Abstract
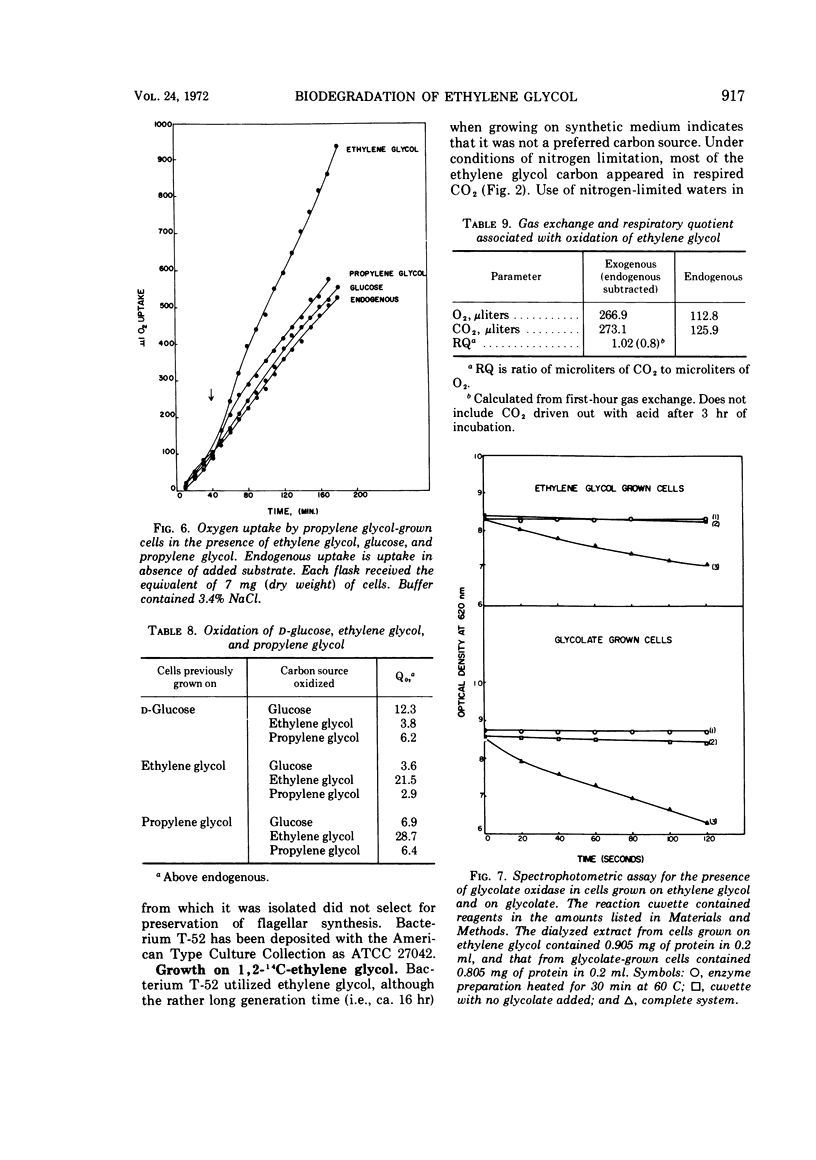
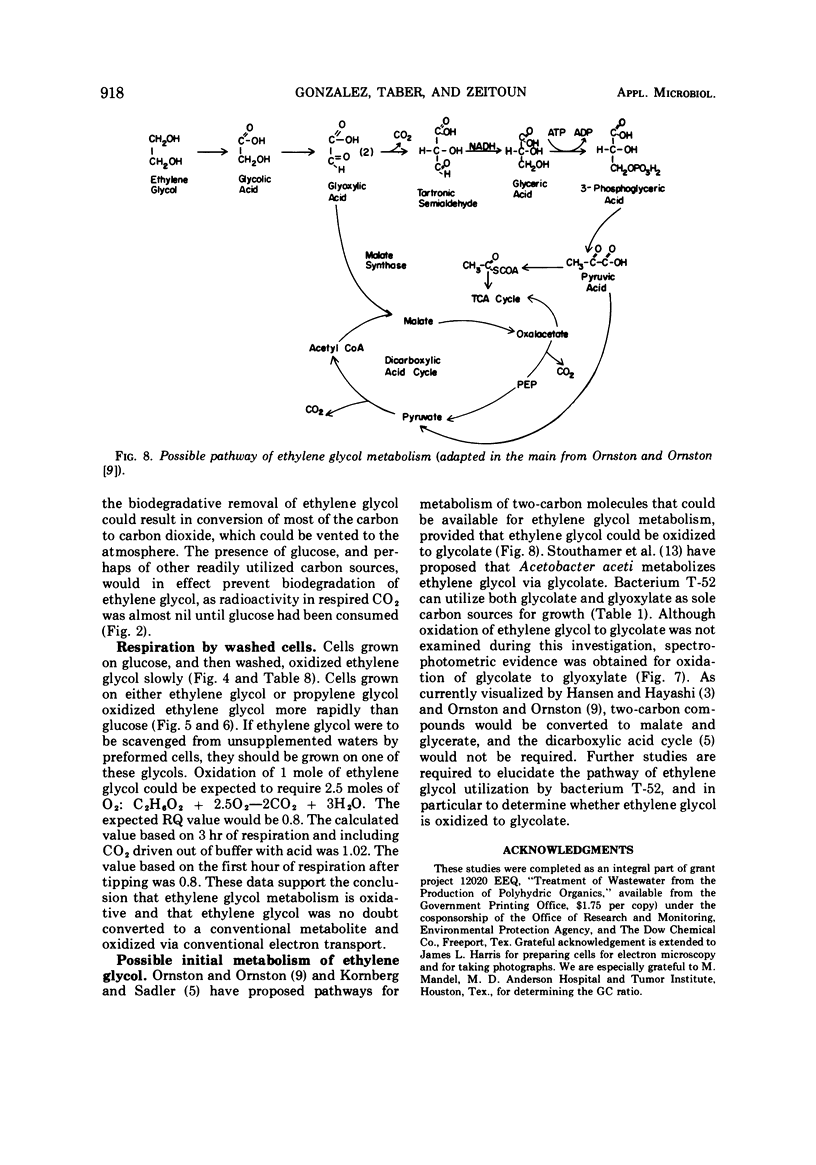
A gram-negative nonmotile rod which was capable of using 1,2-14C-ethylene glycol as a sole carbon source for growth was isolated from a brine pond, Great Salt Lake, Utah. The bacterium (ATCC 27042) required at least 0.85% NaCl for growth and, although the chloride ion was replaceable by sulfate ion, the sodium ion was not replaceable by potassium ion. The maximal concentration of salt tolerated for growth was approximately 12%. The bacterium was oxidase-negative when N,N-dimethyl-p-phenylenediamine was used and weakly positive when N,N,N′,N′-tetramethyl-p-phenylenediamine was used. It grows on many sugars but does not ferment them, it does not have an exogenous vitamin requirement, and it possesses a guanine plus cytosine ratio of 64.3%. Incorporation of ethylene glycol carbon into cell and respired CO2 was quantitated by use of radioactive ethylene glycol and a force-aerated fermentor. Glucose suppressed ethylene glycol metabolism. Cells grown on ethylene and propylene glycol respired ethylene glycol in a Warburg respirometer more rapidly than cells grown on glucose. Spectrophotometric evidence was obtained for oxidation of glycolate to glyoxylate by a dialyzed cell extract.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann L., Baumann P., Mandel M., Allen R. D. Taxonomy of aerobic marine eubacteria. J Bacteriol. 1972 Apr;110(1):402–429. doi: 10.1128/jb.110.1.402-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUYA A., HAYASHI J. A. GLYCOLIC ACID OXIDATION BY ESCHERICHIA COLI ADAPTED TO GLYCOLATE. J Bacteriol. 1963 May;85:1124–1131. doi: 10.1128/jb.85.5.1124-1131.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSEN R. W., HAYASHI J. A. Glycolate metabolism in Escherichia coli. J Bacteriol. 