Summary
The diagnosis and management of idiopathic normal-pressure hydrocephalus (iNPH), a disorder of gait impairment, incontinence, and dementia that affects elderly patients, incorporates an organized approach using familiar principles for neurologists. The starting point is a comprehensive history and neurologic examination, review of neuroimaging, and evaluation of the differential diagnosis. Coexisting disorders should be treated before specific iNPH testing is performed. Specific iNPH testing includes assessing patient response to temporary CSF removal and testing CSF hydrodynamics. In properly selected patients, all iNPH symptoms, including dementia, can improve after shunt surgery. The longitudinal care of iNPH patients with shunts includes evaluation of the differential diagnosis of worsening iNPH symptoms and treatment of coexisting disorders. Evaluation of shunt obstruction is often indicated, and if it is found, surgical correction is likely to result in symptomatic improvement.
Idiopathic normal-pressure hydrocephalus (iNPH) is a disorder of the elderly with symptoms of impaired gait and mobility, urinary urgency and incontinence, and mild cognitive impairment or dementia in the presence of ventriculomegaly. The clinical presentation alone is usually not sufficient to diagnose iNPH and recommend shunt surgery, as each of the primary iNPH symptoms can have multiple potential etiologies, and enlarged ventricles can be seen with either hydrocephalus or brain atrophy.
Organized approach to diagnosis and treatment
The goal of the evaluation of possible iNPH is to predict whether shunt surgery is likely to benefit the patient sufficiently to justify the risks of surgery and postoperative morbidities. Following is an organized approach to evaluation.
Clinical evaluation
Treatment of other disorders before undertaking specific testing for iNPH
Testing that is specific for prognosticating treatment response in iNPH
Shunt surgery
Longitudinal follow-up
Clinical evaluation
Key neurologic features
