Abstract
Bladder cancer (BC) is the second most common malignancy of urological organs. However, patients with non-muscle-invasive BC are at high risk of recurrence and progression into muscle-invasive BC, and the prognosis of patients with muscle-invasive BC is limited by the high rate of metastasis. The epithelial-mesenchymal transition (EMT) is characterized by loss of cell-to-cell adhesion and cell polarity and is closely associated with the invasion and metastasis of several cancers. Given the multifocality and high rates of relapse, progression, and metastasis of BC, the EMT is likely to participate in BC as well. Numerous factors associate with the EMT, and the key regulators of the EMT are E-cadherin, N-cadherin, Twist, Snail, Slug, Zeb-1, Zeb-2, vimentin, and microRNAs. This review focuses on the current concepts regarding the EMT in cancer and the evidence for involvement of the EMT in BC. Several potential EMT targets that may be useful in the treatment of BC are also described.
Keywords: Epithelial-mesenchymal transition, Prognosis, Urinary bladder neoplasms
INTRODUCTION
Bladder cancer (BC) is responsible for the deaths of 150,000 people annually and is the seventh most prevalent type of cancer worldwide [1,2]. At the time of the first diagnosis, about 70% to 80% of BCs are non-muscle-invasive BCs (NMIBCs) and the remaining 20% to 30% are muscle-invasive BCs (MIBCs). Although both BCs originate from the urothelium in the urinary bladder, they have distinct clinical characteristics. Whereas the overall survival rate of patients with NMIBC is excellent compared with that in other malignancies, 30% to 50% of these patients have recurrences after transurethral resection of the primary tumor, and 10% to 20% progress to MIBC [3]. In the case of MIBC, although only 20% of BC patients are diagnosed with MIBCs, these cancers are responsible for the vast majority of BC-specific deaths. Moreover, nearly 50% of patients with MIBC already have occult distant metastases at the time of diagnosis [4,5]. Thus, patients with NMIBC are at risk of recurrence or progression into MIBC, and the prognosis of patients with MIBC is determined by the presence of metastasis.
The epithelial-mesenchymal transition (EMT) is a multistep process in which epithelial cells lose their epithelial characteristics and gain mesenchymal characteristics, such as motility and invasive properties [6]. Numerous in vitro and in vivo studies suggest that the EMT is associated with cancer cell invasion and metastasis in various malignancies, including BC. This review describes the role of the EMT in the recurrence, progression, and metastasis of BC. Furthermore, potential targets in the EMT for BC therapy are discussed.
GENERAL CONCEPTS REGARDING THE EMT IN CANCER
The EMT was first observed during embryonic development [7], but since then interest in this phenomenon has mainly been related to its role in neoplastic progression [8,9]. The EMT is defined as the dynamic switch of a sessile, epithelial cell into a motile cell that has a mesenchymal phenotype (Fig. 1) [10]. It is categorized into three different subtypes [6] on the basis of its function and the pathways that are involved. Type 1 EMT associates with implantation, embryo formation, and organ development and is an organized process that generates diverse cell types that share common mesenchymal phenotypes. Type 2 EMT associates with inflammation and ceases once inflammation is attenuated, as can be seen during wound healing and tissue regeneration. Type 3 or oncogenic EMT occurs in neoplastic cells that have previously undergone genetic and epigenetic changes, specifically in genes that favor clonal outgrowth and the development of localized tumors. Carcinoma cells undergoing type 3 EMT may invade and metastasize, thereby generating the final, life-threatening manifestations of cancer progression. However, the EMT is usually not an irreversible transition, because the cells can return to their epithelial phenotype. This is known as the mesenchymal-epithelial transition (MET) [11].
FIG. 1.
