participants scored below 26 on the MMSE. With regard to Hoehn & Yahr stage of PD, 12.5% were in stage 1, 12.5% were in stage 3, and the remainder were in stage 2. These 87 eligible participants were randomized to either SOPT (n = 44) or control (n = 43) conditions (figure 1).
Table.
Characteristics of participants and outcomes by condition

Figure 1. CONSORT flow diagram.
Standard protocol approvals, registrations, and patient consents.
The USF Institutional Review approved the study, and written informed consent was obtained from all participants. The study was registered at clinicaltrials.gov as NCT01155349, “Cognitive Speed of Processing Training Among Persons With Parkinson's Disease.”
Measures.
Motor speed.
The finger-tapping task was used to measure motor speed. Participants alternated pressing a key with the index finger of each hand for 60 seconds. The average number of key presses across all trials was calculated.24
Health rating.
Self-rated health was rated on a 5-point Likert scale ranging from “excellent” to “poor.”
Mental status.
The Montreal Cognitive Assessment (MoCA) is a brief screening tool used to detect mild cognitive impairment and dementia.25 Given prior research findings that persons with mild cognitive impairment can benefit from SOPT,26 this measure was examined as a moderator rather than inclusion criterion.
Medication use.
Participants provided a list of all prescription medications taken regularly. Medications, dosages, and dose frequencies were recorded. Levodopa dose equivalency was calculated for each participant.27
Parkinson disease.
Age at disease onset, duration of disease, and whether or not tremors were a first symptom was recorded. The Unified Parkinson's Disease Rating Scale (UPDRS)28 was administered in the neurologist's office by experienced raters.
Cognitive speed of processing.
The primary outcome, UFOV, is a well-validated and reliable29 computer-administered measure that objectively evaluates speed of processing under increasing cognitive demand for visual attention tasks. Three subtests required the examinee to identify and localize targets with presentation durations ranging between 17 and 500 ms,29 which are varied by a staircase method to derive the 75% threshold. Scores were combined into a single composite with a possible range of 51 to 1,500 ms.
Cognitive self-perceptions.
The Cognitive Self-Report Questionnaire was a secondary self-administered outcome to measure perceptions of cognitive and everyday functioning.30 Twenty-five items are rated on a 5-point Likert scale. The total score was examined in analyses.
Depressive symptoms.
The Center for Epidemiological Studies Depression Scale (CES-D) (short form)31 measured depressive symptoms and was examined as a secondary outcome and a moderator. Participants indicated the number of days from the prior week that they felt or behaved in ways indicative of depression across 20 items (e.g., feeling down or blue) with ratings ranging from 0 (less than 1 day) to 3 (5 to 7 days). Ratings were summed into a composite with a possible range of 0 to 60.
Cognitive speed of processing training.
The self-administered version of SOPT, InSight (purchased from Posit Science, Inc., San Francisco, CA), was completed by participants at home. The InSight version of SOPT includes 5 exercises designed to improve information processing speed in realistic visual contexts. Across exercises, the visual stimuli change in a graduated fashion, moving from simple to complex visual stimuli. Within exercises, the stimuli become degraded or less discriminable and speed of presentation increases as user performance improves. Participants alternated training sessions between choosing the recommended schedule menu, which includes 5 different exercises (detailed below), and choosing only the Road Tour exercise. These instructions were given based on the efficacy of an earlier version of SOPT, which only used stimuli like the Road Tour exercise. The Road Tour exercise requires discrimination of a center target and localization of a peripheral target with increasing speed and background complexity. The InSight program includes 4 additional exercises: Sweep Seeker requires identification of visual gratings direction; Bird Safari requires visual discrimination of peripheral targets in degrading visual conditions with increasing presentation speed; Jewel Diver involves tracking and recalling multiple, moving visual targets; and Master Gardener requires detecting and recalling multiple visual targets with increasing speed and background complexity.
Procedure.
