Abstract
Objective:
To investigate predictors of trial start-up times, high attrition, and poor protocol adherence in amyotrophic lateral sclerosis (ALS) trials.
Methods:
Retrospective analysis of start-up times, retention, and protocol adherence was performed on 5 clinical studies conducted by the Northeast ALS Consortium and 50 ALS clinical trials identified by PubMed search. Predictors of start-up times were estimated by accelerated failure time models with random effects. Predictors of retention and protocol deviations were estimated by mixed-model logistic regression.
Results:
Median times for contract execution and institutional review board (IRB) approval were 105 days and 125 days, respectively. Contract execution was faster at sites with more ongoing trials (p = 0.005), and more full-time (p = 0.006) and experienced (p < 0.001) coordinators. IRB approval was faster at sites with more ongoing trials (p = 0.010) and larger ALS clinics (p = 0.038). Site activation after IRB approval was faster at sites with more full-time (p = 0.038) and experienced (p < 0.001) coordinators. Twenty-two percent of surviving participants withdrew before completing the trial. Better participant functional score at baseline was an independent predictor of trial completion (odds ratio 1.29, p = 0.002) and fewer protocol deviations (odds ratio 0.86, p = 0.030).
Conclusion:
Delays in IRB review contribute the most to prolonged trial start-up times, and these timelines are faster in sites with more experienced staff. Strategies to improve protocol adherence and participants' retention may include enrolling people at early disease stages.
The translation of basic research to clinical practice depends on the conduct of well-designed and efficient clinical trials. Delays in study start-up, high participant attrition, and poor adherence to the study protocol reduce trial validity and inflate the timelines and costs of drug development. In published clinical trials, enrollment and retention data are routinely reported; however, factors affecting study start-up times, retention, and adherence to the protocol are rarely mentioned.1,2
Conducting efficient clinical trials involves completing study start-up processes in a timely manner. This includes execution of the site contracts, obtaining institutional review board (IRB) approvals, and completing protocol, pharmacy, and outcomes trainings. Delays in the study start-up process can extend trial duration and costs, threaten trial feasibility, and delay answering important clinical questions.
The internal validity of a clinical trial depends largely on good conduct including retention and maintaining good protocol adherence (i.e., complying with the study protocol). In addition, high attrition requires larger sample sizes to maintain statistical power and subsequently inflates trial duration and costs.3,4
Amyotrophic lateral sclerosis (ALS) is a rare neurologic disorder that has many potential therapies currently in development.5 With a small patient population and limited funds, it is critical that clinical trials are well designed and efficiently run. To better characterize these issues, we investigated factors that may correlate with study start-up, retention, and protocol adherence in ALS clinical trials and proposed steps to improve the efficiency of trial initiation and conduct.
METHODS
Study start-up times, retention rates, and adherence to study protocol (number of protocol deviations) were estimated from 5 ALS clinical studies conducted by the Northeast ALS (NEALS) Consortium and 49 previously published ALS trials. A list of factors that might contribute to differential efficacy of trial conduct were proposed by study authors and categorized into site factors, participant factors, and trial factors. Finally, the associations among the proposed factors and start-up times, protocol adherence, and retention were investigated.
NEALS Consortium studies.
Study start-up.
Retrospective analysis of 4 ALS clinical studies conducted by the NEALS Consortium was performed to determine the time required for sites’ contract execution and IRB approval, and enrollment of the first participant at each site. The ALS studies examined include the following: 1) ceftriaxone (63 sites) (NCT00349622), 2) lithium (21 sites),6 3) KNS-760704 (20 sites),7 and 4) biomarkers study (29 sites)8 (table 1).
Table 1.