A comparison of key clinical findings for iNPH from the International and Japanese guidelines is presented in table 1.1–4 By definition, iNPH is idiopathic; however, the neurologic history should include known risk factors for communicating hydrocephalus, including meningitis, encephalitis, traumatic brain injury (including concussion), subarachnoid hemorrhage, and brain radiation. Enlarged head circumference is also a risk factor that may indicate a congenital component to the disorder.5 Patients with secondary communicating hydrocephalus should be evaluated for the need for shunt surgery in the manner outlined below, except in clinically obvious cases, such as the development of hydrocephalus during hospitalization for subarachnoid hemorrhage.
Table 1.
Key clinical features of idiopathic normal-pressure hydrocephalus: Comparison between the International and the Japanese guidelines
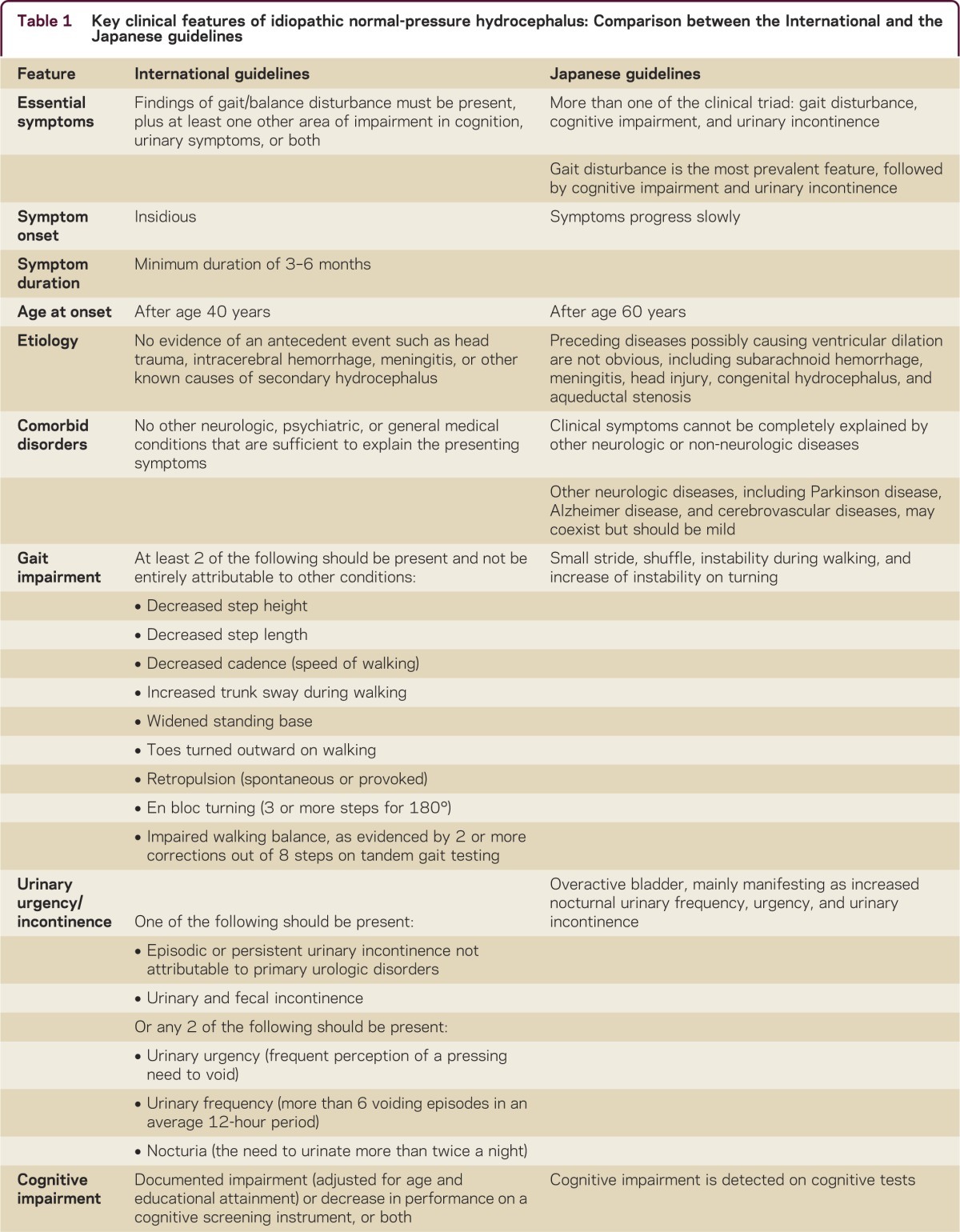
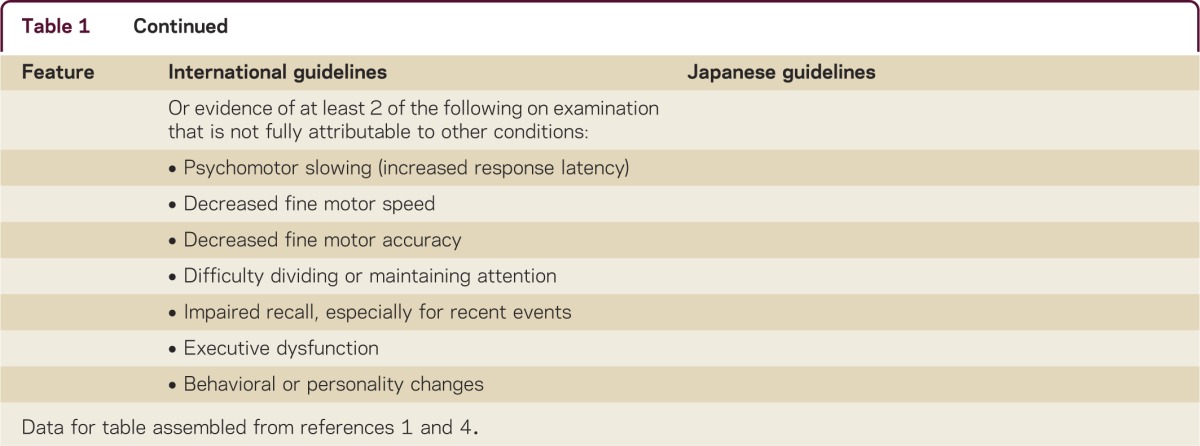
Symptom onset
The onset of iNPH symptoms is insidious and should have been evident for at least 6 months. Some patients and families are not aware of symptoms until a precipitating event occurs (e.g., a fall or a change in symptoms after a surgical procedure). Careful questioning can clarify the nature of symptom onset.
Gait
In iNPH, a higher-level gait disorder is seen with disturbed postural and locomotor reflexes in the absence of primary sensorimotor deficits.6 Findings include difficulty with transitional movements (sitting to standing or standing to sitting); gait initiation failure; poor foot clearance with shuffling, tripping, falling, or festination; multistep turns with instability; and retropulsion or anteropulsion of stance.7 The use of a standardized gait evaluation (e.g., the Tinetti score, Boon Scale, or the timed up-and-go test) can be helpful. Spasticity, hyperreflexia, and other upper motor neuron findings are not typical. Symptoms of iNPH are symmetric; therefore, lateralizing findings should increase suspicion of other disorders.
Bladder dysfunction
The bladder dysfunction of iNPH is usually urinary urgency with difficulty inhibiting bladder emptying.8 In the early stages, patients may experience urgency without incontinence or with loss of a few drops of urine before reaching the toilet. Nighttime urinary frequency is common. Patients are usually aware of their need to urinate and are concerned about their accidents. Incontinence without awareness of urinary urge or that one's clothes are wet is not characteristic of iNPH.
