Abstract
Context
Human epidermal growth factor receptor 2 (HER2/neu) is overexpressed in a proportion of gastroesophageal (GE) adenocarcinomas, and trastuzumab treatment results in significant improvement in overall survival in patients with HER2/neu-overexpressing GE tumors. Grading of HER2/neu expression in GE tumors and its clinical application is different from that of breast cancer. HER2/neu immunohistochemistry (IHC) image analysis (IA), widely used in breast cancer, has not been studied in GE tumors.
Objective
To evaluate the correlation between manual HER2/neu IHC scoring and HER2/neu IHC image analysis in GE adenocarcinomas with characterization of associated clinicopathologic features.
Design
Tumor grade, growth pattern, and stage were evaluated in 116 cases of primary GE adenocarcinoma biopsy and resection specimens. Using anti-HER2/neu antibody and the proposed HER2/neu scoring system for gastric cancer, HER2/neu IHC expression was recorded after manual scoring and automated IA interpretation.
Results
HER2/neu overexpression (IHC 3+) was detected in 19% (10 of 54) of gastric tumors, and overall correlation between manual HER2/neu IHC interpretation and IA interpretation was 78% (42 of 54). HER2/neu overexpression (IHC 3+) was detected in 26% (16 of 62) of GE junction tumors, and the overall correlation between manual HER2/neu IHC interpretation and IA interpretation was 84% (52 of 62).
Conclusions
The HER2/neu IHC scoring system for GE adenocarcinomas differs from that of breast carcinoma. Automated IA, validated for scoring of HER2/neu IHC in breast cancer, has a low correlation between HER2/neu IHC 2+ and IHC 3+ cases scored by conventional light microscopy and cannot be reliably used in the interpretation of HER2/neu IHC expression in GE adenocarcinomas.
The incidence of primary gastric and esophageal/gastroesophageal junction (GEJ) adenocarcinomas is increasing, and these tumors are estimated to make up the third and fifth most common cause of cancer deaths worldwide, respectively.1,2 Many patients with gastroesophageal cancer present with advanced stage or metastatic disease, and the prognosis of patients with these tumors remains poor, with a median overall survival of less than 1 year and a 5-year survival ranging between 5% to 25%.3–5 Improvements in the treatment of gastric cancer, including combination chemotherapy, have resulted in improved overall survival versus single-agent chemotherapy alone.6
Additional therapy aimed at specific targets in cancer has shown a survival benefit in certain tumors.7 One of these cellular targets, human epidermal growth factor receptor 2 (HER2/neu) protein, a 185-kDa transmembrane tyrosine kinase receptor, is associated with tumor proliferation, migration, and differentiation.8 The overexpression of HER2/neu on tumor cells versus normal cells allows selective targeting of malignant cells with anti-HER2/neu therapy.7,9,10 HER2/neu overexpression has been identified in a variety of neoplasms but has been most widely studied in breast cancer.9 Approximately 25% to 30% of breast cancers overexpress HER2/neu.11 HER2/neu-positive status is associated with more aggressive disease and is an important predictive factor of response to therapy with trastuzumab (Herceptin, Genentech, Inc., South San Francisco, California).11 Recently, studies have demonstrated that a proportion of gastroesophageal adenocarcinomas overexpress HER2/neu, with an average of 19% and 22% of gastric and gastroesophageal junction adenocarcinomas displaying overexpression, respectively.12,13 The clinical significance of HER2/neu overexpression in gastroesophageal adenocarcinoma is not yet fully elucidated, and studies have shown conflicting results between HER2/neu status and prognosis.4,14 Some data indicate that HER2/neu positivity may be associated with advanced disease, suggesting a significant prognostic value of HER2/neu-status.4,7,10,12,15,16
Trastuzumab, a recombinant monoclonal antibody targeting HER2/neu, inhibits HER2/neu-related tumor proliferation, induces antibody-dependent cellular cytotoxicity,13,17 and displays significant antitumor activity in HER2/neu+ animal models of gastric cancer.18,19 The use of trastuzumab is an effective treatment option in HER2/neu-overexpressing breast cancer, and a standardized and validated HER2/neu immunohistochemical scoring system, along with other methodologies (ie, fluorescence in situ hybridization [FISH], chromogenic in situ hybridization), has been developed to establish HER2/neu status and determine eligibility for trastuzumab treatment in breast cancer.4 However, in addition to the histologic and biologic variation, gastroesophageal adenocarcinomas demonstrate different HER2/neu immunoreactivity patterns, compared to breast adenocarcinomas. In contrast to breast cancer, HER2/neu staining in gastroesophageal tumors is heterogeneous, with incomplete membrane staining. On the basis of these differences, Hofmann et al13 reported the recommendations of a consensus panel for HER2/neu scoring in gastroesophageal adenocarcinoma, modified from the HercepTest (Dako North America Incorporated, Carpinteria, California) scoring system for breast cancer, which evaluates patterns of membranous reactivity on tumor cells to determine HER2/neu overexpression. The concordance between this modified HER2/neu immunohistochemistry (IHC) scoring system and HER2/neu testing using FISH has been reported to be between 86.9% and 93.5%.13,20
This HER2/neu IHC scoring system for gastroesophageal carcinomas was applied to cases that may be eligible for a clinical trial studying the use of trastuzumab in gastroesophageal cancer. In 2010, the international, multicenter, phase III randomized controlled ToGA trial (Trastuzumab for Gastric Cancer) reported that the addition of trastuzumab to combination chemotherapy prolonged overall survival and improved response rate and median progression-free survival in patients with advanced stage or metastatic gastric or GEJ adenocarcinomas that overexpressed HER2/neu protein (HER2/neu IHC 3+ or HER2/neu+/FISH+).21 The results of this trial further encouraged pathologists to routinely evaluate HER2/neu expression status in gastric or GEJ adenocarcinomas.