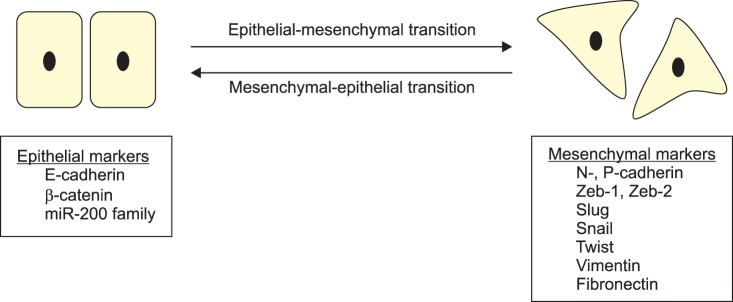
Molecular markers of the epithelial-mesenchymal transition and the mesenchymal-epithelial transition.
Epithelial cells have important barrier functions that are facilitated by their tight cell-to-cell interactions [12]. Loss of these cell-to-cell interactions can induce morphological changes in epithelial cells and increase their cellular motility. The most important mediator of cell-to-cell adhesion in epithelial tissues is cadherin, which is a family of cell-surface adherence junctional proteins. The first cadherins to be identified were E-, P-, and N-cadherin [13]. E-cadherin plays an essential role in epithelial cell-to-cell interactions because it mediates the connections between adjacent epithelial cells and maintains the phenotype and apical-base polarity of epithelial cells [14]. To exert these functions, the cytoplasmic part of E-cadherin interacts with β-catenin, which anchors E-cadherin to the actin cytoskeleton, thereby providing mechanical stability to the cell-to-cell junctions [12]. Due to these functions of E-cadherin, this protein is a key tumor suppressor that suppresses the invasiveness of cancer cells [15,16]. A key change that occurs during EMT is the "cadherin switch," in which the normal expression of E-cadherin is replaced by the abnormal expression of N- or P-cadherin [17,18]. This down-regulation of E-cadherin is associated with the release of β-catenin, which then migrates to the nucleus and activates WNT signaling, thereby resulting in the EMT and metastasis [10].
The EMT is also controlled by a group of transcriptional repressors, namely, Zeb-1, Zeb-2, Twist, Snail, and Slug. Snail1, Snail2, Zeb-1, and Zeb-2 are zinc-finger transcription factors that bind directly to the E-boxes of the promoter of the E-cadherin-encoding gene (CDH1), thereby repressing CDH1 expression [19]. Another important EMT regulator is transforming growth factor (TGF)-β: the TGF-β family members may upregulate Snail and Slug and are potent initiators of the EMT in cancer cells [20-22].
Recent studies suggest that miR-200 family members also participate in the EMT [23-25]. Loss of miR-200 expression leads to the accumulation of Zeb-1 and Zeb-2, which is sufficient to silence CDH1 and promote the EMT and tumor invasion [26]. TGF-beta also negatively regulates the miR-200 family. Although the mechanisms involved are still poorly characterized, this negative regulation leads to the accumulation of Zeb-1 and Zeb-2 and the subsequent suppression of E-cadherin expression [27].
Vimentin is an intermediate filament protein that is characteristically upregulated in cells undergoing the EMT. Consequently, it is frequently used as a marker of cells undergoing the EMT during both normal development and metastatic progression [28]. During the EMT, vimentin expression induces epithelial cell changes, including their adoption of a mesenchymal shape and their increased motility.
EVIDENCE FOR THE EMT IN BC
1. Cadherin
Cadherin is the most important mediator of cell-to-cell adhesion in epithelial tissues, and E-, P-, and N-cadherin were the first cadherins to be identified [13]. The roles of these proteins in BC-related EMT have been investigated extensively. After reviewing numerous reports on cadherin expression in BC, Bryan and Tselepis [29] summarized the patterns of P-cadherin and N-cadherin expression in the bladder during the EMT as follows. In the normal urothelium, P-cadherin but not N-cadherin is expressed by the basal layer. However, during the EMT, P-cadherin expression by the BC is upregulated along with N-cadherin expression; these events occur either independently or synchronously. E-cadherin expression in BC cells is lost after these P- and/or N-cadherin expression changes, and invasion and metastasis are induced. This cadherin switching event is an important process that occurs late in the molecular pathogenesis of BC, although the precise timing and nature of these events remain unknown.
