Abstract
Objective. To utilize a comprehensive, pharmacist-led warfarin pharmacogenetics service to provide pharmacy students, residents, and fellows with clinical and research experiences involving genotype-guided therapy.
Design. First-year (P1) through fourth-year (P4) pharmacy students, pharmacy residents, and pharmacy fellows participated in a newly implemented warfarin pharmacogenetics service in a hospital setting. Students, residents, and fellows provided genotype-guided dosing recommendations as part of clinical care, or analyzed samples and data collected from patients on the service for research purposes.
Assessment. Students’, residents’, and fellows’ achievement of learning objectives was assessed using a checklist based on established core competencies in pharmacogenetics. The mean competency score of the students, residents, and fellows who completed a clinical and/or research experience with the service was 97% ±3%.
Conclusion. A comprehensive warfarin pharmacogenetics service provided unique experiential and research opportunities for pharmacy students, residents, and fellows and sufficiently addressed a number of core competencies in pharmacogenetics.
Keywords: pharmacogenetics, warfarin, pharmacy service, research
INTRODUCTION
Pharmacogenetics was recognized as an important component of the doctor of pharmacy (PharmD) curriculum with the 2007 revision of the standards and guidelines for the professional program of pharmacy by the Accreditation Council for Pharmacy Education (ACPE).1 Curricular content considered essential includes the genetic basis for drug action, alteration of drug metabolism, and individualizing drug doses. The report from the 2007-2008 American Association of Colleges of Pharmacy (AACP) Argus Commission identified advances in personalized medicine as one of the top challenges of academic pharmacy and posed that the pharmacy curricula must adequately address issues related to the topic.2
While pharmacogenetic content is included in the PharmD curriculum of most colleges, whether this content is sufficient to prepare pharmacy graduates to manage personalized therapy is questionable.3,4 The majority of pharmacists agree that pharmacists should be capable of applying pharmacogenetic data to drug therapy decisions; however, few feel prepared to do so.5 An intensive educational effort would probably be necessary to improve pharmacists’ knowledge and comfort level with pharmacogenetics, and a continuing education program alone, without hands-on experience probably would be insufficient.6 Similarly, some pharmacy students feel that exercises involving the direct application of genetic data to drug therapy would increase their comfort level with interpreting and applying pharmacogenetic test results.4
Participation on a clinical pharmacogenetics service would provide pharmacy students, residents, and fellows with firsthand experience with interpreting and applying genetic information to drug management. In addition, a pharmacogenetics service could serve as a platform for unique research opportunities. At the University of Illinois at Chicago College of Pharmacy, we used a novel and comprehensive warfarin pharmacogenetics service to provide pharmacy students, residents, and fellows with unique practical experiences and research opportunities involving genotype-guided therapy. We examined the extent to which students, residents, and fellows participating in pharmacogenetic service activities met proposed competencies in pharmacogenetics.
DESIGN
The Warfarin Pharmacogenetics Service
Beginning in August 2012, genotype-guided warfarin dosing became the standard of care for all patients newly starting warfarin during hospitalization at the University of Illinois Hospital & Health Sciences System (UI-Health). Clinical decision support tools were built into the electronic health record (EHR) to trigger an automatic order for genetic testing in response to a new warfarin order for a hospitalized patient without a recent (<6 months) history of warfarin use. Each genotype order was accompanied by a consultation with the pharmacogenetics service, which was jointly staffed by faculty members from the University of Illinois at Chicago Colleges of Pharmacy and Medicine. The pharmacist on the service was responsible for screening each order for appropriateness and canceling orders deemed unnecessary (eg, prior warfarin use from an outside facility at the time of admission, genotype results already available in the EHR) or inappropriate (eg, history of liver transplantation, active cancer where low molecular weight heparin is preferred). Genotype was determined in the university’s molecular pathology laboratory, with results made available to the service/health care team prior to the second warfarin dose. The genotypes tested include vitamin K epoxide reductase complex 1 (VKORC1) c.-1639G>A and cytochrome P450 2C9 (CYP2C9) *2, *3, *5, *6, and *11. The VKORC1 enzyme is the target protein of warfarin, while CYP2C9 metabolizes the more potent S-enantiomer of warfarin. The pharmacist on the pharmacogenetics service provided a genotype-guided warfarin dose recommendation, using algorithms by Gage and colleagues7 or the International Warfarin Pharmacogenetics Consortium8 as a guide. Both algorithms are freely available through the http://www.warfarindosing.org Web site. In the event that a patient had the CYP2C9*11 genotype, which is not included in current dosing algorithms, the dose recommended by the algorithm was reduced by approximately 30%.9 The pharmacist provided a daily dose recommendation, refined based on international normalized ratio (INR) response to previous warfarin doses, for the initial 7 days of warfarin therapy or until the patient was discharged (whichever came first).