HER2/neu immunohistochemical scoring in breast cancers, using manual and automated techniques, has been well established, standardized, and validated. However, manual light microscopic evaluation of HER2/neu IHC staining intensity and uniformity in breast cancer specimens is subjective, which is a major limitation in accuracy and reproducibility and results in significant interobserver variability.22,23 Automated image analysis allows for objective enumeration of staining intensity and has been recommended by the College of American Pathologists/American Society of Clinical Oncology as an effective tool for achieving consistent interpretation of HER2/neu IHC scoring in breast cancer specimens with pathologist confirmation of results.22,24 The HER2/neu immunohistochemical scoring system for gastroesophageal carcinomas is still being refined, and recent studies indicate that pathologists need special and specific training with guidelines for determining HER2/neu IHC status in gastroesophageal cancer.25 Immunohistochemical evaluation of HER2/neu expression is now routine for cases of gastroesophageal cancer as the most predictive test for response to trastuzumab-based therapy and as the primary testing modality with subsequent FISH testing for IHC equivocal 2+ cases5,25 or each 1+ case.5
Therefore, our study aimed to add additional validity to recently published literature regarding the HER2/neu IHC scoring system in gastroesophageal cancer by evaluating the HER2/neu IHC status and clinicopathologic features in biopsy and resection specimens of primary gastric and GEJ adenocarcinomas. We also evaluated the concordance between manual HER2/neu IHC scoring in gastroesophageal carcinomas and automated computer image analysis to determine if image analysis interpretation can be used for the objective enumeration and consistent interpretation of HER2/neu expression in gastroesophageal tumors.
MATERIALS AND METHODS
This study was approved by the institutional review board at the University of Florida College of Medicine, Gainesville. We retrospectively analyzed 116 cases of primary gastric (n = 54) and esophageal/gastroesophageal junction adenocarcinomas (n = 62) from biopsy and resection specimens received from individual patients between 2006 and 2010. Primary gastric adenocarcinomas arose within the cardia, body, or antrum. Primary esophageal/gastroesophageal junction adenocarcinomas arose from Barrett esophagus either proximal to the gastroesophageal junction or with a tumor epicenter located directly at the gastroesophageal junction (histologically confirmed by presence of both overlying squamous and glandular mucosa). Histologic parameters, including tumor grade (well, moderately, or poorly differentiated) and growth pattern (Lauren classification—intestinal, diffuse, mixed), were identified and recorded for hematoxylin-eosin slides reviewed by 3 pathologists (J.J., R.P., L.V.). We reevaluated the cases that had been staged according to the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) tumor-node-metastasis (TNM) classification, 6th edition, and updated the stage to reflect the AJCC/UICC TNM classification, 7th edition, if applicable.
Slide Preparation/Immunohistochemistry Staining
Biopsy and resection tissue samples were fixed in 10% neutral buffered formalin and processed to paraffin blocks by using standard histologic techniques, and 4-μm-thick paraffin sections were stained routinely with hematoxylin-eosin. Immunohistochemistry was performed by routine methodology on 4-μm-thick, formalin-fixed, unstained, paraffin-embedded tissue sections. HER2/neu immunohistochemical staining using the commercially available rabbit anti-human HER2/neu protein and reagent kit (HercepTest, prediluted) was performed according to the manufacturer’s guidelines.