The two pathological types of BC also have different cadherin protein expression patterns [29]. While the mucosal layer in normal urothelium expresses E-cadherin strongly, 20% of Ta and up to 60% of T1 tumors show reduced E-cadherin expression in NMIBC. However, in more than 80% of MIBCs, E-cadherin expression is reduced or completely absent. Similarly, while the normal urothelium usually shows weak basal membranous staining of P-cadherin, and 80% of Ta/T1 tumors also have this weak basal membranous expression, 50% to 88% of advanced MIBCs demonstrate strong P-cadherin expression throughout the tumor mass. In terms of N-cadherin, the normal urothelium does not express this protein at all and over 80% of Ta/T1 tumors also lack N-cadherin expression. However, over 60% of advanced MIBCs express N-cadherin [29].
2. Transcriptional repressors
Several transcription factors are differentially expressed in BC. Twist expression is associated with a poorer prognosis in BC. Moreover, Fondrevelle et al. [30] demonstrated that Twist expression correlates with reduced E-cadherin expression and higher BC stages and grades and is significantly upregulated in metastases compared with primary BCs. This suggests that Twist may play an important role in BC progression and metastasis. Fondrevelle et al. [30] also found that Twist expression is associated with smoking, which is an important risk factor for BC. Wallerand et al. [31] also suggested that Twist expression is linked to variables that associate with a poor prognosis.
Unlike Twist, the roles of Snail, Zeb-1, and Zeb-2 in BC are not clear. However, when Yu et al. [32] investigated Slug, Snail, and Twist expression in BC, they found that Slug and Twist expression is increased in BCs, whereas that of Snail seems to be reduced. Twist expression also rises as the tumor stage, grade, and progression increase. Moreover, as nodal involvement rises, Slug expression increases while Snail expression drops. In addition, E-cadherin expression drops as the tumor grade rises. Positivity for Twist, Slug, and E-cadherin expression is clearly predictive of poorer survival. However, a study by Bruyere et al. [33] showed contrary results in terms of Snail expression in BC. They reported that high Snail expression in NMIBC is a strong predictor of tumor recurrence and could be used to improve risk stratification and prognostication. A study by Kenny et al. [34] that examined the role of Zeb-1 and Zeb-2 expression in BC also returned inconsistent results. First, they showed the novel expression of Zeb-1 in BC but found that it did not associate with any clinical variables of change, including metastasis and survival. However, their in vitro assays then showed that when Zeb-1 expression was induced in BC cell lines by forced expression or blocked by shRNA knockdown, the migration and invasion abilities of the cells were enhanced and reduced, respectively. Kenny et al. [34] concluded that despite the differences between the BC cell lines and tissues, Zeb-1 expression is a prognostic indicator of BC progression.
3. microRNAs
microRNAs may regulate the EMT, with the miR-200 family being the most well-known EMT regulator. An in vitro study with several BC cell lines found that miR-200 levels were increased in epithelial BC cell lines (UMUC5, UMUC9, UMUC6, and UMUC16) and decreased in mesenchymal BC cell lines (KU7, UMUC2, UMUC3, and UMUC13) [35]. This study also showed that increased expression of the miR-200 family directly suppresses Zeb-1 and Zeb-2 expression and the EMT. Thus, the miR-200 family may be a treatment target that reverses the EMT.
Like the miR-200 family, miR-205 also associates with EMT regulation. Wiklund et al. [36] reported that in MIBCs and undifferentiated BC cell lines, miR-200 and miR-205 loci are often silenced and have promoter hypermethylation. They also suggested that miR-200c expression correlates significantly with NMIBC progression. Thus, miR-200 and miR-205 silencing and DNA hypermethylation may be prognostic markers in BC. In addition, TWIST1 binds directly to miR-200 and miR-205 promoters and thus may act as a repressor of miR-200 and miR-205 expression. Recently, Tran et al. [37] reported that high miR-205 expression is associated with adverse clinical outcomes in patients with MIBC, such as poor cancer-specific and overall survival.