Pharmacists also led the research efforts involving the service. Most patients served at UI-Health were of African descent or Hispanic ethnicity for whom limited data on warfarin pharmacogenetics are available. Existing pharmacogenetic dosing algorithms perform less well in African Americans compared to Europeans.10,11 This is likely because these algorithms do not include variants with effects on dose requirements that occur specifically in persons of African descent. Research by pharmacists on the warfarin genetics service was aimed at identifying unique genetic associations with warfarin dose requirements in African Americans and Hispanics in order to optimize warfarin pharmacogenetics for these populations.12-16
Training Opportunities for Students and Residents
The warfarin pharmacogenetics service was first offered as a clinical and research training opportunity for pharmacy students, residents, and fellows in August 2012. Students in their P1 through P3 years could enroll in an elective independent study course to obtain credit for their work with the service, which provided between 1 and 3 hours of credit depending on the time committed. Students in their P4 year could either complete an elective clerkship practice experience on the service, providing the opportunity to work with the service on a full-time basis, or spend 1 or more mornings per week with the service as part of their anticoagulation clerkship experience. Residents could complete a month-long clinical rotation on the service, working under the direct supervision of a pharmacy faculty member. In the absence of a resident, a residency-trained pharmacy fellow was involved in the day-to-day operations (in the same capacity as the resident), also working under the direct supervision of pharmacy faculty members.
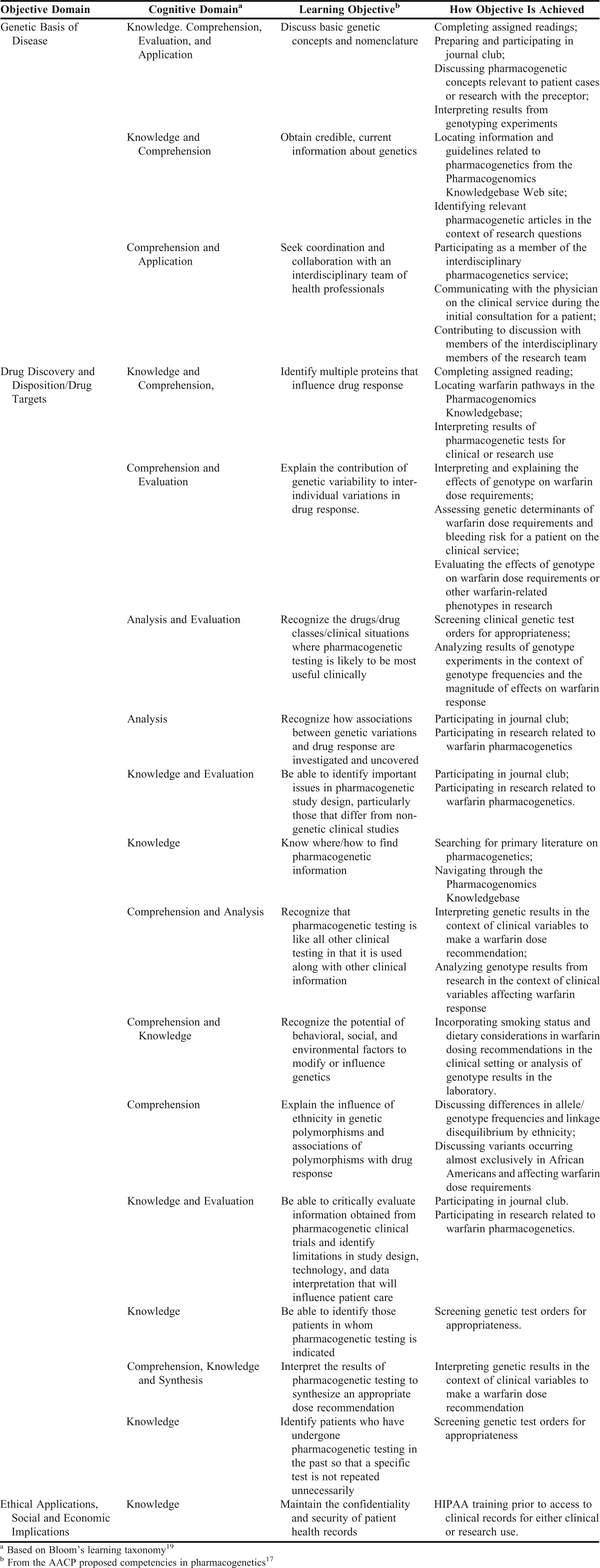