Dementia
The dementia of iNPH includes apathy or amotivation, daytime sleepiness, psychomotor slowing, and other features of frontal-subcortical dysfunction.9–11 Functional losses with dementia in iNPH overlap with those of other dementias, including difficulty managing finances, taking medications properly, driving, and keeping track of appointments. Impaired expressive or receptive language, impaired naming, agnosia, poor immediate recall that does not benefit from cueing, hallucinations, and failure to recognize close family or friends should raise concern for other causes of dementia. Delirium implies the presence of a concomitant disorder or medication side effect.
Neuroimaging
The terms hydrocephalus and ventriculomegaly are not synonymous. Although all patients with iNPH should have enlarged ventricles, not all elderly patients with enlarged ventricles have iNPH. In neurodegenerative disorders, cerebral atrophy results in ventriculomegaly (so-called hydrocephalus ex vacuo). Neuroimaging can be used to raise or lower suspicion of iNPH; however, it can rarely exclude it entirely. A comparison of key neuroimaging findings from the International and Japanese guidelines is presented in table 2.1–4
Table 2.
Key imaging and CSF pressure features of idiopathic normal-pressure hydrocephalus: Comparison between the International and the Japanese guidelines
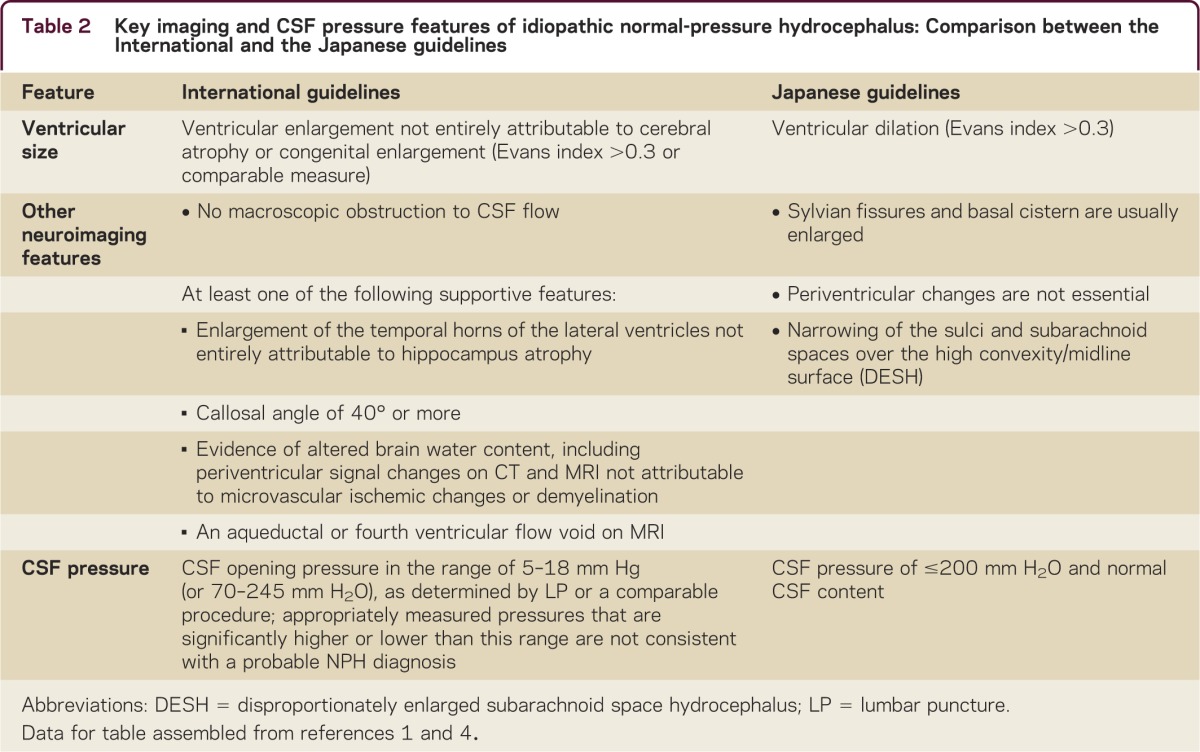
The distinction between normal and enlarged ventricular size for age is difficult to ascertain; however, for screening purposes, the Evans ratio (the ratio of the widest diameter of the frontal horns to the widest diameter of the brain on the same axial slice) suffices (figure). The International and Japanese guidelines use a threshold of ≥0.3, but research on normal elderly subjects suggests a threshold of ≥0.33.1–4,12
Figure. MRI of a 73-year-old woman with impairment of gait and balance, bladder control, and cognition for 3 years.