A priori power analyses were conducted to determine a target sample size of 85 to yield a final sample of 50 with 80% power to detect a SOPT effect size of d = 0.33. The study design was a parallel-group randomized trial in which an equal number of participants were allocated to the experimental and control conditions. The majority of participants were recruited by staff at the coinvestigators' movement disorders clinics (at the University of South Florida [USF], Tampa) and identified as potentially eligible. Participants were also recruited through support groups in Tampa, Florida, and surrounding areas and were evaluated for eligibility at the clinics. Recruitment and enrollment were conducted by research assistants (RAs) between October 2009 and July 2011. Participants provided informed consent and completed the MMSE and UPDRS at the clinic. Those eligible were scheduled for a baseline testing visit at the USF Cognitive Aging Laboratory. At the laboratory, visual acuity was assessed to further determine eligibility, and those meeting the eligibility requirements completed questionnaires and measures of motor and cognitive functioning. Participants were enrolled in the study and randomized to either SOPT or the control condition at the end of the baseline visit by an RA. An RA (M.L.O.) prepared randomization chips prior to the study start, which were blindly selected from a box by participants at enrollment to determine condition. Neither the experimenters nor participants were blinded to condition. Both randomized conditions completed the post-test visit at the laboratory approximately 3 months (mean = 13.56 weeks) after baseline (by December 2011). Participants were encouraged to complete post-test regardless of intervention compliance. Training participants were provided with the InSight training software as well as detailed instructions on how to install the program (control participants received the program at the end of the study). An RA (trainer who did not conduct testing visits [E.G.V.]) contacted the participants within 1 week to confirm installation of the program, and contacted participants approximately every 2 weeks to check on their progress. Control participants were contacted midway through the waiting period. The software program logged the amount of time spent working on the program and the exercises completed. Participants were instructed to complete 20 hours of training, with every other session spent working on only the Road Tour exercise. Participants were encouraged to work on the program 3 times per week for 1 hour per session, but were told that they could vary the schedule of training based on their needs (given prior research demonstrating that the interval between training sessions did not alter efficacy32). Participants were compensated up to $100 for completing the study.
Classification of evidence.
The primary question of interest was whether SOPT could enhance UFOV performance among persons in the early to moderate stages of PD. This interventional study presents Class III level of evidence that randomization to 20 hours of the InSight SOPT program over a 3-month period improves UFOV performance. This study is a prospective randomized trial with objective primary outcome assessment, including a sample representative of patients in the mild to moderate stages of PD, with equivalent samples in the treatment and control conditions.
Analyses.
Multivariate analysis of variance (MANOVA) compared participants who did and did not complete the study as well as the randomized conditions across baseline demographics and measures. χ2 tests compared the randomized conditions across sex and Hoehn & Yahr stage. Repeated-measures MANOVA compared the randomized conditions across pre- to post-test to examine the effects of SOPT first for the primary outcome, and then for secondary outcomes. Multiple regression explored moderators of SOPT effects.
RESULTS
Seventy-four participants completed the immediate post-test visit, reflecting an attrition rate of 15%. Thirteen participants did not complete the study (figure 1). MANOVA revealed that participants who did and did not complete the study were not significantly different across any demographic characteristic or baseline measure (p > 0.05).
On average, SOPT participants completed 21.37 hours of InSight training (SD = 13.24), with an average of 10.01 hours (SD = 7.26) of the Road Tour exercise. Sixty-nine percent of the participants completed the recommended amount of training (at least 20 hours).
Missing baseline data (MoCA, motor speed, and CES-D) were substituted for less than 3% of the data points using multiple regression techniques (no substitutions were made for post-test data).
The randomized conditions (training and control) were compared at baseline on sex and Hoehn & Yahr stage using χ2 tests, and across age, education, motor speed, MoCA scores, depressive symptoms, MMSE scores, visual acuity, age at diagnosis, disease duration, Schwab and England Scale, and UFOV performance using MANOVA. Results indicated that there were no significant differences; the 2 randomized conditions were equivalent at baseline, sex χ2 = 0.09, p = 0.761; Hoehn & Yahr stage χ2 = 2.19, p = 0.700; Wilks λ = 0.927, F9,64 < 1, p = 0.823, partial η2 = 0.073.
To examine efficacy of training, UFOV performance of those randomized to training was compared to controls from baseline to post-test using repeated-measures analysis of variance. Results indicated a significant randomization group by testing occasion interaction, Wilks λ = 0.938, F1,72 = 4.79, p = 0.032, partial η2 = 0.062. Those randomized to SOPT experienced significantly greater improvements on UFOV performance than control participants. Post hoc Fisher least significant difference test indicated that both groups experienced significant improvements in UFOV performance from baseline to post (p < 0.01). Means and SDs are reported in the table.