Learning objectives for students, residents, and fellows from either a clinical or research standpoint are listed in Appendix 1 according to objective domain, Bloom’s learning taxonomy, and objective achievement.17-19 The objectives were based on proposed core competencies in pharmacogenetics for pharmacists proposed by AACP and derived in part from core competencies in genetics for health-care professionals proposed by the National Coalition for Health Professional Education in Genetics.20 Twenty learning objectives were developed that covered 3 domains: genetic basis of disease; drug discovery and disposition/drug targets; and ethical applications, social and economic implications.20
Prior to working with the service from either a clinical or research perspective, students, residents, and fellows were assigned background reading material on pharmacogenetics in general and warfarin pharmacogenetics in particular.21-23 Because an understanding of warfarin pharmacology was essential, P1 and P2 students who had not yet completed therapeutics coursework were assigned additional reading on anticoagulation management.24,25 Additional optional reading on warfarin pharmacogenetic dosing algorithms and genotype-guided warfarin dosing in minority populations was recommended.7,8,26 The student, resident, or fellow and the faculty member spent time discussing the readings prior to applying them to patient care or research. Further, because most of the patients at the medical center were of minority descent, the faculty member devoted significant time to discussing the effect of ancestry on warfarin genetics, emphasizing the concepts of allele and genotype frequencies, haplotypes, and linkage disequilibrium. Through these discussions, ethnic differences in genetic architecture and the importance of considering ethnicity in pharmacogenetics were covered. Students and residents were also introduced to the Pharmacogenomics Knowledgebase (PharmGKB, www.pharmgkb.org) and taught how to navigate through the Web site to examine warfarin pathways, access the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for warfarin and other drugs, and review important publications related to warfarin pharmacogenetics.27 Through this activity, the students, residents, or fellows were exposed to an important centralized pharmacogenetic resource that could be used to stay abreast of important pharmacogenetic discoveries and the release of additional CPIC guidelines.