1962 Mar;83:679–687. doi: 10.1128/jb.83.3.679-687.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., SADLER J. R. The metabolism of C2-compounds in micro-organisms. VIII. A dicarboxylic acid cycle as a route for the oxidation of glycollate by Escherichia coli. Biochem J. 1961 Dec;81:503–513. doi: 10.1042/bj0810503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACS N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956 Sep 29;178(4535):703–703. doi: 10.1038/178703a0. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K. Regulation of glyoxylate metabolism in Escherichia coli K-12. J Bacteriol. 1969 Jun;98(3):1098–1108. doi: 10.1128/jb.98.3.1098-1108.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES M. E. The cytology of Pseudomonas spp. as revealed by a silver-plating staining method. J Gen Microbiol. 1958 Jun;18(3):639–648. doi: 10.1099/00221287-18-3-639. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STOUTHAMER A. H., VAN BOOMJ, BASTIAANSE A. J. METABOLISM OF C2 COMPOUNDS IN ACETOBACTER ACETI. Antonie Van Leeuwenhoek. 1963;29:393–406. doi: 10.1007/BF02046092. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- TABER W. A., VINING L. C. The influence of certain factors on the in vitro production of ergot alkaloids by Claviceps purpurea (Fr.) Tul. Can J Microbiol. 1958 Dec;4(6):611–626. doi: 10.1139/m58-067. [DOI] [PubMed] [Google Scholar]
- Taber W. A. Simultaneous assimilation and respiration of exogenous 2, 3-14C-succinate and exogenous glucose by ergot alkaloid producing cultures of Claviceps purpurea. Mycologia. 1968 Mar-Apr;60(2):345–355. [PubMed] [Google Scholar]
- ZELITCH I., OCHOA S. Oxidation and reduction of glycolic and glyoxylic acids in plants. I. Glycolic and oxidase. J Biol Chem. 1953 Apr;201(2):707–718. [PubMed] [Google Scholar]