List of NEALS Consortium studies examined for start-up times, adherence, and retention
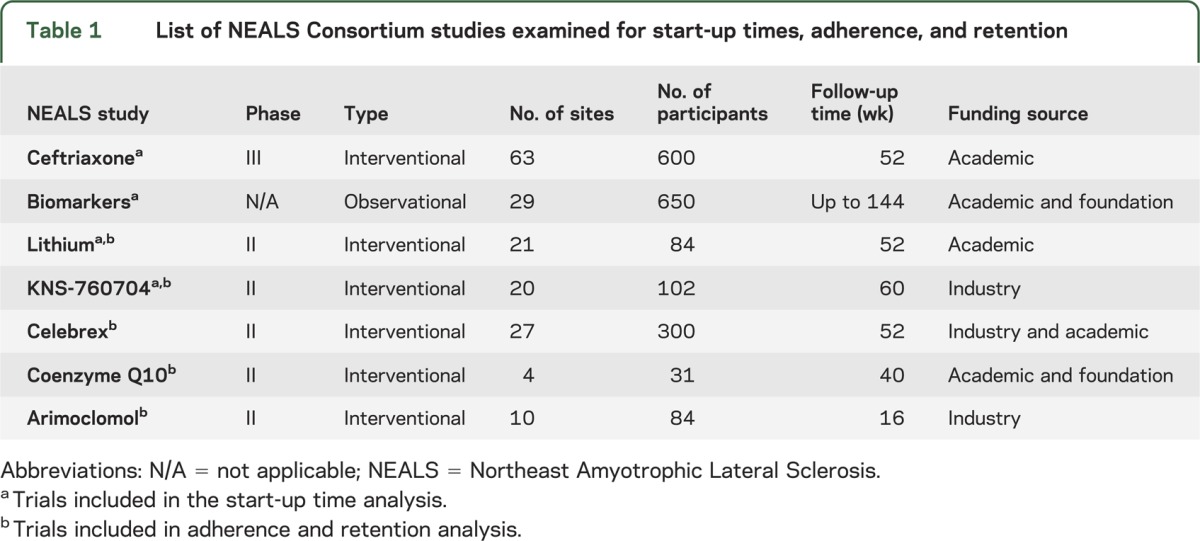
Contract execution time was defined as the time from when the trial contract was initially sent to sites to full execution of the agreement; IRB approval time as the time from when the initial protocol was sent to sites to site IRB approval; and IRB to site activation time as the time from IRB approval of protocol to the site enrolling the first participant. Finally, total time for first participant enrollment was defined as total time from when the initial protocol was sent to sites for IRB preparation to time of first participant enrollment at the site.
Site factors that may contribute to study start-up were investigated. Participating NEALS sites were surveyed on the presence and size of a multidisciplinary ALS clinic, the number of investigators (1, 2, 3, >3), and the number of research coordinators devoted to clinical trials, the years of experience of site coordinators (<2, 2–3, >3 years), the number of ALS trials completed by site primary investigator, the time site primary investigator devotes to clinical research (<20%, 20%–40%, >40%), and the number of ongoing ALS clinical trials at the site.
Retention and adherence.
Retention and protocol adherence data were collected on 601 participants enrolled in 5 ALS clinical trials conducted by the NEALS Consortium: 1) celebrex,9 2) arimoclomol,10 3) coenzyme Q10,11 4) lithium,6 and 5) KNS-7607047 (table 1).
Retention was defined as trial completion on study drug. Attrition rates were estimated by the percentage of participants that withdrew from a trial, and reasons for attrition were collected for all participants including death. Adherence was estimated by the number of protocol deviations, defined as any change, divergence, or departure from the study protocol, for each participant in these trials. Deviation rates were measured by counts of deviations per participant during their time in the study.
Participant factors that may contribute to retention and adherence were examined, including age, sex, race, disease duration (time from symptom onset to enrollment), time from symptom onset to diagnosis, familial ALS status, and baseline disease state measured by forced vital capacity and the ALS Functional Rating Scale–Revised.
Literature review.
To further explore retention rates and causes of attrition in a large number of ALS trials, a PubMed search was conducted for phase II and III ALS trials published between 1979 and 2011. Fifty published ALS clinical trials were identified, 49 of which reported participant attrition data. Available trial factors that may contribute to retention rates were collected including treatment to placebo ratio, open-label option, trial sponsor (industry vs academic), route of administration, off-label availability, and rate of serious adverse events.