Repeated-measures MANOVA was conducted to examine whether SOPT transferred to improved perceptions of cognitive performance or depressive symptoms from baseline to post-test. Results indicated no significant randomization group by testing occasion interaction, Wilks λ = 0.987, F2,70 < 1, p = 0.637, partial η2 = 0.013. Although the training significantly enhanced UFOV performance, it did not transfer to immediate improvement on secondary outcomes. Descriptives are presented in the table.
The second aim was to explore potential moderators of the effects of SOPT among persons with PD. The effect of potential moderating factors (age, age at diagnosis, disease duration, Hoehn & Yahr stage, MoCA score, visual acuity, depressive symptoms, l-dopa equivalent dose, or self-rated health) on training gains was explored through correlation and regression analyses. The dependent variable was training gain gauged by baseline to post UFOV performance.
Pearson correlations among the SOPT condition indicated that training gain was significantly correlated with disease duration (r = 0.52, p = 0.002), diagnosis age (r = −0.46, p = 0.008), and l-dopa equivalent dose (r = 0.40, p = 0.02) only. Those with longer disease duration, earlier age at diagnosis, or higher l-dopa equivalent doses tended to experience greater training gains.
For regression analyses, randomized condition (training or control) was first entered as a predictor, followed by the moderator of interest and an interaction term of moderator by randomization condition. A significant interaction would reflect moderation of benefit from SOPT. Results indicated significant interactions for training condition and age at diagnosis, as well as disease duration (R2 = 0.123−0.142, p < 0.05), but no significant interactions of training condition with age, visual acuity, MoCA score, Hoehn & Yahr stage, depressive symptoms, l-dopa equivalent, or self-rated health (p > 0.05). These results are depicted in figure 2. Younger age at PD diagnosis and longer duration of PD were significantly associated with greater training gains.
Figure 2. Significant moderators of cognitive speed of processing training.
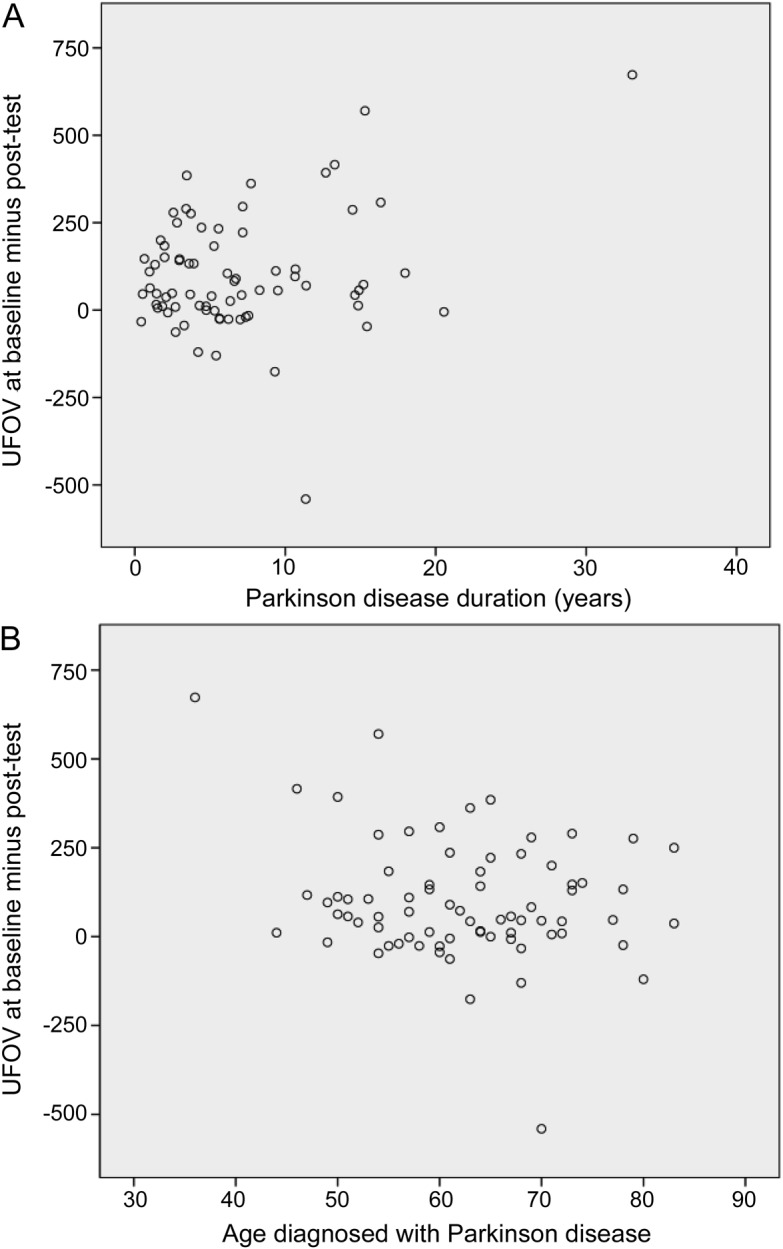
The y-axis represents training gain measured by useful field of view test (UFOV) improvement from baseline to post-test, and the x-axis represents duration of Parkinson disease (PD) (A) and age at PD diagnosis (B). Those with longer disease duration and those who were diagnosed with PD at younger ages benefited the most.