HER2/neu Immunohistochemical Quantitation
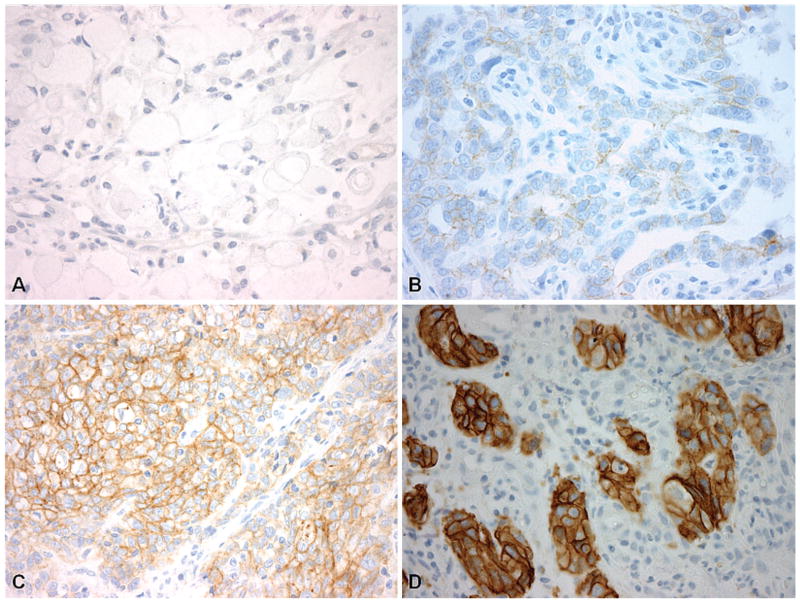
The original HER2/neu immunostained glass slides were concurrently reviewed by 3 pathologists (R.P., J.J., L.V.) at a multiheaded microscope, and the consensus HER2/neu immunoreactivity was manually scored by conventional microscopy as 0, 1+, 2+, or 3+ according to the proposed HER2/neu scoring system for gastric cancer13,25 (Table 1 and Figure 1, A through D). HER2/neu IHC scoring in gastroesophageal cancer versus breast cancer included differences in the pattern of HER2/neu IHC staining, intensity of HER2/neu IHC (for 3+ score criteria), and number or percentage of tumor cells demonstrating HER2/neu IHC staining. The requirement for complete membranous HER2/neu IHC staining in breast cancer (weak/moderate for IHC 2+ score and strong for IHC 3+ score) in at least 10% of tumor cells in both biopsy and resection specimens was modified for HER2/neu IHC scoring in GE cancer cases according to the consensus panel guidelines proposed by Hoffman et al13 and Rüschoff et al.25 In comparison to HER2/neu IHC scoring in breast cancer, HER2/neu IHC scoring in GE adenocarcinomas includes the following differences: both complete and basolateral/lateral membranous HER2/neu immunoreactivity was accepted as staining (weak/moderate staining for IHC 2+ score, modified to include both moderate/strong staining for IHC 3+ score); and the pattern and intensity of HER2/neu immunoreactivity was applicable to 5 or more cohesive tumor cells for biopsy specimens and at least 10% of tumor cells in resection specimens.
Table 1.
Consensus Panel Recommendations on HER2/neu Immunohistochemistry Scoring for Gastroesophageal Adenocarcinoma Biopsy and Resection Specimensa
| Reactivity Characteristics | Score/Classification |
|---|---|
| Resection specimens | |
| No reactivity (at ×40 magnification) | 0/negative |
| Membranous reactivity in <10% of cells | |
| Faint/barely perceptible partial membranous reactivity in >10% of tumor cells (at ×40 magnification) | 1+/negative |
| Weak/moderate complete OR basolateral/lateral membranous reactivity in >10% of tumor cells (at ×10–×20 magnification) | 2+/equivocal |
| Moderate/strong complete OR basolateral/lateral membranous reactivity in >10% of tumor cells | 3+/positive |
| Unequivocal membranous staining at low magnification (×2.5/×5) | |
| Biopsy specimens | |
| Any single cluster of tumor cells (≥5 cells) demonstrating IHC 3+ staining characteristics | 3+/positive |
Figure 1.
Examples of quantitative human epidermal growth factor receptor 2 (HER2/neu) immunohistochemistry (IHC) scoring in gastroesophageal specimens based on consensus guidelines. A, Gastric adenocarcinoma specimen with HER2/neu IHC score of 0. B, Gastric adenocarcinoma specimen demonstrating HER2/neu IHC 1+ score. C, Example of gastric adenocarcinoma displaying HER2/neu IHC 2+ score. D, Gastroesophageal junction adenocarcinoma specimen demonstrating HER2/neu overexpression (IHC 3+) (HER2/neu IHC stain [HercepTest, Dako, Carpinteria, California], original magnifications ×400 [A through D]).
Image Analysis
Digital image analysis was performed after HER2/neu immunostaining by using the Automated Cellular Imaging System III (ACIS III) (Dako). Whole slide images were captured automatically by the ACIS III scanner at low-power magnification, and the images were viewed on a monitor. The areas with the greatest immunohistochemical staining intensity and highest percentage of immunoreactive cells were selected for automated analysis. The results of all selected fields were automatically calculated and reported as both a numerical staining intensity average (mean region score for all studied regions, from 0.0 to >3.0) and as a digital interpretation result of favorable/negative (≤1.7), equivocal/intermediate (>1.7 to <3.0), or unfavorable/positive (≥3.0), based on the system parameters for evaluation of HER2/neu expression in breast carcinomas. The tumor areas with highest immunohistochemical staining intensity and highest percentage of immunoreactive cells (a minimum of one ×40 circular field with only tumor for biopsy samples and six ×40 fields for resection specimens) were then selected by a pathologist. The regions selected for automated evaluation contained only invasive carcinoma and corresponded to areas that were representative of most of the grade/pattern of the tumor that had the highest percentage of immunoreactive cells with the greatest staining intensity. Areas that were evaluated in the computer-selected automated analysis and were omitted after pathologist review included the following: necrotic tissue and crush or edge artifact with nonspecific HER2/neu staining, tumor cells with cytoplasmic staining, staining within normal tissue elements, and other staining nontumor tissue (including intestinal metaplasia and/or dysplasia).