Students, residents, and fellows received access to the EHR once they completed required training on the use and protection of medical information and signed a confidentiality agreement regarding use and disclosure of medical data. Under a faculty member’s tutelage and supervision, the students, residents, or fellows involved in clinical activities were taught how to assess genetic test orders for appropriateness, how to interact with the physician on the service to discuss new patients, and how to interpret genetic test results in the context of clinical factors to provide appropriate warfarin dose recommendations. The students, residents, and fellows received instruction on using available warfarin pharmacogenetic dosing algorithms, namely the algorithms available by Gage and colleagues7 and the International Warfarin Pharmacogenetics Consortium.8 By entering both clinical and genetic data into the algorithm, the student, resident, or fellow gained perspective on how factors interact to influence warfarin dosing. There were additional interacting medications (eg, metronidazole), diseases (eg, renal dysfunction), and genotypes (eg, CYP2C9*11) that are not accounted for in available dosing algorithms. Thus, the students, residents, and fellows were exposed to the limitations of pharmacogenetic dosing algorithms and how clinical judgment was still necessary in final dose recommendations. Finally, the student, resident, or fellow drafted a clinical note for the patient, which was reviewed and discussed with the faculty member. Once approved, the initial consult note was entered into the patient’s EHR with the faculty member’s co-signature and forwarded to the physician on the pharmacogenetics service, who added the physical and medical assessment. Because patients on the service were followed daily until discharge or day 7 (whichever was sooner), with a daily dose recommendation provided based on the INR in response to previous warfarin doses, the students, residents, and fellows gained insight into how genotype affects INR response. The students and residents composed a follow-up note with the refined dose recommendation that was cosigned by the attending pharmacist.
All PGY1 residents were involved in the service’s pharmacy on-call program, and the resident was responsible for carrying the service pager during evenings, overnight, and on weekends. Involvement in this capacity included fielding calls from the clinical laboratory about the appropriateness of a genetic test order, answering pharmacogenetic-related questions, and interpreting and applying genetic test results that came in over the weekend. A pharmacy faculty member was present to supervise the service every weekend and assist or guide the on-call resident with dose recommendations as needed. Residents wishing to gain additional experience could complete an elective clinical rotation on the service. In this capacity, the resident was involved with all clinical aspects of the service under the mentorship and supervision of the attending pharmacy faculty member.
During clinical practice experiences, pharmacy students, residents, and fellows were also encouraged to attend a pharmacology journal club in which a pharmacogenetic research article was discussed. Through this activity, concepts such as population variability in genetic effects, sample size considerations for genetic studies, and the strengths and limitations of various pharmacogenetic study designs were taught. This also provided an opportunity for students, residents, and fellows to further interact with various faculty members interested in pharmacogenetics, potentially stimulating interest in research.
There were a number of research opportunities within the service. Students were generally involved with an ongoing study in which data and DNA samples were collected from consenting patients on the service to learn more about genetic determinants of warfarin response. DNA samples were brought to the pharmacy faculty investigator’s research laboratory for genotyping for research purposes. The ultimate goal of this study was to tailor warfarin pharmacogenetics to urban patient populations, mainly through incorporating variants of importance in African Americans or Hispanics onto the genotyping platform. Thus, in addition to experience gained in clinical research, students learned how research findings could be directly translated to improve patient care.
Prior to beginning research, in addition to the reading described above, students and residents had to complete human subjects research training and prepare an amendment to be added as an investigator on the IRB-approved protocol. These activities provided exposure to the regulatory aspects of research. Once added to the protocol, students had an opportunity to participate in many facets of research, including patient recruitment, the informed consent process, data collection, genotyping in the investigator’s laboratory, and data analysis. Students were involved in clinical-based (eg, patient recruitment, enrollment, and data collection) or laboratory-based (eg, DNA extraction, genotyping) activities. For students participating in laboratory-based research, additional training in blood-borne pathogens and laboratory safety was required. Patient recruitment activities provided students with experience interacting with and explaining difficult genetics concepts to the patient in a manner the patient could understand. Through data collection activities, the student was taught how to navigate through the EHR, an activity in which most first- and second-year pharmacy students had not participated. In the investigator’s laboratory, students learned how to apply the concepts of genotyping learned from the classroom to the laboratory. Working in the research laboratory also helped students develop their reasoning skills through trouble-shooting laboratory problems or failed experiments.