DISCUSSION
The results indicate that it is feasible for adults with mild to moderate PD to self-administer and complete approximately 20 hours of SOPT at home using commercially available software to improve cognitive speed of processing for visual attention tasks. Translational benefits include the ability to immediately implement this treatment without requiring extensive personnel time or costs.
The hypothesis that SOPT would enhance UFOV performance among persons with PD was supported. SOPT did not result in immediate improvements in either cognitive self-perceptions or depressive symptoms. Prior study among older adults found that SOPT protected against depressive symptoms 1 year post training.33 Longer follow-up is needed to determine if this applies in PD. This study is the first to examine cognitive self-perceptions in relation to SOPT. Because SOPT is a process-based training technique that is procedural rather than declarative,34 training-related improvements may not be recognized by participants. More feedback on performance while training may be warranted to promote continued engagement.
Our results uncovered several moderators of training gains. Those with longer disease duration and those who were diagnosed with PD at younger ages (both of whom tended to perform worse on UFOV at baseline) benefited the most. Similarly, higher doses of l-dopa were associated with greater training gains. Given that there were no moderation effects of disease stage, the study findings are generalizable to persons with PD in Hoehn & Yahr stages 1–3. Further study with a larger and more diverse sample of persons with PD and inclusion of other factors such as side of PD onset, daytime sleepiness, or anxiety is needed to clearly determine what factors influence benefit from SOPT.
These results are similar to those found among older adults in general. Among older adults, SOPT has shown several longitudinal benefits, despite the lack of immediate transfer to other cognitive or functional measures. For example, SOPT has resulted in a 48% reduced risk of at-fault crash involvement and decreased risk of driving cessation over 3–5 years.14 Given that impaired UFOV predicts driving cessation in PD,2 SOPT may delay loss of vehicular mobility, as observed in normal aging.14,15 In addition, SOPT has protective effects longitudinally with regard to depressive symptoms, health-related quality of life, and estimated medical care costs.15,34,35 Overall, present and prior findings indicate potential for SOPT to similarly enhance quality of life in PD. It is important that these research results be replicated and that the potential longitudinal effects of SOPT are examined in PD.
There are limitations to the current study. Our sample included only Caucasian subjects who were highly educated and in the mild to moderate stages of PD; therefore, results may not generalize to less educated individuals, minorities, or those in later stages of the disease. However, prior study indicates that race and education are not likely to moderate SOPT gains.10 The longitudinal effects of the intervention were not examined, and it is not clear from this study whether one particular exercise of InSight is more effective than the others. The required dose to obtain benefit is also not known. The outcomes included in this study were somewhat limited in that measures of executive decline were not included. It is important to note, however, that a great deal of executive decline in aging can be attributed to speed of processing difficulties.36 Although overall attrition rates were lower than expected between baseline and post-test, attrition in the training condition was greater than in the control condition. The most common reason for drop out reported by participants was being too busy to continue the study. Importantly, no differences were evident between those who did and did not complete the study. Further study should examine individual factors associated with patients' willingness to participate in cognitive training, which have yet to be determined. A potential limitation to the study is that participants and RAs were not blinded to condition. However, 7 prior clinical trials among healthy older adults have demonstrated that SOPT improves UFOV performance regardless of blinding, thereby lessening this concern.10 Due to the lack of blinding, this study presents Class III level of evidence.