Statistical Analysis
Statistical analyses were performed with Microsoft Excel (Microsoft Corporation, Redmond, Washington) and the Student t test, with P < .05 considered statistically significant. To assess the concordance rate between the results of manual HER2/neu IHC score and automated HER2/neu IHC image analysis interpretation for gastroesophageal adenocarcinoma biopsy and resection specimens, we compared the positive, intermediate, and negative results for each examination. Confidence intervals were computed by using the normal approximation to the binomial distribution.
RESULTS
Clinicopathologic Findings and Manual Quantitation of HER2/neu Expression in Gastroesophageal Cancer Biopsy Specimens and Surgically Resected Tumors
The HER2/neu status was evaluated by immunohistochemistry and conventional light microscopy in a total of 116 samples. The tumor stage, tumor grade, tumor growth pattern, and HER2/neu IHC quantitation for the gastric and gastroesophageal junction adenocarcinoma biopsy and resection cases are summarized in Tables 2 through 5. The primary gastric adenocarcinomas (total n = 54) consisted of 27 biopsy specimens and 27 resection cases from different patients. The intestinal tumor types demonstrated greater HER2/neu positivity than diffuse or mixed types (29% versus 15% versus 0%, P = .12 and P = .15). For the gastric cancer biopsy specimens, HER2/neu immunoreactivity was classified as negative (0 or 1+) in 18 cases (67%), equivocal (2+) in 2 cases (7%), and positive (3+) in 7 cases (26%) (Table 2). For the gastric cancer resection cases, HER2/neu immunoreactivity was classified as negative (0 or 1+) in 22 cases (81%), equivocal (2+) in 2 cases (7%), and positive (3+) in 3 cases (11%) (Table 3). Only the lower-stage tumors (IA and IIA) were found to overexpress HER2/neu protein, and none of the higher-stage tumors overexpressed HER2/neu. Overall in gastric tumors, HER2/neu overexpression was identified in 19% (10 of 54) of total cases, with 26% (7 of 27) of biopsy specimens and 11% (3 of 27) of resection cases demonstrating overexpression.
Table 2.
HER2/neu Expression in Gastric Adenocarcinoma Biopsy Specimens
| Manual Quantitative HER2/neu IHC Score | No.
|
Total, No. (%) | ||
|---|---|---|---|---|
| Intestinal | Diffuse | Mixed | ||
| 0 | 6 | 8 | 2 | 16 (59) |
| 1+ | 2 | 0 | 0 | 2 (7) |
| 2+ | 1 | 1 | 0 | 2 (7) |
| 3+ | 4 | 3 | 0 | 7 (26) |
| Total | 13 | 12 | 2 | 27 (100) |
| HER2/neu overexpression (IHC 3+), % | 31 | 25 | 0 | |
| Overall cases with HER2/neu overexpression: 7/27 (26%) | ||||
Abbreviations: HER2/neu, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
Table 5.
HER2/neu Expression in Esophageal/Gastroesophageal Junction Adenocarcinoma Resection Specimens
| Manual Quantitative HER2/neu IHC Score
|
Total | Positive Rate (HER2/neu IHC 3+), % | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |||
| Stagea | ||||||
| IA | 4 | 3 | 0 | 2 | 9 | 22 |
| IB | 3 | 1 | 0 | 0 | 4 | 0 |
| IIA | 1 | 0 | 0 | 0 | 1 | 0 |
| IIB | 10 | 0 | 0 | 0 | 10 | 0 |
| IIIA | 5 | 1 | 0 | 0 | 6 | 0 |
| IIIB | 0 | 1 | 0 | 1 | 2 | 50 |
| IIIC | … | … | … | … | 0 | … |
| IV | … | … | … | … | 0 | … |
| Total | 23 | 6 | 0 | 3 | 32 | |
| Grade 1 | 4 | 1 | 0 | 1 | 6 | 17 |
| Grade 2 | 6 | 3 | 0 | 2 | 11 | 18 |
| Grade 3 | 13 | 2 | 0 | 0 | 15 | 0 |
| Total | 23 | 6 | 0 | 3 | 32 | |
| Overall cases with HER2/neu overexpression: 3/32 (9%) | ||||||
Abbreviations: HER2/neu, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
American Joint Committee on Cancer/International Union Against Cancer tumor-node-metastasis (AJCC/UICC TNM) classification, 7th edition.
Table 3.
HER2/neu Expression in Gastric Adenocarcinoma Resection Specimens
| Manual Quantitative HER2/neu IHC Score
|
Total | Positive Rate (HER2/neu IHC 3+), % | ||||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |||
| Stagea | ||||||
| IA | 4 | 0 | 0 | 2 | 6 | 33 |
| IB | 3 | 3 | 1 | 0 | 7 | 0 |
| IIA | 2 | 1 | 1 | 1 | 5 | 20 |
| IIB | 1 | 0 | 0 | 0 | 1 | 0 |
| IIIA | 1 | 0 | 0 | 0 | 1 | 0 |
| IIIB | 3 | 0 | 0 | 0 | 3 | 0 |
| IIIC | 1 | 0 | 0 | 0 | 1 | 0 |
| IV | 3 | 0 | 0 | 0 | 3 | 0 |
| Total | 18 | 4 | 2 | 3 | 27 | |
| Intestinalb | 3 | 3 | 0 | 2 | 8 | 25 |
| Diffuseb | 12 | 0 | 1 | 1 | 14 | 7 |
| Mixedb | 3 | 1 | 1 | 0 | 5 | 0 |
| Total | 18 | 4 | 2 | 3 | 27 | |
| Overall cases with HER2/neu overexpression: 3/27 (11%) | ||||||
Abbreviations: HER2/neu, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
American Joint Committee on Cancer/International Union Against Cancer tumor-node-metastasis (AJCC/UICC TNM) classification, 7th edition.