Statistical analysis.
NEALS Consortium studies.
Study start-up.
Median time intervals were estimated from Kaplan-Meier product-limit estimates of the survival function. Point-wise confidence intervals (CIs) were obtained using a log-log transformation of the survival function. Predictors of differential times to events of interest were analyzed using accelerated failure time frailty models assuming Weibull-distributed event times, controlling for study, and with principal investigator as a random effect. Estimates are expressed as a percent difference in mean time to event. Predictors originally collected on an ordinal scale (e.g., mean coordinator experience <2 years, 2–3 years, and >3 years) were dichotomized at their medians.
Adherence and retention.
Predictors of attrition odds were tested using mixed-model logistic regression with random effects for study and site within each study. Predictors of causes of attrition, categorized as death, consent withdrawal for an unknown reason, adverse events, or other, were tested by multinomial regressions using a generalized logit link. Multinomial models with random effects of study and site within each study failed to converge. Predictors of protocol deviation rates were tested by mixed-model negative binomial regression with random effects for study and site within each study and an offset equal to the log of time on study.
Literature review.
Weighted linear regression models were used to estimate associations between trial factors and attrition rates as the reported proportion of the enrolled cohort who survived but did not complete the study in published ALS trials. The total enrollment in each trial was used as sample weights. Inference was confirmed in mixed-model log-linear regressions with random effects for each trial.
RESULTS
NEALS Consortium studies.
Study start-up.
The median total time for first participant enrollment was 252 days (95% CI 227–264 days) after the initial protocol was submitted to the sites. Within this time frame, the median time for contract execution was 105 days (95% CI 81–118 days), for IRB approval 125 days (95% CI 102–139 days), from IRB approval to site activation 63 days (95% CI 50–73 days), and from site activation to first participant enrollment 33 days (95% CI 22–54 days) (figure). Contract execution time was shorter at sites with more ongoing trials and more full-time and more experienced coordinators. Contract execution time decreased by 16% (95% CI 5%–25%, p = 0.005) for each additional ongoing trial at the site, by 21% (95% CI 7%–33%, p = 0.006) for each additional full-time coordinator, and by 48% (95% CI 32%–60%, p < 0.001) for sites whose coordinator(s) had 2 or more years of experience.
Figure. Median start-up timelines for 4 Northeast Amyotrophic Lateral Sclerosis multicenter studies.
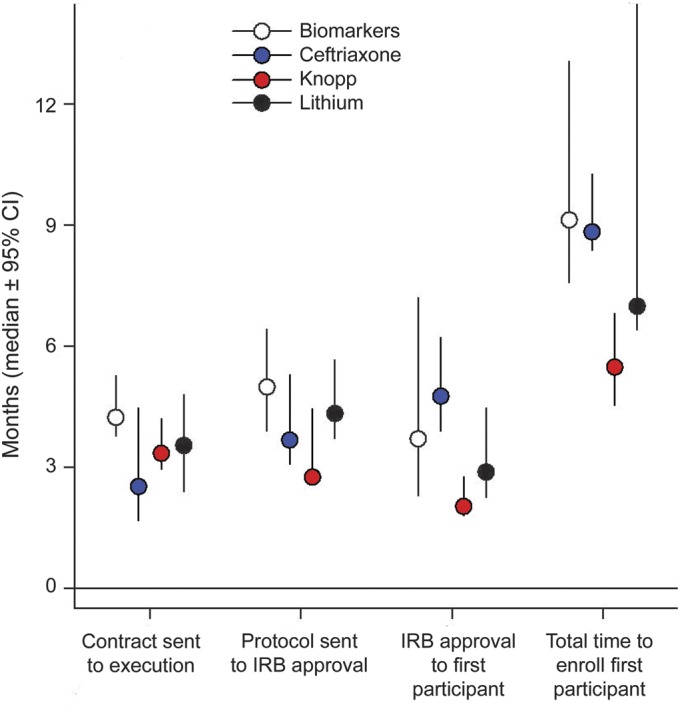
CI = confidence interval; IRB = institutional review board.