Urinary microRNAs are of particular interest in BC research because BC tissues are directly bathed with urine. When we assessed the diagnostic value of urinary miR-200a in 207 patients with primary urothelial cancer in the urinary bladder, we found that the patients with lower miR-200a levels had a higher risk of recurrence than did the patients with higher miR-200a levels [38]. In addition, univariate and multivariate Cox regression analyses showed that the urinary miR-200a level was an independent predictor of NMIBC recurrence. Similarly, when another study investigated the expression of microRNAs in the urine sediment of patients with BC, many markers of the EMT (including Zeb1, vimentin, TGF-β1, and RhoA) associated negatively with several miRNA targets whose urine levels were significantly lower in patients with BC (miR-200a, miR-200b, miR-200c, miR-141, miR-429, miR-205, and miR-192) [39].
4. Other lines of evidence for the EMT in BC
Several reports suggest that vimentin associates with BC grade and stage. When Baumgart et al. [40] subjected 825 BC samples to immunohistochemical staining for E-cadherin, plakoglobin, β-catenin, N-cadherin, and vimentin, they found that vimentin expression was mainly detected in invasive BC (31% in MIBC vs. 7% in NMIBC) and was positively associated with tumor grade and stage. Paliwal et al. [41] also suggested that the immunohistochemical expression of cytokeratin, E-cadherin, vimentin, and Twist correlates with BC stage and grade.
THE EMT AS A THERAPEUTIC TARGET FOR BC
The various lines of evidence discussed above indicate clearly that the EMT is strongly associated with aggressive BC behavior, such as recurrence, progression, and metastasis. This raises the possibility that the EMT may be a target for BC treatment. As mentioned above, E-cadherin is the first and most important regulator of the EMT. In BC, loss of E-cadherin expression is a marker of poor responses to the monoclonal antibody cetuximab, which blocks EGFR binding and thereby down-regulates BC proliferation [42]. Thus, E-cadherin expression levels can predict responsiveness to EGFR-targeting therapy. In addition, when we investigated the chemo-responsiveness of patients with MIBC to cisplatin-based chemotherapy (manuscript submitted for publication), EGFR and S100A9 levels were found to be predictive of chemo-responsiveness. Moreover, the inhibition of EGFR and S100A9 re-sensitized BC cells to cisplatin-based chemotherapy. One of the possible mechanisms of this effect may be the reversal of the EMT. Another possible EMT target in BC may be miR-200 family members, which can control the EMT and resistance to EGFR inhibitors in human BC cells. Adam et al. [35] suggested that miR-200 family expression can be used to identify EGFR inhibitor-sensitive urothelial cancers and that EGFR inhibitor resistance can be reversed by reintroducing miR-200 expression. Another study also showed that exogenous miR-205 can repress Zeb-1 and Zeb-2 levels in BC cells and reverse the EMT by enhancing E-cadherin expression [37]. Therefore, miR-205 may be useful as a BC treatment.
EMT reversal may be associated with the responsiveness of high-risk patients with NMIBC to Bacillus Calmette-Guérin (BCG) treatment. This association may relate to tumor necrosis-related apoptosis-inducing ligand (TRAIL), because Ludwig et al. [43] reported that whereas BCG-treated patients initially had undetectable TRAIL levels, these levels were high after later induction treatments. Moreover, the patients who responded to BCG therapy had significantly higher urine TRAIL levels, which killed BC cells in an in vitro study. Therefore, TRAIL plays a key role in the antitumor effects of BCG treatment. Although there is no evidence of a direct association between TRAIL and the EMT in BC to date, Srivastava et al. [44] demonstrated that a histone deacetylase inhibitor sensitizes TRAIL-resistant breast cancer cells and reverses the EMT. Other studies have also reported a relationship between TRAIL and the EMT in pancreatic and nasopharyngeal cancers [45,46]. Therefore, controlling the EMT and elevating TRAIL levels may enhance the response of NMIBCs to BCG treatment as well as reversing their BCG resistance.
The mesenchymal-associated protein N-cadherin is upregulated during the cadherin switch in the EMT. Whether blocking N-cadherin can reverse the EMT has been assessed in several cancers [47,48]. Shintani et al. [47] reported that in a mouse model of pancreatic cancer, treatment with an N-cadherin-blocking peptide (ADH-1) prevented N-cadherin-mediated tumor progression. Thus, it is possible that such N-cadherin-targeting therapy may be beneficial for patients with BC, especially those with high-risk NMIBC or MIBC.