Lauren classification.
The primary esophageal/GEJ adenocarcinomas (total n = 62) consisted of 30 biopsy specimens and 32 resection cases. For the GEJ cancer biopsy specimens, HER2/neu immunoreactivity was classified as negative (0 or 1+) in 15 cases (50%), equivocal (2+) in 2 cases (7%), and positive (3+) in 13 cases (43%) (Table 4). For the GEJ resection cases, HER2/neu immunoreactivity was classified as negative (0 or 1+) in 29 cases (91%), equivocal (2+) in 0 cases, and positive (3+) in 3 cases (9%) (Table 5). There was no significant difference in HER2/neu expression between different grade or tumor stage. Overall in esophageal/GEJ tumors, HER2/neu overexpression was identified in 26% (16 of 62) of total cases, with 43% (13 of 30) of biopsy specimens and 9% (3 of 32) of resection cases demonstrating overexpression.
Table 4.
HER2/neu Expression in Esophageal/Gastroesophageal Junction Adenocarcinoma Biopsy Specimens
| Manual Quantitative HER2/neu IHC Score | No.
|
Total, No. (%) | ||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | ||
| 0 | 1 | 4 | 4 | 9 (30) |
| 1+ | 2 | 3 | 1 | 6 (20) |
| 2+ | 1 | 1 | 0 | 2 (7) |
| 3+ | 3 | 4 | 6 | 13 (43) |
| Total | 7 | 12 | 11 | 30 (100) |
| HER2/neu overexpression (IHC 3+), % | 43 | 33 | 55 | |
| Overall cases with HER2/neuoverexpression: 13/30 (43%) | ||||
Abbreviations: HER2/neu, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
Quantitation of HER2/neu Expression by Automated Image Analysis and Concordance With Manual HER2/neu IHC Quantitation in Gastroesophageal Cancer Biopsy Specimens and Surgically Resected Tumors
Automated image analysis of HER2/neu expression in the gastric cancer biopsy specimens resulted in a negative value in 25 cases (93%) and an intermediate and positive value in 1 case (4%) each. There was 100% (18 of 18 cases) concordance between the IHC 0/1+ cases and IA negative category, 0% (0 of 2 cases) concordance between the IHC 2+ and IA intermediate category, and 14% (1 of 7 cases) concordance between the IHC 3+ and IA positive category (Table 6). The overall concordance between manual HER2/neu IHC scoring and automated HER2/neu IHC analysis for gastric cancer biopsies was 70% (19 of 27). Automated image analysis of HER2/neu expression in the gastric resection cases returned a negative value in 24 cases (89%), an intermediate value in 2 cases (7%), and a positive value in 1 case (4%). There was 100% (22 of 22 cases) concordance between the IHC 0/1+ cases and IA negative category, 0% (0 of 2 cases) concordance between the IHC 2+ and IA intermediate category, and 33% (1 of 3 cases) concordance between the IHC 3+ and IA positive category (Table 7 and Figure 2, A and B). The overall concordance between manual HER2/neu IHC scoring and automated HER2/neu IHC analysis for gastric resection specimens was 85% (23 of 27) (95% confidence interval [CI], 72%–98%), and for all gastric cancer cases it was 78% (42 of 54) (95% CI, 67%–89%).
Table 6.
Concordance Between the Results of Manual HER2/neu Immunohistochemistry (IHC) Score and Automated HER2/neu IHC Image Analysis Interpretation in Gastric Adenocarcinoma Biopsy Specimens
| Manual HER2/neu IHC Score | HER2/neu IHC Image Analysis Interpretation
|
Concordance Rate, % | ||
|---|---|---|---|---|
| Negative/Favorable (≤1.7) | Equivocal/Intermediate (>1.7 to <3.0) | Positive/Unfavorable (≥3.0) | ||
| 0 | 16 | 0 | 0 | 100 |
| 1+ | 2 | 0 | 0 | 100 |
| 2+ | 2 | 0 | 0 | 0 |
| 3+ | 5 | 1 | 1 | 14 |
| Average digital score | 0.6 | 2.3 | 4.0 | |
Abbreviation: HER2/neu, human epidermal growth factor receptor 2.
Table 7.