IRB approval time was shorter at sites with more ongoing trials and larger clinics. IRB approval time decreased by 15% (95% CI 3.5%–22%, p = 0.010) for each additional ongoing study, and by 15% (95% CI 1.0%–28%, p = 0.038) for each additional 100 patients followed in clinic. As with contract execution times, IRB to site activation time was shorter at sites with more full-time coordinators (21% decrease/coordinator, 95% CI 1.3%–36%, p = 0.038) and those that had coordinators with more than 2 years of experience (44% decrease, 95% CI 24%–59%, p < 0.001). None of the tested factors had a significant impact on the time to enrollment of first participant (table e-1 on the Neurology® Web site at www.neurology.org).
Retention and protocol adherence.
The 3 most common reasons for attrition were consent withdrawal for no specific cause (37%), death (28%), and withdrawal secondary to an adverse event (17%). Other causes for attrition were loss to follow-up (4%), disease progression (3%), difficulty traveling to the site (2%), and perceived lack of drug efficacy (2%); no reason was given in 7% of the cases. The overall mean rate of attrition for causes other than death was 22%. A better functional status at baseline measured using the ALS Functional Rating Scale–Revised was an independent predictor of completing the trial (odds ratio [OR] 1.38/5-unit increase, 95% CI 1.13–1.68, p = 0.002). Longer time from diagnosis to enrollment also predicted higher odds of study completion (OR 1.30/year, 95% CI 1.03–1.64, p = 0.026).
The average number of protocol deviations across all 5 NEALS trials was 4.7 deviations/participant/year. Omitting parts of the protocol, such as outcomes and safety procedures, and out of window visits were the most common form of nonadherence, together accounting for 84% of all deviations. Other more serious reasons for nonadherence included deviations related to eligibility (6%), study medication (4%), IRB/regulatory (3%), consenting procedure (2%), and other (1%). A better respiratory status at baseline measured by forced vital capacity was a predictor of fewer protocol deviations (OR 0.86/10-unit increase, 95% CI 0.74–0.99, p = 0.030). Caucasian race was predictive of a lower rate of deviations (OR 0.17, 95% CI 0.05–0.54, p = 0.003). None of the other participant factors had an impact on retention or adherence (table e-1).
Literature review: Retention.
The mean attrition rate across 49 studies was 33% ± 19%; 15% was due to death. Among surviving participants, the 2 most common reasons for attrition were adverse events (30%) and consent withdrawal for no specific reason (21%). Other reasons included disease progression (11%), study termination (9%), loss to follow-up (6%), perceived lack of drug efficacy (4%), and poor compliance (4%). Twenty-six percent of the reviewed trials offered open-label extensions. Trials offering open-label extension had a 9% higher attrition rate (95% CI 3.9%–14.7%, p = 0.041) after adjusting for follow-up time. No other trial factors were significantly associated with the attrition rate.
DISCUSSION
Similar to published studies in other disorders,12–15 study start-up processes for multicenter ALS studies were long (252 days) and variable (range 70–596). Most of the start-up time was spent obtaining site IRB approvals (125 days) and executing site contracts (105 days). These 2 processes were sequential at some sites and parallel at others. Sites that follow more patients in clinic, conduct more trials, and have more experienced staff had faster study start-up. This is reminiscent of the positive correlation between volume and improved health care delivery outcomes.16
There is very little published data on start-up times in neurologic clinical trials, and none of the 50 ALS clinical trials reviewed reported start-up timelines. Study start-up has been examined in more detail in oncology clinical trials.12–14 These studies also observed that administrative processes, including review of a protocol by multiple IRBs, lengthen study start-up times.14 Reduction in start-up time can be accomplished by adopting paperless technologies such as central Web portal systems for document sharing, and establishing central IRBs (CIRBs) and master contract templates.