Residents and fellows could complete an independent project related to the service. For example, one resident project involved comparing the accuracy of various warfarin pharmacogenetic dosing methods, such as use of the FDA-approved dose recommendations along with clinician judgment by a pharmacist to estimate warfarin dose requirements. A fellow was involved in a more comprehensive project to identify novel genetic determinants of warfarin dose requirements in African Americans.
EVALUATION AND ASSESSMENT
From August, 2012, to December, 2012, 5 students (1 P1, 2 P2, and 2 P4 students), 2 residents (1 PGY1 and 1 PGY2), and 3 fellows completed practice experiences or elective courses involving the service. A PGY2 resident was involved in both clinical activities and a research project with the service. Two students (both P2 students) and 1 fellow were involved in research, and the remaining students, PGY1 resident, and fellows were involved exclusively in clinical activities. Both P4 students completed a practice experience in anticoagulation management and spent 20% of their time with the service.
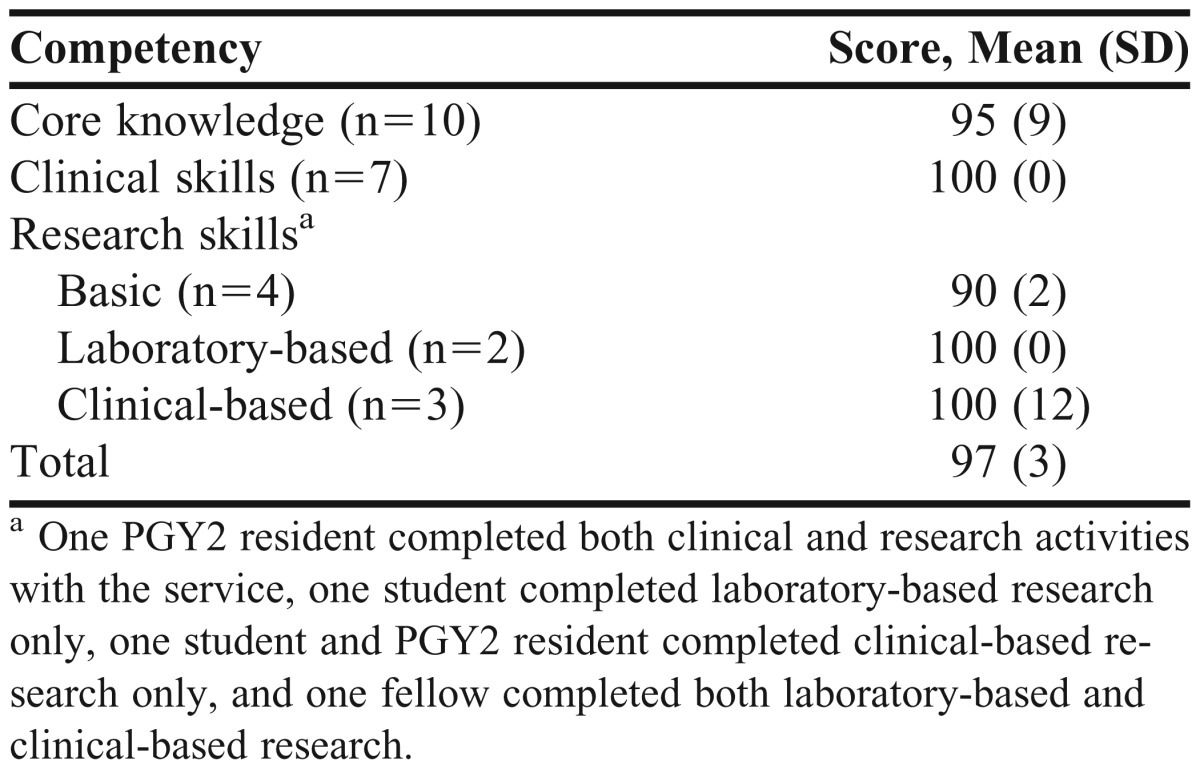
A competency checklist (Appendix 2), was developed to assess achievement of learning objectives. The checklist was divided into core knowledge, clinical, and research competencies. Students and residents had to achieve 90% of the relevant competencies to earn a letter grade of A for elective course credit. For example, those participating in clinical aspects of the service had to achieve 90% of knowledge-based and clinical competencies, while those involved in research had to achieve 90% of knowledge-based and research competencies. At least 80% of the competencies had to be met to earn a B, and 70% had to be met to earn a C or passing/satisfactory grade for the course. Scores for fellows were not needed for grading purposes but rather to provide faculty with a means of assessing the effectiveness of the learning opportunity. Students and residents received a midpoint and final assessment via the checklist, with the midpoint evaluation used to identify areas of deficiency that are focused on for the remainder of the experience. Competency scores are shown in Table 1; the mean competency score among all students and residents was 97% ± 3%. All achieved a score >90%.