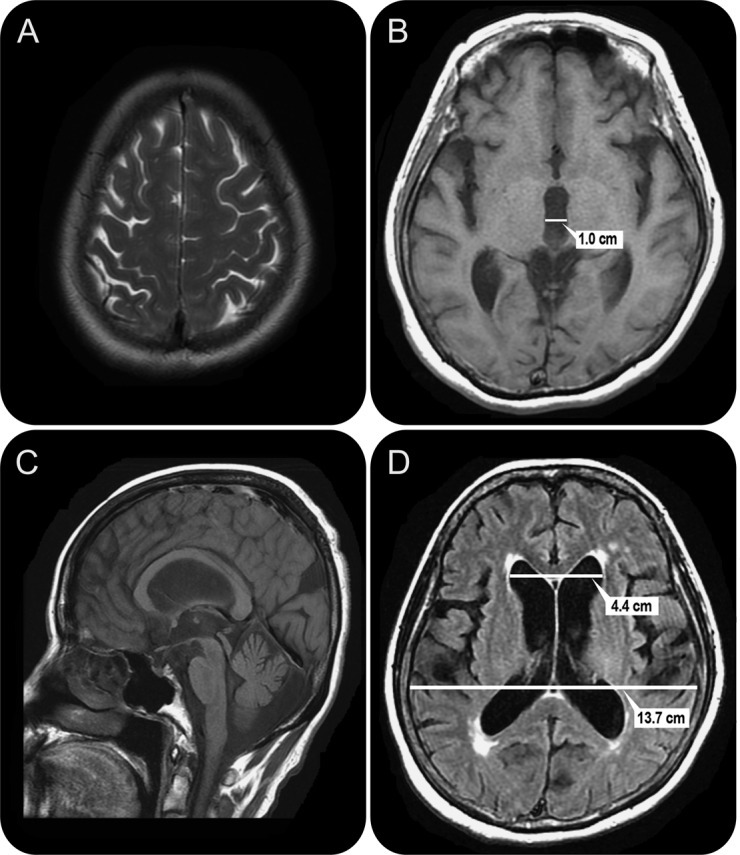
The patient had not improved with treatment for parkinsonism. A trial of CSF removal via external lumbar drainage produced substantial gait improvement, and she was treated with a ventriculoperitoneal shunt. At baseline, the Tinetti score was 12–16/28, which is significantly impaired; 9 months after shunt surgery, the score was 26/28, and the patient walked effortlessly. (A) Axial T2 imaging consistent with the Japanese “high and tight” criteria for the convexity. The interhemispheric fissure is effaced. (B) Axial T1 imaging shows a widened IIIrd ventricle with a span of 10 mm. (C) Sagittal T1 imaging shows bowing of the corpus callosum and a pulsation artifact (flow void) in the Sylvian aqueduct. (D) Axial fluid-attenuated inversion recovery imaging shows measurement of the Evans ratio. The diameter of the frontal horns is 4.4 cm, the widest brain diameter is 13.7 cm, and the Evans ratio is 0.32.
MRI is considered superior to CT in terms of providing more information on diagnostic relevance and avoiding exposure to ionizing radiation. High-speed and high-resolution MRI techniques can better identify aqueductal stenosis, and MRI phase-contrast techniques show the hyperdynamic aqueductal CSF flow that has been associated with shunt-responsive iNPH.
Features that distinguish hydrocephalus from atrophy
The Japanese guidelines for iNPH4 identify several features that distinguish iNPH from atrophy, including disproportionately enlarged subarachnoid spaces (particularly in the Sylvian fissure and basal cisterns) and the “tight high convexity,” which is effacement of the subarachnoid space over the convexity.13,14 These findings may suggest a block of CSF flow between the basal cisterns and the arachnoid granulations, a condition that has been termed disproportionately enlarged subarachnoid space hydrocephalus (DESH). By implication, the absence of DESH on brain imaging is suggestive of brain atrophy, but it does not exclude the possibility of iNPH.4,14
Enlargement of the ventricles from iNPH will change the shape of the corpus callosum, with bowing and effacement seen on sagittal views (figure),15 and impingement against the falx cerebri, resulting in an acute callosal angle (≤90°) seen on coronal views.
Cisternography
Radionuclide cisternography has a high false-positive rate, and the International and Japanese guidelines recommend against it.1–4
Differential diagnosis
Each of the primary symptoms of iNPH has multiple potential etiologies (table 3). Most patients with iNPH have other conditions contributing to their symptoms, and it is uncommon to see “pure” iNPH. Further, patients without iNPH may appear to have the iNPH syndrome because of multiple comorbidities. The first step is to identify or exclude other disorders that should be treated before evaluating iNPH.
Table 3.