Some institutions require completion of the contract execution process before submission of a protocol to their local IRB. This sequential approach adds to start-up delays. Either working in parallel with IRB review or using a standardized contract template could reduce the timeline for study start-up. In our study, 90% of sites used their local IRB, whereas a CIRB system may have speeded up the approval process by eliminating duplication of efforts at local sites.15 For multicenter clinical trials in Parkinson disease, Ravina et al.15 reviewed the impact of local IRB reviews on safety and costs and found that local IRB reviews did not result in any significant changes to the protocol, and that these minor changes made no difference to a person's decision to participate in a trial. In addition to start-up delays, the cost of this process exceeded $100,000.15 Implementation of a CIRB model in oncology trials was associated with faster review and less IRB staff effort.17
The main mission of the IRB is to protect the welfare of study participants; however, the prolonged IRB approval timelines contribute to inflating the cost of clinical trials and delaying finding treatments for people with devastating diseases such as ALS. The cost of developing a new drug is approximately $800 million18 and the principal driver of this cost is late-stage multicenter clinical trials, where IRB delays have the most negative impact on cost.
The National Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT) was established by the National Institute of Neurological Disorders and Stroke to improve the efficiency of neurology clinical trials. Two key initiatives utilized by NeuroNEXT to accomplish this goal were to implement the use of a CIRB along with standardized master clinical trial agreements between the clinical study sites.19
The average nondeath attrition rates in ALS clinical trials measured in our literature review and NEALS trials were 18% and 22%, respectively. The most common causes for nondeath attrition were consent withdrawal and adverse events. Of the 27% who withdrew consent without giving any specific reasons, it is conceivable that there was a combination of issues such as disease progression, travel difficulties, and caregiver burden. One limitation of our study is that the original trials did not fully explore reasons for consent withdrawal.
Our results suggest that enrolling people with better functional status may improve retention. We also found that people with longer disease duration had better retention in ALS trials. The combination of longer disease duration and preserved functional status at enrollment represent a subgroup of people with slowly progressive disease. Enrolling people at early disease stages and with good functional status will not only improve retention in clinical trials, but will also increase the chances of seeing the biological benefits of neuroprotective treatments.
Participant retention is a challenge in any clinical trial. In a recent review of Alzheimer disease clinical trials, retention ranged from 46% to 95%.20 Non-Caucasian race, lower education level, depression, being unmarried, and being recruited by commercial sites were among the risk factors for higher attrition.21 In one trial of antiepileptic therapy, the 1-year retention rate was 47% and the most common reasons for early study treatment withdrawal were adverse events and perceived lack of efficacy.22
We could not identify specific trial design factors that predict retention. This highlights the limitations of using published literature to study trial retention and emphasizes the need for conducting studies dedicated to characterizing this problem in various neurologic disorders. We propose a few modifications in clinical trial design that could affect retention. For example, retention could be improved by designing shorter trials with a simpler schedule of activities that include telephone calls between visits, sending newsletters to study participants, more frequent communications with sites, use of performance measurement tools to the sites, using telemedicine tools for ease of communication between sites and participants, and developing reliable self-administered trial outcomes.23,24
Higher attrition in trials offering open-label extensions is a surprising finding in our study. This could be a false-positive result attributable to the relatively small number of trials that offered open-label extension (12/49 trials) or a true finding that reflects an inherent difference in the design and conduct of trials that offer open-label extension or the types of participants who enroll in such trials. Those trials could be more complex, require more frequent visits, or test invasive treatments with more adverse events. Unfortunately, the available published data were not sufficient to verify these hypotheses.
Our results suggest that suboptimal adherence to trial protocol, measured by the incidents of protocol deviations, is common (4.7 deviations/participant/year). Although the majority of these protocol deviations were minor, 15% were serious deviations related to trial eligibility, the consenting process, and study drug administration. Similar to retention, enrolling people at earlier stages of the disease was associated with better protocol adherence.