Concordance Between the Results of Manual HER2/neu Immunohistochemistry (IHC) Score and Automated HER2/neu IHC Image Analysis Interpretation in Gastric Adenocarcinoma Resection Specimens
| Manual HER2/neu IHC Score | HER2/neu IHC Image Analysis Interpretation
|
Concordance Rate, % | ||
|---|---|---|---|---|
| Negative/Favorable (≤1.7) | Equivocal/Intermediate (>1.7 to <3.0) | Positive/Unfavorable (≥3.0) | ||
| 0 | 18 | 0 | 0 | 100 |
| 1+ | 4 | 0 | 0 | 100 |
| 2+ | 2 | 0 | 0 | 0 |
| 3+ | 0 | 2 | 1 | 33 |
| Average digital score | 0.5 | 2.0 | 3.8 | |
Abbreviation: HER2/neu, human epidermal growth factor receptor 2.
Figure 2.
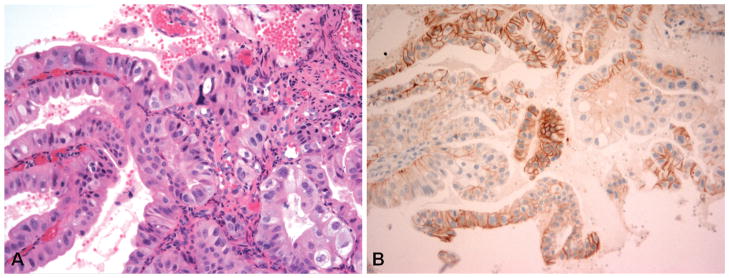
Example of a case with discordance between manual human epidermal growth factor receptor 2 (HER2/neu) immunohistochemistry (IHC) score and HER2/neu image analysis interpretation. A, Biopsy specimen of gastroesophageal junction adenocarcinoma. B, The tumor demonstrates scattered tumor clusters (>5 cells) with HER2/neu IHC 3+ staining, which would be considered overexpression in this biopsy sample. The Automated Cellular Imaging System III (ACIS III) image analysis HER2/neu value was 1.2, which corresponds to a negative interpretation (hematoxylin-eosin, original magnification ×400 [A]; HER2/neu IHC stain [HercepTest, Dako, Carpinteria, California], original magnification × 200 [B]).
Automated image analysis of HER2/neu expression in the GEJ cancer biopsy specimens returned a negative value in 23 cases (77%), an intermediate value in 2 cases (7%), and a positive value in 5 cases (17%). There was 100% (15 of 15 cases) concordance between the IHC 0/1+ cases and IA negative category, 0% (0 of 2 cases) concordance between the IHC 2+ and IA intermediate category, and 38% (5 of 13 cases) concordance between the IHC 3+ and IA positive category (Table 8). The overall concordance between manual HER2/neu IHC scoring and automated HER2/neu IHC analysis for the GEJ biopsy specimens was 67% (20 of 30). Automated image analysis of HER2/neu expression in the GEJ resection cases returned a negative value in 29 cases (91%), no intermediate values, and a positive value in 3 cases (9%) each. There was 100% (29 of 29 cases) concordance between the IHC 0/1+ cases and IA negative category, and 100% (3 of 3 cases) concordance between the IHC 3+ and IA positive category (Table 9). The overall concordance between manual HER2/neu IHC scoring and automated HER2/neu IHC analysis for GEJ resection specimens was 100% (32 of 32), and for all GEJ cancer cases it was 84% (52 of 62) (95% CI, 75%–93%).
Table 8.
Concordance Between the Results of Manual HER2/neu Immunohistochemistry (IHC) Score and Automated HER2/neu IHC Image Analysis Interpretation in Esophageal/Gastroesophageal Junction Adenocarcinoma Biopsy Specimens
| Manual HER2/neu IHC Score | HER2/neu IHC Image Analysis Interpretation
|
Concordance Rate, % | ||
|---|---|---|---|---|
| Negative/Favorable (≤1.7) | Equivocal/Intermediate (>1.7 to <3.0) | Positive/Unfavorable (≥3.0) | ||
| 0 | 9 | 0 | 0 | 100 |
| 1+ | 6 | 0 | 0 | 100 |
| 2+ | 2 | 0 | 0 | 0 |
| 3+ | 6 | 2 | 5 | 38 |
| Average digital score | 0.7 | 2.5 | 4.0 | |
Abbreviation: HER2/neu, human epidermal growth factor receptor 2.
Table 9.
Concordance Between the Results of Manual HER2/neu Immunohistochemistry (IHC) Score and Automated HER2/neu IHC Image Analysis Interpretation in Esophageal/Gastroesophageal Junction Adenocarcinoma Resection Specimens
| Manual HER2/neu IHC Score | HER2/neu IHC Image Analysis Interpretation
|
Concordance Rate, % | ||
|---|---|---|---|---|
| Negative/Favorable (≤1.7) | Equivocal/Intermediate (>1.7 to <3.0) | Positive/Unfavorable (≥3.0) | ||
| 0 | 23 | 0 | 0 | 100 |
| 1+ | 6 | 0 | 0 | 100 |
| 2+ | … | … | … | … |
| 3+ | 0 | 0 | 3 | 100 |
| Average digital score | 0.3 | … | 3.1 | |
Abbreviation: HER2/neu, human epidermal growth factor receptor 2.