It is feasible for patients with mild to moderate stage PD to self-administer and benefit from SOPT. Such training improves UFOV performance, which is associated with safe driving. Further research should establish if there are longitudinal benefits of such training for persons with PD such as prolonged and safer driving mobility.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the study participants and the other contributors to this study including, but not limited to, Holly Delgado, Eden Feldman, Christine Haley, Elizabeth Hudak, Dr. Israt Jahan, Theresa McClain, Chelsea McNee, Adina Mears, Amber Miller, Carol Peronto, Judy Smolk, and Kelly Sullivan, who helped to recruit, test, and coordinate study participants; Neveen Nawaway and Hope Smith for clerical assistance; and the undergraduate student Research Assistants at the USF Cognitive Aging Lab for data entry assistance.
GLOSSARY
- CES-D
Center for Epidemiological Studies Depression Scale
- MANOVA
multivariate analysis of variance
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson disease
- RA
research assistant
- SOPT
speed of processing training
- UFOV
useful field of view
- UPDRS
Unified Parkinson's Disease Rating Scale
- USF
University of South Florida
Footnotes
Editorial, page 1278
AUTHOR CONTRIBUTIONS
Dr. Edwards, Dr. Hauser, Dr. Zesiewicz, and Dr. Uc contributed to the study design and conceptualization. Statistical analyses and interpretation were performed by Dr. Edwards, Dr. O'Connor, and E.G. Valdés. All authors contributed to drafting or revising the manuscript.
STUDY FUNDING
Supported by a grant from the NIH National Institute on Aging 1R21AG033332 to Dr. Edwards, Dr. Hauser, Dr. Zesiewicz, and Dr. Uc. NIH/NIA funded the study, but did not play a role in conceptualization, design, analyses, or interpretation. The InSight training software was purchased from Posit Science, Inc., which played no role in the study conceptualization, design, analyses, or interpretation.
DISCLOSURE
J. Edwards worked as a limited consultant to Posit Science, Inc., the company that markets InSight, from June to August 2008. R. Hauser received honoraria or payments for consulting, advisory services, or speaking services in 2012 from Abbott Laboratories, Allergan, Inc., AstraZeneca, Biotie Therapies Corporation, Ceregene, Inc., Chelsea Therapeutics, Inc., GE Healthcare, Impax Laboratories, Inc., Ipsen Biopharmaceuticals, Inc., Lundbeck, Med-IQ, Merck/MSD, Noven Pharmaceuticals, Inc., Straken Pharmaceuticals, Ltd., Targacept, Inc., Teva Pharmaceuticals Industries, Ltd., Teva Neuroscience, Inc., Upsher-Smith Laboratories, UCB, Inc., UCB Pharma SA, and Xenoport, Inc. Dr. Hauser received royalties in 2012 from the University of South Florida. In addition, Dr. Hauser has consulted in litigation with lawyers representing various current and former manufacturers of welding consumables. M. O'Connor and E. Valdés report no disclosures. T. Zesiewicz reports receives research support from FARA, Astellas, Allon, GlaxoSmithKline, Edison, and UCB Pharma, and honoraria from UCB Pharma, Pfizer, TEVA, and GE Healthcare. E. Uc was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development (1-B6261R, Merit Review Award, PI: E.Y.U., 2009–2012; 2-I01 RX000 170-01, Merit Review Award, PI: E.Y.U., 2010–2012), and National Institute of Neurological Disorders and Stroke grant R01 NS044930 (PI: E.Y.U., 2003–2010) from NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 2003;16:193–210 [DOI] [PubMed] [Google Scholar]
- 2.Uc EY, Rizzo M, Johnson AM, et al. Real-life driving outcomes in Parkinson disease. Neurology 2011;76:1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karayanidis F. Parkinson's disease: a conceptualization of neuropsychological deficits with an information processing framework. Biol Psychol 1989;29:149–179 [DOI] [PubMed] [Google Scholar]
- 4.Lee C, Grossman M, Morris J, Stern MB, Hurtig HI. Attentional resource and processing speed limitations during sentence processing in Parkinson's disease. Brain Lang 2003;85:347–356 [DOI] [PubMed] [Google Scholar]
- 5.Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology 2005;65:1907–1913 [DOI] [PubMed] [Google Scholar]
- 6.Uc EY, Rizzo M, Johnson AM, Dastrup E, Anderson SW, Dawson JD. Road safety in drivers with Parkinson disease. Neurology 2009;73:2112–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Classen S, McCarthy DP, Shecthman O, et al. Useful field of view as a reliable screening measure of driving performance in people with Parkinson's disease: results of a pilot study. Traffic Inj Prev 2009;10:593–598 [DOI] [PubMed] [Google Scholar]