As previously mentioned, Twist associates clearly with the clinical outcomes of BC, and 40% to 60% of BCs overexpress Twist protein [31]. Moreover, Twist associates with chemo-resistance in BC and ovarian cancer [49,50]. Therefore, Twist-targeting therapy may not only improve chemo-sensitivity, but may also be an important component of cancer treatment.
CONCLUSIONS
The EMT is regarded as a key evolutional change in cancer cells that allows them to migrate to adjacent organs or metastasize to distant sites. In BC, the EMT is closely associated with grave clinical characteristics, such as recurrence, progression, metastasis, and poorer survival. However, the EMT is usually not an irreversible process and strategies that induce EMT reversal (which is known as the MET) may be able to suppress cancer cell migration and metastasis. Therefore, a better understanding of the molecular biological mechanisms of the EMT in BC is likely to greatly expand the development of new therapeutic modalities for this cancer. Moreover, EMT-targeting therapy may also be useful as a personalized medicine approach that complements conventional BC treatments or reduces chemo-resistance, although more studies on this issue are needed.
ACKNOWLEDGMENTS
This work was supported by the research grant of Chungbuk National University in 2012.
Footnotes
The authors have nothing to disclose.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–465. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 4.Messing EM. Urothelial tumors of the urinary tract. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell's urology. 8th ed. Philadelphia: Saunders; 2002. pp. 2750–2751. [Google Scholar]
- 5.Messing EM. Urothelial tumors of the bladder. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh's urology. 9th ed. Philadelphia: Saunders; 2007. pp. 17–2430. [Google Scholar]
- 6.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savagner P, Boyer B, Valles AM, Jouanneau J, Thiery JP. Modulations of the epithelial phenotype during embryogenesis and cancer progression. Cancer Treat Res. 1994;71:229–249. doi: 10.1007/978-1-4615-2592-9_12. [DOI] [PubMed] [Google Scholar]
- 8.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 9.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. [DOI] [PubMed] [Google Scholar]
- 10.van der Horst G, Bos L, van der Pluijm G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res. 2012;10:995–1009. doi: 10.1158/1541-7786.MCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Han Q, Pei D. EMT and MET as paradigms for cell fate switching. J Mol Cell Biol. 2012;4:66–69. doi: 10.1093/jmcb/mjr045. [DOI] [PubMed] [Google Scholar]
- 12.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 13.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 14.Rangel MC, Karasawa H, Castro NP, Nagaoka T, Salomon DS, Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. Am J Pathol. 2012;180:2188–2200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stemmler MP. Cadherins in development and cancer. Mol Biosyst. 2008;4:835–850. doi: 10.1039/b719215k. [DOI] [PubMed] [Google Scholar]
- 17.De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, et al. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130:481–494. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 19.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 20.Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 22.Choi J, Park SY, Joo CK. Transforming growth factor-beta1 represses E-cadherin production via slug expression in lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2708–2718. doi: 10.1167/iovs.06-0639. [DOI] [PubMed] [Google Scholar]