COMMENT
The results of the 2010 ToGA study indicate that trastuzumab-based therapy offers a significant survival advantage for patients with HER2-neu overexpressing gastroesophageal adenocarcinomas.21 On the basis of these data, pathologists now routinely evaluate HER2/neu protein expression in gastroesophageal tumors to identify patients who may benefit from the addition of trastuzumab.13,25 Evaluation of HER2/neu expression in gastroesophageal adenocarcinoma requires accurate application of the guidelines for HER2/neu IHC scoring in GE cancer. To our knowledge, this is the first study to evaluate the concordance between manual quantitation of HER2/neu IHC expression and automated HER2/neu IHC image analysis.
In this study, HER2/neu protein overexpression was demonstrated in 19% and 26% of overall gastric and GEJ adenocarcinoma specimens, respectively. These findings are similar to those of other studies that have reported that the mean HER2/neu (IHC 3+) positivity rate for gastric cancer is 17.6% (range, 6.8%–34.0%) and 22% for esophageal/GEJ tumors and that HER2/neu positivity rates tend to be higher in GEJ adenocarcinomas.12,13,21,25 Similar to other studies, more cases of the intestinal histologic subtype were found to overexpress HER2/neu than diffuse and mixed subtypes,5,20 although our results did not reach statistical significance, most likely secondary to a small sample size of gastric adenocarcinoma cases. In our study, many of the tumors displayed heterogeneous reactivity with areas of tumor displaying moderate/strong membranous staining, while other adjacent tumor foci with the same morphology demonstrated weak to absent reactivity. Interestingly, we noted that a greater number of biopsy cases demonstrated HER2/neu overexpression than did resection specimens for both gastric (26% versus 11%) and GEJ (43% versus 9%) specimens.
The ability to evaluate HER2/neu expression in small gastroesophageal cancer biopsy specimens is important, since these samples can be obtained endoscopically and can facilitate the determination of HER2/neu status in unresectable cases or in recurrent tumors after gastrectomy. HER2/neu IHC expression in gastroesophageal tumor biopsy specimens has not been well studied since Hofmann et al13 published the proposed standardized guidelines for evaluation of HER2/neu expression in GE cancer, and many of the studies evaluating HER2/neu IHC expression in gastroesophageal tumors10,15,20,26 were published before these proposed standardized guidelines. The cases evaluated in the validation study by Hoffman et al13 and in the ToGA trial were composed entirely of resection specimens, and the study by Rüschoff et al25 was composed entirely of microarray tissue core samples of only gastric cancer cases without reference to GEJ cases. For biopsy specimens, the presence of 5 or more cohesive tumor cells displaying 3+ HER2/neu immunoreactivity is considered a positive result according to Rüschoff et al.25 Gastroesophageal adenocarcinomas are heterogeneous and biopsy specimens may not be indicative of the entire tumor or tumor behavior.20 We attribute the larger number of HER2/neu-overexpressing biopsy specimens than resection specimens by IHC for both gastric and GEJ tumors to the possible sampling bias of a small biopsy of tumor compared to the entire surgical resection. The biopsy and resection specimens were from individual patients, and we did not correlate biopsy findings with subsequent resection results. Future studies correlating the HER2/neu expression in biopsy specimens with the subsequent resection specimens both with and without prior treatment, using the new proposed HER2/neu scoring guidelines in GE tumors, would add validity to evaluation of HER2/neu IHC expression in GE tumor biopsy specimens.
The ACIS III automated image analysis system used in our study has been widely used in practice for interpretation of HER2/neu immunohistochemical expression in breast cancer as a means of objective enumeration of staining intensity. Studies show a correlation between manual quantitation and image analysis interpretation of 86.5%.27 A major aim of our study was to correlate the manual interpretation of HER2/neu IHC quantitation with that of automated image analysis in gastroesophageal tumors. The correlation was perfect (100%) for cases that were considered negative by manual scoring (HER2/neu IHC 0 or 1+), but discordant for HER2/neu IHC 2+ (0%) and HER2/neu IHC 3+ (20%–50%) cases. Most of the discordant results were found in biopsy samples. The algorithm used for scoring by ACIS III has been validated for use in detecting the HER2/neu IHC expression patterns in breast carcinoma; therefore, we attribute the discordant results to the strong, but incomplete, membranous HER2/neu immunoreactivity in GE tumors not being recognized as true staining by the system. Additionally, although most of our HER2/neu-overexpressing biopsy specimens had many more tumor cells than the minimum cluster of 5 recommended by Rüschoff et al,25 the number of HER2/neu positive cells and the strong incomplete membranous staining of these cells did not reach the threshold of the image analysis system for interpretation as a positive result.
Additional problems encountered with the use of automated HER2/neu quantitation included false-positive reactivity such as staining of reactive mucosa surrounding ulcers, staining of normal gastric mucosa, diffuse cytoplasmic reaction with or without nuclear staining, strong membranous reactivity in intestinal metaplasia or dysplastic epithelium, staining of edge/crush artifacts affecting tumor cells, and staining within necrotic tissue. The use of automated image analysis systems requires the expertise of pathologists to define invasive tumor and discriminate between tumor and nontumor in immunoreactive tissue.