- 8.Crizzle AM, Classen S, Uc EY. Parkinson disease and driving: an evidence-based review. Neurology 2012;79:2067–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball KK, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA 2002;288:2271–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball KK, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. J Gerontol B Psychol Sci Soc Sci 2007;62B:19–31 [DOI] [PubMed] [Google Scholar]
- 11.Ball KK, Sekuler R. Improving visual perception in older observers. J Gerontol 1986;41:176–182 [DOI] [PubMed] [Google Scholar]
- 12.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296:2805–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roenker DL, Cissell GM, Ball KK, Wadley VG, Edwards JD. Speed-of-processing and driving simulator training result in improved driving performance. Hum Factors 2003;45:218–233 [DOI] [PubMed] [Google Scholar]
- 14.Ball KK, Edwards JD, Ross LA, McGwin G., Jr Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc 2010;58:2107–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards JD, Myers C, Ross LA, et al. The longitudinal impact of speed of processing training on driving mobility of older drivers. Gerontologist 2009;49:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuter I, Mehnert S, Sammer G, Oechsner M, Engelhardt M. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson's disease. J Aging Res 2012;2012;235765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson's disease. J Neurol Sci 2006;248:115–119 [DOI] [PubMed] [Google Scholar]
- 18.Caparros-Lefebvre D, Pecheux N, Petit V, Duhamel A, Petit H. Which factors predict cognitive decline in Parkinson's disease? J Neurol Neurosurg Psychiatry 1995;58:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzen HL, Levin BE, Llabre ML. Age of disease onset influences cognition in Parkinson's disease. J Int Neuropsychol Soc 1998;4:285–290 [PubMed] [Google Scholar]
- 20.Cohen S, Freedman M. Cognitive and behavioral changes in the Parkinson-plus syndromes. Adv Neurol 2005;96:166–186 [PubMed] [Google Scholar]
- 21.Hermanowicz N. Drug therapy for Parkinson's disease. Semin Neurol 2007;27:97–105 [DOI] [PubMed] [Google Scholar]
- 22.Molloy SA, Rowan EN, O'Brien JT, McKeith IG, Wesnes K, Burn DJ. Effect of levodopa on cognitive function in Parkinson's disease with and without dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesnes KA, McKeith I, Edgar C, Emre M, Lane R. Benefits of rivastigmine on attention in dementia associated with Parkinson disease. Neurology 2005;65:1654–1656 [DOI] [PubMed] [Google Scholar]
- 24.Lezak MD. Neuropsychological Assessment, 3rd ed New York: Oxford University Press; 1995 [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 26.Valdes EG, O'Connor ML, Edwards JD. The effects of speed of processing training among older adults with psychometrically-defined mild cognitive impairment. Curr Alzheimer Res 2012;9:999–1009 [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653 [DOI] [PubMed] [Google Scholar]
- 28.Fahn S, Elton RL, Members of the UPDRS development committee: Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Lieberman A, eds. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information; 1987:153–163 [Google Scholar]
- 29.Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. Reliability and validity of the useful field of view test scores as administered by personal computer. J Clin Exp Neuropsychol 2005;27:529–543 [DOI] [PubMed] [Google Scholar]
- 30.Smith GE, Housen P, Yaffe K, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. J Am Geriatr Soc 2009;57:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health 1993;5:179–193 [DOI] [PubMed] [Google Scholar]
- 32.Vance DE, Dawson J, Wadley VG, et al. The accelerate study: the longitudinal effect of speed of processing training on cognitive performance of older adults. Rehabil Psychol 2007;52:89–96 [Google Scholar]
- 33.Wolinsky FD, Mahncke HW, Vander Weg MW, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci 2009;64A:468–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolinsky FD, Mahncke HW, Vander Weg MW, et al. Speed of processing training protects self-rated health in older adults: enduring effects observed in the multi-site ACTIVE randomized controlled trial. Int Psychogeriatr 2010;22:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolinsky FD, Mahncke HW, Kosinski M, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res 2009;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhaeghen P. Aging and executive control: reports of a demise greatly exaggerated. Curr Dir Psychol Sci 2011;20:174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