Table 1.
Competency Scores of Students and Trainees Involved With the Pharmacogenetics Service
DISCUSSION
A pharmacist-led comprehensive warfarin pharmacogenetics service provided experiential and research opportunities to students and residents that addressed many of the core competencies in pharmacogenetics proposed by AACP. Based on assessment via a competency checklist, most students completing experiences and residents completing rotations with the service achieved core competencies in pharmacogenetics. To our knowledge, this is one of the first examples of an experiential approach to instruction in pharmacogenetics.
While some colleges and schools of pharmacy provide comprehensive classroom and laboratory instruction in genetics and pharmacogenetics,28 this appears to be the exception rather than the norm.3,4 While the opportunity to solidify clinical knowledge gained from lectures by participating in case discussion or small group activities is available for many therapeutic areas, pharmacogenetics is notably absent in most curricula.28 This is despite the desire by students for clinical experience in the area, which is presumably more ideal than classroom instruction alone.4
Other academic medical centers are now implementing pharmacogenetics into clinical practice, and others are expected to follow.29-32 Thus, it is more important now than ever for pharmacy graduates to possess the knowledge and skill to manage genotype-guided therapy. Elective and advanced pharmacy practice experiences in pharmacogenetics, such as those we describe, are expected to better prepare students, residents, and fellows to manage genotype-guided drug therapy. Even though the experience centers on warfarin pharmacogenetics, the principle of applying genetic data to drug therapy decisions is applicable across therapeutic areas.
The ACPE standards that govern accreditation emphasize scholarship and research as important components of pharmacy education.33 In addition to achieving competency in many aspects of pharmacogenetics, there are other benefits for students participating in research with the pharmacogenetics service. In addition to leading to important scholarly contributions, involvement in clinical research helps students, residents, and fellows foster relationships with faculty members who will be in a position to provide future letters of recommendation. Through participation in pharmacogenetic research-related activities, students and trainees may gain a better understanding and appreciation of the evidence necessary to inform genotype-guided therapy in addition to the nuances and limitations of such an approach. In particular, students, residents, and fellows may gain a better grasp on study design for pharmacogenetic research, including the importance of considering allele frequencies and genotype distribution in interpreting study results.
Moving forward, the university plans to expand both its pharmacogenetic efforts and opportunities for clinical and research training with the service. Along these lines, we recently began offering CYP2C19 genotyping to guide antiplatelet therapy. We also plan to offer training opportunities for visiting pharmacists from within and outside the United States. Thus, a variety of clinical and research opportunities centered on pharmacogenetic principles will be available for students and pharmacy graduates to gain important experience in managing genotype-guided therapies.