Differential diagnosis of idiopathic normal-pressure hydrocephalus (iNPH)
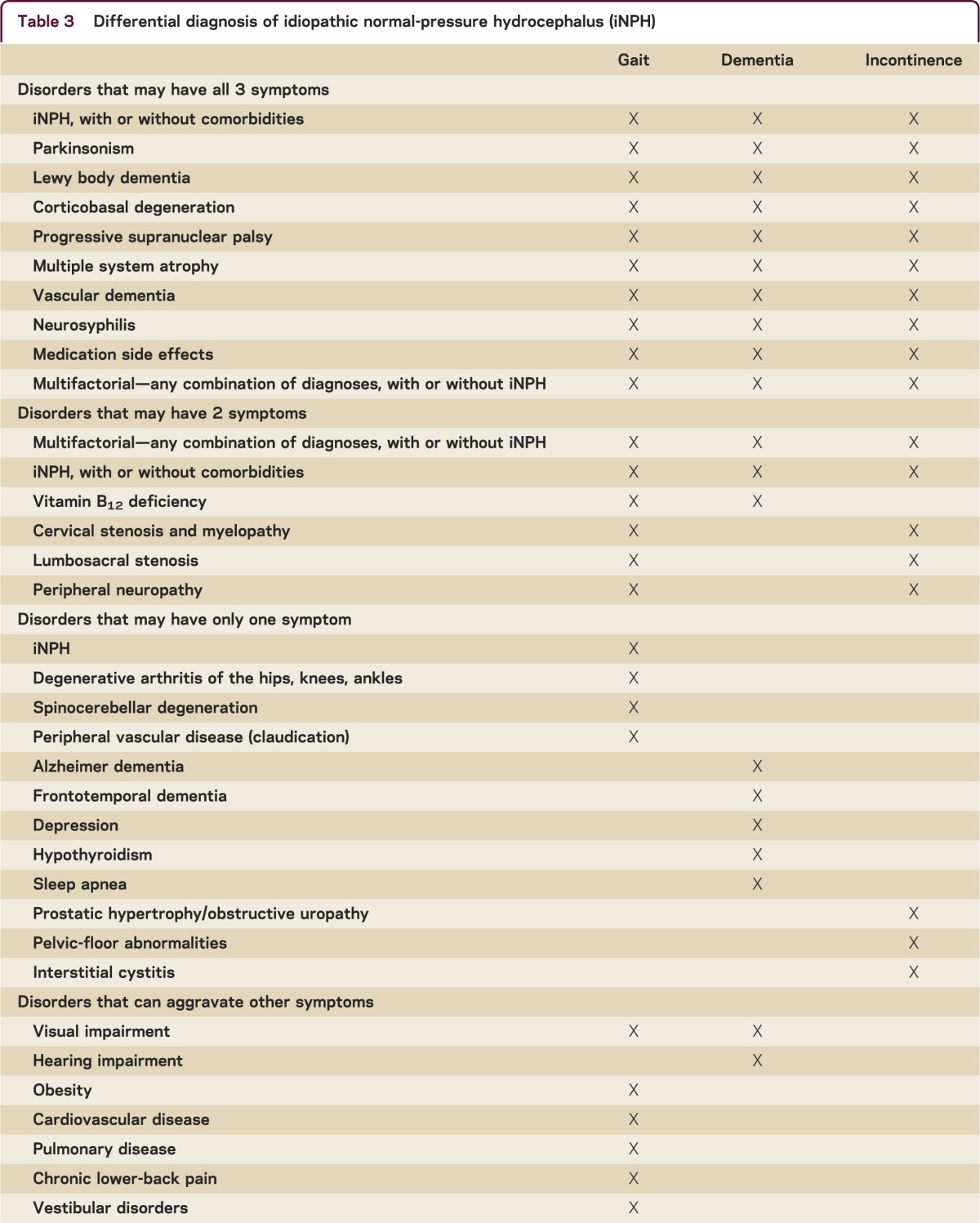
Although iNPH is described as a symptom “triad,” patients need not have all 3 symptoms. However, most published series and guidelines indicate that nearly all patients have gait impairment. A patient who has only dementia or incontinence should first be evaluated for other disorders. Patients with gait impairment and urinary symptoms but no cognitive impairment may need evaluation for spinal cord disorders. Although any of the primary iNPH symptoms may be the initial symptom, gait impairment is usually either the first or worst symptom.
The tests ordered to evaluate the differential diagnosis include the so-called “dementia bloodwork” (complete blood count, biochemical profile, B12, folate, thyroid-stimulating hormone, and when indicated, rapid plasma reagin, Lyme, vitamin D); neuropsychological testing; MRI of the cervical, thoracic, or lumbar spine; EMG/nerve conduction velocity; and urology consultation.