A limitation of this study was the sample size. We included a roughly equal number of both biopsy and resection specimens from both gastric and gastroesophageal adenocarcinomas, which resulted in a small number of higher-stage resection specimens and an unequal distribution of tumors of different growth patterns and grades. Therefore, we were unable to determine statistical significance between HER2/neu expression and specific clinicopathologic features.
Gastroesophageal cancer currently remains one of the leading causes of cancer deaths worldwide. The addition of trastuzumab offers new benefits to patients with HER2/neu-overexpressing GE cancers and solidifies the integral role of pathologists in the appropriate use of treatment and patient care. The results from recent studies add validity to the proposed standardized guidelines for HER2/neu IHC scoring in GE cancers. Currently, the use of automated HER2/neu IHC image analysis in the determination of HER2/neu status in GE tumors is not practical for clinical use, and HER2/neu IHC quantitation in GE tumors still requires the expertise of pathologists knowledgeable of the standardized scoring guidelines.
Acknowledgments
This study was supported in part by the Clinical Research Committee, Department of Pathology, University of Florida. Dr Liu is supported by a grant (K26RR023976) from the National Institutes of Health.
Footnotes
The manuscript was presented in poster format at the 2011 United States & Canadian Academy of Pathology (USCAP) annual meeting in San Antonio, Texas, February 28, 2011.
References
- 1.American Cancer Society. Cancer facts & figures 2010. [Accessed March 19, 2011];American Cancer Society Web site. 2010 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf.
- 2.Albarello L, Pecciarini L, Doglioni C. Her2 testing in gastric cancer. Adv Anat Pathol. 2011;18(1):53–59. doi: 10.1097/PAP.0b013e3182026d72. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Moelans CB, van Diest PJ, Milne ANA, Offerhaus GJA. HER-2/neu testing and therapy in gastroesophageal adenocarcinoma. Patholog Res Int. 2011;2011:1–10. doi: 10.4061/2011/674182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, Hu Y, Fan J, et al. Clinicopathologic significance of HER-2/neu protein expression and gene amplification in gastric carcinoma. World J Gastroenterol. 2011;17(11):1501–1506. doi: 10.3748/wjg.v17.i11.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara H, Morita S, Kawai M, et al. Expression of HER2 in human gastric cancer cells directly correlates with antitumor activity of a recombinant disulfide-stabilized anti-HER2 immunotoxin. J Surg Res. 2002;102(2):169–177. doi: 10.1006/jsre.2001.6305. [DOI] [PubMed] [Google Scholar]
- 8.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 9.Koeppen HKW, Wright BD, Burt AD, et al. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology. 2001;38(2):96–104. doi: 10.1046/j.1365-2559.2001.01084.x. [DOI] [PubMed] [Google Scholar]
- 10.Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIa gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 205;16(2):273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 12.Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19(5):554–568. doi: 10.1081/cnv-100103852. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 14.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. Her2 expression in gastric cancer: rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol. 2010;32(1–2):57–65. doi: 10.3233/CLO-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonemura Y, Ninomiya I, Yamaguchi A, et al. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51(3):1034–1038. [PubMed] [Google Scholar]
- 16.Barros-Silva JD, Leitao D, Afonso L, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100(3):487–493. doi: 10.1038/sj.bjc.6604885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 18.Kasprzyk PG, Song SU, Di Fiore PP, King CR. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 1992;52(10):2771–2776. [PubMed] [Google Scholar]
- 19.Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, Moriya Y, Mori K, Tanaka Y. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59(6):795–805. doi: 10.1007/s00280-006-0337-z. [DOI] [PubMed] [Google Scholar]
- 20.Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15(1):65–71. [PubMed] [Google Scholar]
- 21.Bang Y, Cutsem EV, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advance gastric or gastrooesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 22.Gavrielides M, Gallas B, Lenz P, Badano A, Hewitt S. Observer variability in the interpretation of HER2/neu immunohistochemical expression with unaided and computer-aided digital microscopy. Arch Pathol Lab Med. 2011;135(2):233–242. doi: 10.1043/1543-2165-135.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu C, Ho D, Yang C, Lai C, Yu I, Chiang H. Interobserver reproducibility of Her-2/neu protein overexpression in invasive breast carcinoma using the Dako HercepTest. Am J Clin Pathol. 2002;118(5):693–698. doi: 10.1309/6ANB-QXCF-EHKC-7UC7. [DOI] [PubMed] [Google Scholar]
- 24.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol LabMed. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 25.Rüschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer—guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457(3):299–307. doi: 10.1007/s00428-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Q, Chen L, Sheng L, Nordgren H, Wester K, Carlsson J. EGFR, HER2 and HER3 expression in esophageal primary tumours and corresponding metastases. Int J Oncol. 2007;31(3):493–499. [PubMed] [Google Scholar]
- 27.Slodkowska J, Filas V, Buskiewicz E, et al. Study on breast carcinoma Her2/neu and hormonal receptors status assessed by automated image analysis systems: ACIS III (Dako) and ScanScope (Aperio) Folia Histochem Cytobiol. 2010;48(1):19–25. doi: 10.2478/v10042-010-0015-1. [DOI] [PubMed] [Google Scholar]