SUMMARY
A pharmacist-led warfarin pharmacogenetics service provided a unique opportunity for pharmacy students, residents, and fellows to gain clinical experience with personalized medicine and become involved in pharmacogenetic research. While the service is one of the first examples of a comprehensive warfarin pharmacogenetic service, other academic centers are beginning to implement pharmacogenetic programs for other medications.29-31 Broader implementation efforts are expected as further data supporting personalized medicine emerge. The experiential and research opportunities described in this paper may thus serve as models for other institutions as they establish clinical pharmacogenetics programs of their own.
ACKNOWLEDGEMENTS
Work by Dr. Nutescu was supported by a grant from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix 1.
Learning Objectives for Students, Residents, and Fellows Completing Experiential or Research Activities With the Warfarin Pharmacogenetics Service
Appendix 2.
Competency Checklist

REFERENCES
- 1.Accreditation Council for Pharmacy Education. Accreditation standards and guidelines for the professional program in pharmacy leading to the doctor of pharmacy degree, 2007. Appendix B: Additional guidance on the science foundation for the curriculum. https://www.acpe-accredit.org/pdf/ACPE_Revised_PharmD_Standards_Adopted_Jan152006.pdf. Accessed February 6, 2013. [Google Scholar]
- 2.Wells BG, Beck DE, Draugalis JR, et al. Report of the 2007-2008 Argus Commission: What future awaits beyond pharmaceutical care? Am J Pharm Educ. 2008;72(Suppl) Article S08. [Google Scholar]
- 3.Murphy JE, Green JS, Adams LA, Squire RB, Kuo GM, McKay A. Pharmacogenomics in the curricula of colleges and schools of pharmacy in the United States. Am J Pharm Educ. 2010;74(1) doi: 10.5688/aj740107. Article 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moen M, Lamba J. Assessment of healthcare students' views on pharmacogenomics at the University of Minnesota. Pharmacogenomics. 2012;13(13):1537–1545. doi: 10.2217/pgs.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough KB, Formea CM, Berg KD, et al. Assessment of the pharmacogenomics educational needs of pharmacists. Am J Pharm Educ. 2011;75(3) doi: 10.5688/ajpe75351. Article 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Formea CM, Nicholson WT, McCullough KB, et al. Development and evaluation of a pharmacogenomics educational program for pharmacists. Am J Pharm Educ. 2013;77(1) doi: 10.5688/ajpe77110. Article 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360(8):753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai G, Farin F, Rieder MJ, et al. In-vitro and in-vivo effects of the CYP2C9*11 polymorphism on warfarin metabolism and dose. Pharmacogenet Genomics. 2005;15(7):475–481. doi: 10.1097/01.fpc.0000162005.80857.98. [DOI] [PubMed] [Google Scholar]
- 10.Limdi NA, Wadelius M, Cavallari L, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood. 2010;115(18):3827–3834. doi: 10.1182/blood-2009-12-255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schelleman H, Chen J, Chen Z, et al. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin Pharmacol Ther. 2008;84(3):332–339. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bress A, Patel SR, Perera MA, Campbell RT, Kittles RA, Cavallari LH. Effect of NQO1 and CYP4F2 genotypes on warfarin dose requirements in Hispanic-Americans and African-Americans. Pharmacogenomics. 2012;13(16):1925–1935. doi: 10.2217/pgs.12.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavallari LH, Langaee TY, Momary KM, et al. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin Pharmacol Ther. 2010;87(4):459–464. doi: 10.1038/clpt.2009.223. [DOI] [PubMed] [Google Scholar]