Treatment of other disorders
Several rationales support excluding or treating other disorders before pursuing iNPH testing. First, no evidence suggests that the time required to identify and treat other disorders diminishes the likelihood of response to shunt surgery. Patients who have had iNPH symptoms for several years can still respond to shunt surgery. Patients can be reassured that it is more beneficial to undertake a thorough evaluation than it is to rush. Second, should the patient's symptoms resolve with treatment of other disorders, then testing for iNPH may no longer be necessary. Finally, initiating treatment of an underlying disorder while testing for iNPH makes it difficult to determine whether the patient's response to CSF removal is a result of 1) the removal of CSF or 2) the treatment of the underlying disorder. Any trials of initiating treatment (e.g., carbidopa/levodopa) or withdrawing treatment (e.g., benzodiazepines or neuroleptics) should be completed or at a stable dosage before tests for iNPH are initiated. If delirium is present, testing for iNPH should be deferred because resolving delirium can create a false-positive response to CSF removal, and a persistent delirium can cause a false-negative response.
Testing specific for iNPH
The International and Japanese guidelines recommend tests of CSF drainage (lumbar puncture [LP] or CSF drainage via spinal catheter); however, the International guidelines also recommend infusion testing.1–4
The rationale for testing a patient's response to CSF drainage is that doing so emulates the physiologic effect of a shunt. If iNPH is present, a response to CSF removal should be seen and shunt surgery should help. However, if iNPH is absent or contributes only minimally, no response to CSF removal is seen and shunt surgery is unlikely to help.
If properly performed, the large-volume LP (also known as “the tap test”) has a high positive predictive value. If improperly done, it can be inaccurate. Proper tap test performance hinges on 2 principles. First, because the test is used to assess the patient's response to CSF removal, the patient must be examined before and after the LP. Gait is most likely to respond, and use of a standardized evaluation of gait, with or without video recording or computer-assisted assessment, is helpful. The evaluation should be done by qualified health care professionals.16 Without pre- and post-LP examinations, the physician must rely on patient and family reporting, which is less accurate and may be influenced by a desire to see improvement. Second, the volume of CSF removed must be adequate to influence NPH symptoms long enough to assess the change. Typical tap test protocols remove 30–50 mL of CSF and observe change in gait and cognition 30 minutes to 4 hours afterward. Although it is standard practice that patients lie flat for 10–30 minutes after the LP, some experts believe the sensitivity of the test is increased by having the patient remain upright and engage in modest activity as tolerated. Although the safety of this approach has not been rigorously evaluated, headache and nausea after LP are uncommon in the elderly iNPH population.
Although the interval between the LP and the follow-up examination can be as little as 30 minutes, observation over several hours is often needed, as the response is variable in latency and only rarely persists for more than a few days. If the response is considerable, shunt surgery can be recommended. Absence of response to the tap test does not exclude shunt responsiveness, and external lumbar drainage (ELD) can be considered if iNPH is still clinically suspected.
ELD has been used for more than 20 years to diagnose iNPH, but it is not widely available.17,18 The procedure requires a brief hospitalization to insert a 16-gauge spinal catheter for controlled CSF drainage of approximately 10 mL/h. The patient's gait should be examined before the procedure, daily during CSF drainage, and after removal of the catheter. Gait testing is standard and neuropsychological testing before and after ELD may also be helpful. Most publications have cited 72 hours of CSF drainage, although some centers drain CSF for shorter periods. ELD is said to be accurate, with both a high positive predictive value and a high negative predictive value. The most frequent serious complication of ELD is bacterial meningitis, seen in 2%–3% of patients.17,18
Infusion testing for assessment of hydrodynamics and Rout is more commonly used in Europe. This test involves infusing artificial CSF (usually Ringer lactate) via one spinal needle while simultaneously recording CSF pressure via a second spinal needle.19 Several variables that characterize intracranial compliance can be calculated, including Rout and CSF conductance. Several methods for infusion testing exist, and the value of Rout is method-dependent.19 Reference values for healthy elderly exist.20 Infusion testing requires specialized equipment and considerable expertise on the part of the physicians who perform it.
Shunt surgery
Shunt surgery is indicated for patients who respond to CSF drainage or who have CSF hydrodynamic variables consistent with iNPH. The International and Japanese guidelines support shunt surgery.1–4 Evidence does not yet support the use of one type of shunt over another. Endoscopic third ventriculostomy has not proven effective in treatment of iNPH. As of 2013, no medical treatments are effective in iNPH.4
The goal of treating with a shunt is to improve the patient's symptoms while avoiding complications of overdrainage, such as subdural effusion or hematoma. The International and Japanese guidelines find that adjustable shunt valves offer the advantage of being able to gradually lower the pressure setting until symptom improvement and to raise the pressure setting if low-pressure symptoms or complications emerge.1–4 Severe complications, such as subdural hematoma with significant mass effect, shunt infection, and shunt obstruction, typically require neurosurgical intervention. Adjustable shunts can be used to safely manage iNPH patients who need chronic anticoagulation.21
The choice of shunt valve and configuration (e.g., ventriculoperitoneal, ventriculoatrial, lumboperitoneal) depends on the neurosurgeon's recommendation and the patient's preference. When compared to other intracranial procedures, the risk of shunt surgery is relatively low. When these risks are placed in the context of the benefits expected on the basis of specific iNPH testing results, most patients will elect to proceed with shunt surgery.