Any clinical trial is likely to have in practice less than 100% adherence, which reduces the opportunity to observe the true effect of the trial intervention. For example, a 20% reduction in drug adherence by study participants may result in the need for a greater than 50% increase in sample size. Unfortunately, to date, little research has been conducted investigating protocol adherence issues in clinical trials.25
Lessons learned from other disorders suggest that 3 major considerations should be addressed when designing and conducting clinical trials. First, it is important to define a study population that is likely to adhere to the study protocol. Drug addiction, psychological problems, cognitive impairment, low literacy, history of missed clinic appointments, advanced disease, and living a long distance from the study site are some of the participant factors that could affect protocol adherence.26 Second, trial design may have an impact on protocol adherence. Involving patients and patient advocates in the trial design phase and designing trials with simplified schedules could all improve protocol adherence.26,27 Third, site staff can have a major impact on maintaining good adherence. Trial adherence can be improved by establishing a good relationship between site staff and the participant, offering parking, flexible visit hours, optional home visits, and sending visit reminders and study update cards.26
As more therapies are developed for neurologic disorders, it is important to systematically study start-up times, retention, and protocol adherence. Current start-up times for ALS clinical trials are long but could be improved by selecting experienced sites with better training, and perhaps by implementing a CIRB review process that is streamlined with contract execution. We attempted to identify factors that may affect retention and adherence in ALS trials, and our results suggest that these can be improved by enrolling people at earlier disease stages. It is important to note that some of the reasons and solutions for retention and adherence challenges are disease-specific and should be addressed in each field separately.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Northeast ALS Consortium (NEALS) and Knopp Biosciences for providing the data for this research, and Craig Amburgey, Jim Mather, and Eva Petzinger at Knopp Neuroscience.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- CI
confidence interval
- CIRB
central institutional review board
- IRB
institutional review board
- NEALS
Northeast Amyotrophic Lateral Sclerosis
- NeuroNEXT
Network for Excellence in Neuroscience Clinical Trials
- OR
odds ratio
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Nazem Atassi: study concept and design. Padmaja Yerramilli-Rao: acquisition of data. Jackie Szymonifka: data analysis. Hong Yu: acquisition of data. Marianne Kearney: critical revision of the manuscript for important intellectual content. Daniela Grasso: acquisition of data. Jing Deng: data analysis. Mark Levine-Weinberg, Jordan Shapiro, Alexandra Lee, and Lucia Joseph: acquisition of data. Eric A. Macklin: data analysis. Merit E. Cudkowicz: critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Supported by institutional funding. This work was conducted with support from the Muscular Dystrophy Association (MDA) Clinical Research Training Grant, research fellowship from the American Academy of Neurology (AAN), the MGH ALS Research Fund, and the National Institute of Neurological Disorders and Stroke U01-NS049640 and U01-NS049640-04S1.
DISCLOSURE
N. Atassi receives fellowship grants from the AAN, MDA, and the Anne Young Fellowship, has research grants from the Harvard NeuroDiscovery Center and ALS Therapy Alliance, and provided consulting for Biogen Idec. P. Yerramilli-Rao is currently employed by Novartis. J. Szymonifka, H. Yu, M. Kearney, D. Grasso, J. Deng, M. Levine-Weinberg, J. Shapiro, A. Lee, and L. Joseph report no disclosures. E. Macklin serves on data and safety monitoring boards for Shire Human Genetic Therapies and Lantheus Medical Imaging and as an unpaid consultant to Knopp Biosciences. M. Cudkowicz served on the data and safety monitoring board for Synapse and Trophos and was a consultant for Teva, Millennium, GlaxoSmithKline, Biogen Idec, and Cytokinetics, Inc. Dr. Cudkowicz is also the primary investigator of the NeuroNEXT Coordination Center. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Newberry A, Sherwood P, Hricik A, et al. Understanding recruitment and retention in neurological research. J Neurosci Nurs 2010;42:47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qureshi AI, Tariq N, Vazquez G, et al. Low patient enrollment sites in multicenter randomized clinical trials of cerebrovascular diseases: associated factors and impact on trial outcomes. J Stroke Cerebrovasc Dis 2012;21:131–142 [DOI] [PubMed] [Google Scholar]