- 14.Cavallari LH, Perera M, Wadelius M, et al. Association of the GGCX (CAA)16/17 repeat polymorphism with higher warfarin dose requirements in African Americans. Pharmacogenet Genomics. 2012;22(2):152–158. doi: 10.1097/FPC.0b013e32834f288f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Jeong H, Takahashi H, et al. Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin Pharmacol Ther. 2012;91(4):660–665. doi: 10.1038/clpt.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perera MA, Gamazon E, Cavallari LH, et al. The missing association: sequencing-based discovery of novel SNPs in VKORC1 and CYP2C9 that affect warfarin dose in African Americans. Clin Pharmacol Ther. 2011;89(3):408–415. doi: 10.1038/clpt.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JA, Bootman JL, Evans WE, et al. Pharmacogenomics: a scientific revolution in pharmaceutical sciences and pharmacy practice. report of the 2001-2002 Academic Affairs Committee. Am J Pharm Educ. 2002;66:12S–15S. [Google Scholar]
- 18.Poirier T, Crouch M, MacKinnon G, Mehvar R, Monk-Tutor M. Updated guidelines for manuscripts describing instructional design and assessment: the IDEAS format. Am J Pharm Educ. 2009;73(3) doi: 10.5688/aj730355. Article 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloom BS. Taxonomy of Educational Objectives. The Classification of Educational Goals. Handbook I: Cognitivie Domain. New York: McKay; 1956. [Google Scholar]
- 20.Jenkins J, Blitzer M, Boehm K, et al. Recommendations of core competencies in genetics essential for all health professionals. Genet Med. 2001;3(2):155–159. doi: 10.1097/00125817-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Cavallari LH, Lam YWF. Pharmacogenetics. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach, Vol 8. Vol. 17 New York: McGraw-Hill Companies, Inc; 2011. [Google Scholar]
- 22.Cavallari LH, Shin J, Perera MA. Role of pharmacogenomics in the management of traditional and novel oral anticoagulants. Pharmacotherapy. 2011;31(12):1192–1207. doi: 10.1592/phco.31.12.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90(4):625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witt DM, Nutescu EA, Haines ST. Venous thromboembolism. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach, Vol 8. New York: McGraw-Hill Companies, Inc; 2011. pp. 327–334. [Google Scholar]
- 25.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th edition: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e152S–184S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavallari LH, Perera MA. The future of warfarin pharmacogenetics in under-represented minority groups. Future Cardiol. 2012;8(4):563–576. doi: 10.2217/fca.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonagh EM, Whirl-Carrillo M, Garten Y, Altman RB, Klein TE. From pharmacogenomic knowledge acquisition to clinical applications: the PharmGKB as a clinical pharmacogenomic biomarker resource. Biomark Med. 2011;5(6):795–806. doi: 10.2217/bmm.11.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brazeau DA, Brazeau GA. A required course in human genomics, pharmacogenomics, and bioinformatics. Am J Pharm Educ. 2006;70(6) doi: 10.5688/aj7006125. Article 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicks JK, Crews KR, Hoffman JM, et al. A clinician-driven automated system for integration of pharmacogenetic interpretations into an electronic medical record. Clin Pharmacol Ther. 2012;92(5):563–566. doi: 10.1038/clpt.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JA, Burkley BM, Langaee TY, Clare-Salzler MJ, Klein TE, Altman RB. Implementing personalized medicine: development of a cost-effective customized pharmacogenetics genotyping array. Clin Pharmacol Ther. 2012;92(4):437–439. doi: 10.1038/clpt.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieb W, Volzke H, Pulley JM, Roden DM, Kroemer HK. Strategies for personalized medicine-based research and implementation in the clinical workflow. Clin Pharmacol Ther. 2012;92(4):443–445. doi: 10.1038/clpt.2012.119. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor SK, Ferreri SP, Michaels NM, et al. Exploratory planning and implementation of a pilot pharmacogenetic program in a community pharmacy. Pharmacogenomics. 2012;13(8):955–962. doi: 10.2217/pgs.12.67. [DOI] [PubMed] [Google Scholar]
- 33.Accreditation Council for Pharmacy Education. Accreditation standards and guidelines for the professional program in pharmacy leading to the doctor of pharmacy degree. Version 2.0. https://www.acpe-accredit.org/pdf/FinalS2007Guidelines2.0.pdf. Accessed February 25, 2013. [Google Scholar]