Longitudinal follow-up
Neurologists have not traditionally been involved in the longitudinal management of patients with iNPH after shunt surgery. Many patients find that neurosurgeons will see them only if they have a shunt complication that requires surgery, and most neurologists are not trained to manage patients after shunt surgery.
All symptoms in iNPH can improve after shunt surgery. The robust improvement in dementia is not widely appreciated, although it has been shown in multiple studies.9–11 Cognitive improvement has been shown not to be the result of the practice effect in properly structured batteries.22
The principle of differential diagnosis is key to the management of patients after shunt surgery. If a patient worsens after initial improvement, the question that most frequently arises is whether the cause is shunt malfunction or concomitant disorders. Typical scenarios are lagging symptom recovery, transient worsening, and insidious worsening.
Some patients recover from only 1 or 2 symptoms after shunt surgery, while 1 symptom lags behind. In most circumstances, another disorder is responsible for the lagging symptom and should be investigated further.
After initial improvement, some patients will have transient worsening of their iNPH symptoms in association with another illness (e.g., urinary tract infection) or with hospitalization or surgery. This phenomenon is similar to the transient worsening of latent symptoms seen in many neurologic disorders when patients experience other illnesses. Often, iNPH symptoms will improve after the underlying illness is identified and treated.
Insidious worsening of symptoms over weeks or months may result from shunt malfunction or the emergence or worsening of a comorbidity. In this circumstance, evaluation of shunt malfunction is indicated. The evaluation of shunt malfunction is straightforward. Disconnection of the shunt components is easily detected by plain x-rays. Depression of the shunt reservoir to assess the rate of refilling is not helpful in distal shunt obstruction.
Radionuclide shunt patency study and CSF infusion testing can be used to assess shunt function.4,23,24 Both of these techniques involve procedural skills for which most neurologists are not trained, and they will need to collaborate with neurosurgeons. If shunt obstruction is detected and treated, approximately 75% of patients will improve.23
The diagnosis and management of iNPH can be accomplished through an organized approach that incorporates familiar principles, including differential diagnosis; treatment of other disorders before specific testing for iNPH; use of specific iNPH tests; and, for patients treated with shunt surgery who have subsequent return of iNPH symptoms, the application of the differential diagnosis process to the worsening symptoms.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
M. Williams has received speaker honoraria from the American Academy of Neurology; is the Associate Editor for Ethics for Continuum; is the President of the International Society for Hydrocephalus and CSF Disorders; is an author on patents re: Shunts and Self-sealing catheter for deformable tissue; performs lumbar punctures and external lumbar drainage as part of his practice at the Adult Hydrocephalus Program at Sinai Hospital of Baltimore (60% clinical effort); receives research support from the National Institute of Neurological Disorders and Stroke, NeuroDx Development, and the national Space Biomedical Research Institute; owns a 5% interest in Mensana Therapeutics, a start-up with intellectual property that is related to CSF shunting for the treatment of Alzheimer dementia; and his spouse owns stock in Ecolab, General Electric, Life Technologies, Medtronic, and Pfizer. N. Relkin is Associate Editor of Neurology Alert; performs NPH-related research, brain imaging, and clinical practice at Weill Cornell Memory Disorders Program (25% of effort); has received research support from the Leon Levy Foundation, O'Neill Foundation, NY Community Trust, the National Institute on Aging, the US Department of Defense, Eisai, Merck, and Baxter Healthcare; serves as a consultant to Eisai and Kyowa Kakko Kirin; is head of the Taskforce on Neuroimaging of Hydrocephalus for the International Society for Hydrocephalus and CSF Disorders; and is inventor on a patent application for use of diffusion-tensor-histogram analysis in the differential diagnosis of NPH. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus: INPH Guidelines, part II. Neurosurgery 2005;57:S4–S16 [DOI] [PubMed] [Google Scholar]