- 3.Cooley ME, Sarna L, Brown JK, et al. Challenges of recruitment and retention in multisite clinical research. Cancer Nurs 2003;26:376–386 [DOI] [PubMed] [Google Scholar]
- 4.Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials 1981;2:93–113 [DOI] [PubMed] [Google Scholar]
- 5.Bedlack RS, Traynor BJ, Cudkowicz ME. Emerging disease-modifying therapies for the treatment of motor neuron disease/amyotrophic lateral sclerosis. Expert Opin Emerg Drugs 2007;12:229–252 [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal SP, Zinman L, Simpson E, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cudkowicz M, Bozik ME, Ingersoll EW, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med 2011;17:1652–1656 [DOI] [PubMed] [Google Scholar]
- 8.Sherman A, Bowser R, Grasso D, et al. Proposed BioRepository platform solution for the ALS research community. Amyotroph Lateral Scler 2011;12:11–16 [DOI] [PubMed] [Google Scholar]
- 9.Cudkowicz M, Shefner J, Schoenfeld D, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol 2006;60:22–31 [DOI] [PubMed] [Google Scholar]
- 10.Cudkowicz ME, Shefner JM, Simpson E, et al. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve 2008;38:837–844 [DOI] [PubMed] [Google Scholar]
- 11.Ferrante KL, Shefner J, Zhang H, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology 2005;65:1834–1836 [DOI] [PubMed] [Google Scholar]
- 12.Dilts DM, Sandler AB, Baker M, et al. Processes to activate phase III clinical trials in a Cooperative Oncology Group: the case of Cancer and Leukemia Group B. J Clin Oncol 2006;24:4553–4557 [DOI] [PubMed] [Google Scholar]
- 13.Dilts DM, Sandler AB. Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol 2006;24:4545–4552 [DOI] [PubMed] [Google Scholar]
- 14.Dilts DM, Sandler A, Cheng S, et al. Development of clinical trials in a cooperative group setting: the Eastern Cooperative Oncology Group. Clin Cancer Res 2008;14:3427–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravina B, Deuel L, Siderowf A, Dorsey ER. Local institutional review board (IRB) review of a multicenter trial: local costs without local context. Ann Neurol 2010;67:258–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 2002;137:511–520 [DOI] [PubMed] [Google Scholar]
- 17.Wagner TH, Murray C, Goldberg J, Adler JM, Abrams J. Costs and benefits of the National Cancer Institute Central Institutional Review Board. J Clin Oncol 2010;28:662–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ 2003;22:151–185 [DOI] [PubMed] [Google Scholar]
- 19.Dolgin E. Trial networks move beyond single-disease strategies. Nat Med 2011;17:1525. [DOI] [PubMed] [Google Scholar]
- 20.Grill J, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer's disease clinical trials. Alzheimers Res Ther 2010;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edland SD, Emond JA, Aisen PS, Petersen RC. Nia-funded Alzheimer centers are more efficient than commercial clinical recruitment sites for conducting secondary prevention trials of dementia. Alzheimer Dis Assoc Disord 2010;24:159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macias FM, Ramsay RE, Rowan AJ. Recruitment and retention in clinical trials of the elderly. In: Ramsay RE, Cloyd JC, Kelly KM, Leppik IE, Perucca E, editors. International Review of Neurobiology. San Diego: Academic Press/Elsevier; 2007:265–272 [DOI] [PubMed] [Google Scholar]
- 23.Dorsey ER, Deuel LM, Voss TS, et al. Increasing access to specialty care: a pilot, randomized controlled trial of telemedicine for Parkinson's disease. Mov Disord 2010;25:1652–1659 [DOI] [PubMed] [Google Scholar]
- 24.DeKosky ST. Maintaining adherence and retention in dementia prevention trials. Neurology 2006;67(9 suppl 3):S14–S16 [DOI] [PubMed] [Google Scholar]
- 25.Sweetman E, Doig G. Failure to report protocol violations in clinical trials: a threat to internal validity? Trials 2011;12:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman L, Furberg C, DeMets D. Fundamentals of Clinical Trials. New York: Springer; 2010:251–268 [Google Scholar]
- 27.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23:1296–1310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