- 23.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelialmesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 26.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 27.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryan RT, Tselepis C. Cadherin switching and bladder cancer. J Urol. 2010;184:423–431. doi: 10.1016/j.juro.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Fondrevelle ME, Kantelip B, Reiter RE, Chopin DK, Thiery JP, Monnien F, et al. The expression of Twist has an impact on survival in human bladder cancer and is influenced by the smoking status. Urol Oncol. 2009;27:268–276. doi: 10.1016/j.urolonc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Wallerand H, Robert G, Pasticier G, Ravaud A, Ballanger P, Reiter RE, et al. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol. 2010;28:473–479. doi: 10.1016/j.urolonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Yu Q, Zhang K, Wang X, Liu X, Zhang Z. Expression of transcription factors snail, slug, and twist in human bladder carcinoma. J Exp Clin Cancer Res. 2010;29:119. doi: 10.1186/1756-9966-29-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruyere F, Namdarian B, Corcoran NM, Pedersen J, Ockrim J, Voelzke BB, et al. Snail expression is an independent predictor of tumor recurrence in superficial bladder cancers. Urol Oncol. 2010;28:591–596. doi: 10.1016/j.urolonc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Kenney PA, Wszolek MF, Rieger-Christ KM, Neto BS, Gould JJ, Harty NJ, et al. Novel ZEB1 expression in bladder tumorigenesis. BJU Int. 2011;107:656–663. doi: 10.1111/j.1464-410X.2010.09489.x. [DOI] [PubMed] [Google Scholar]
- 35.Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiklund ED, Bramsen JB, Hulf T, Dyrskjot L, Ramanathan R, Hansen TB, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 37.Tran MN, Choi W, Wszolek MF, Navai N, Lee IL, Nitti G, et al. The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J Biol Chem. 2013;288:3275–3288. doi: 10.1074/jbc.M112.408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun SJ, Jeong P, Kim WT, Kim TH, Lee YS, Song PH, et al. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int J Oncol. 2012;41:1871–1878. doi: 10.3892/ijo.2012.1622. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Chan ES, Kwan BC, Li PK, Yip SK, Szeto CC, et al. Expression of microRNAs in the urine of patients with bladder cancer. Clin Genitourin Cancer. 2012;10:106–113. doi: 10.1016/j.clgc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Baumgart E, Cohen MS, Silva Neto B, Jacobs MA, Wotkowicz C, Rieger-Christ KM, et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res. 2007;13:1685–1694. doi: 10.1158/1078-0432.CCR-06-2330. [DOI] [PubMed] [Google Scholar]
- 41.Paliwal P, Arora D, Mishra AK. Epithelial mesenchymal transition in urothelial carcinoma: twist in the tale. Indian J Pathol Microbiol. 2012;55:443–449. doi: 10.4103/0377-4929.107777. [DOI] [PubMed] [Google Scholar]
- 42.Black PC, Brown GA, Inamoto T, Shrader M, Arora A, Siefker-Radtke AO, et al. Sensitivity to epidermal growth factor receptor inhibitor requires E-cadherin expression in urothelial carcinoma cells. Clin Cancer Res. 2008;14:1478–1486. doi: 10.1158/1078-0432.CCR-07-1593. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig AT, Moore JM, Luo Y, Chen X, Saltsgaver NA, O'Donnell MA, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava RK, Kurzrock R, Shankar S. MS-275 sensitizes TRAIL-resistant breast cancer cells, inhibits angiogenesis and metastasis, and reverses epithelial-mesenchymal transition in vivo. Mol Cancer Ther. 2010;9:3254–3266. doi: 10.1158/1535-7163.MCT-10-0582. [DOI] [PubMed] [Google Scholar]
- 45.Gao F, Zhao ZL, Zhao WT, Fan QR, Wang SC, Li J, et al. miR-9 modulates the expression of interferon-regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2013;431:610–616. doi: 10.1016/j.bbrc.2012.12.097. [DOI] [PubMed] [Google Scholar]
- 46.Zhang G, He P, Gaedcke J, Ghadimi BM, Ried T, Yfantis HG, et al. FOXL1, a novel candidate tumor suppressor, inhibits tumor aggressiveness and predicts outcome in human pancreatic cancer. Cancer Res. 2013;73:5416–5425. doi: 10.1158/0008-5472.CAN-13-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shintani Y, Fukumoto Y, Chaika N, Grandgenett PM, Hollingsworth MA, Wheelock MJ, et al. ADH-1 suppresses N-cadherin-dependent pancreatic cancer progression. Int J Cancer. 2008;122:71–77. doi: 10.1002/ijc.23027. [DOI] [PubMed] [Google Scholar]
- 48.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 49.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–283. [PubMed] [Google Scholar]
- 50.Chen Y, Li L, Zeng J, Wu K, Zhou J, Guo P, et al. Twist confers chemoresistance to anthracyclines in bladder cancer through upregulating P-glycoprotein. Chemotherapy. 2012;58:264–272. doi: 10.1159/000341860. [DOI] [PubMed] [Google Scholar]