- 2.Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus: INPH Guidelines, part III. Neurosurgery 2005;57:S17–S28 [DOI] [PubMed] [Google Scholar]
- 3.Bergsneider M, Black PM, Klinge P, Marmarou A, Relkin N. Surgical management of idiopathic normal-pressure hydrocephalus: INPH Guidelines, part IV. Neurosurgery 2005;57:S29–S39 [DOI] [PubMed] [Google Scholar]
- 4.Mori E, Ishikawa M, Kato T, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir 2012;52:775–809 [DOI] [PubMed] [Google Scholar]
- 5.Wilson RK, Williams MA. Evidence that congenital hydrocephalus is a precursor to idiopathic normal pressure hydrocephalus (INPH) in only a subset of patients. J Neurol Neurosurg Psychiatry 2007;78:508–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology 1993;43:268–279 [DOI] [PubMed] [Google Scholar]
- 7.Stolze H, Kuhtz-Buschbeck JP, Drucke H, et al. Gait analysis in idiopathic normal pressure hydrocephalus: which parameters respond to the CSF tap test? Clin Neurophysiol 2000;111:1678–1686 [DOI] [PubMed] [Google Scholar]
- 8.Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn 2008;27:507–510 [DOI] [PubMed] [Google Scholar]
- 9.Thomas G, McGirt MJ, Woodworth G, et al. Baseline neuropsychological profile and cognitive response to CSF shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 2005;20:163–168 [DOI] [PubMed] [Google Scholar]
- 10.Katzen H, Ravdin LD, Assuras S, et al. Post shunt cognitive and functional improvement in idiopathic normal pressure hydrocephalus. Neurosurgery 2011;68:416–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellström P, Klinge P, Tans J, Wikkelsø C. The neuropsychology of iNPH: findings and evaluation of tests in the European multicentre study. Clin Neurol Neurosurg 2012;114:130–134 [DOI] [PubMed] [Google Scholar]
- 12.Ambarki K, Israelsson H, Wåhlin A, Birgander R, Eklund A, Malm J. Brain ventricular size in healthy elderly: comparison between Evans index and volume measurement. Neurosurgery 2010;67:94–99 [DOI] [PubMed] [Google Scholar]
- 13.Kitagaki H, Mori E, Ishii K, Yamaji S, Hirono N, Imamura T. CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR Am J Neuroradiol 1998;19:1277–1284 [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto M, Ishikawa M, Mori E, Kuwana N. Study of INPH on neurological improvement (SINPHONI): diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D. Diagnosis, treatment and analysis of long-term outcomes in idiopathic normal pressure hydrocephalus. Neurosurgery 2005;57:699–705 [DOI] [PubMed] [Google Scholar]
- 16.Williams MA, Thomas G, de Lateur BJ, et al. Objective assessment of gait in normal-pressure hydrocephalus. Am J Phys Med Rehabil 2008;87:39–45 [DOI] [PubMed] [Google Scholar]
- 17.Greenberg BM, Williams MA. Infectious complications of temporary spinal catheter insertion for diagnosis of adult hydrocephalus and idiopathic intracranial hypertension. Neurosurgery 2008;62:431–436 [DOI] [PubMed] [Google Scholar]
- 18.Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg 2005;102:987–997 [DOI] [PubMed] [Google Scholar]
- 19.Eklund A, Smielewski P, Chambers I, et al. Assessment of cerebrospinal fluid outflow resistance. Med Biol Eng Comput 2007;45:719–735 [DOI] [PubMed] [Google Scholar]
- 20.Malm J, Jacobsson J, Birgander R, Eklund A. Reference values for CSF outflow resistance and intracranial pressure in healthy elderly. Neurology 2011;76;903–909 [DOI] [PubMed] [Google Scholar]
- 21.Goodwin CR, Kharkar S, Wang P, Pujari S, Rigamonti D, Williams MA. Evaluation and treatment of patients with suspected normal pressure hydrocephalus on long term warfarin anticoagulation therapy. Neurosurgery 2007;60:497–501 [DOI] [PubMed] [Google Scholar]
- 22.Solana E, Poca MA, Sahuquillo J, Benejam B, Junqué C, Dronavalli M. Cognitive and motor improvement after retesting in normal-pressure hydrocephalus: a real change or merely a learning effect? J Neurosurg 2010;112:399–409 [DOI] [PubMed] [Google Scholar]
- 23.Kharkar S, Shuck J, Kapoor S, Batra S, Williams MA, Rigamonti D. Radionuclide shunt patency study for evaluation of suspected ventriculo-peritoneal shunt malfunction in adults with normal pressure hydrocephalus. Neurosurgery 2009;64:909–918 [DOI] [PubMed] [Google Scholar]
- 24.Lundkvist B, Koskinen LO, Birgander R, Eklund A, Malm J. Cerebrospinal fluid dynamics and long-term survival of the Strata valve in idiopathic normal pressure hydrocephalus. Acta Neurol Scand 2011;124:115–121 [DOI] [PubMed] [Google Scholar]


