Abstract
Purpose
The utility of the expression of glucose-regulated protein 78 (GRP78) in the evaluation of prognosis depends on the type of tumor. Hence, we aimed to examine the impact of expression of GRP78 and Bcl-2, which are used in the existing prognostic evaluation of ureter tumors, in the evaluation of recurrence and survival rates of ureter tumors.
Materials and Methods
In 53 patients who had undergone radical nephroureterectomy for a ureter tumor from March 2002 to March 2012, age, sex, T stage, nuclear grade, bladder recurrence, and survival rate were analyzed at the time of the patient's surgery depending on the extent of immunohistochemical expression of GRP78 and Bcl-2.
Results
GRP78 was overexpressed in 25 patients (47.2%). When GRP78 was overexpressed, there was a high T stage (p=0.001) and nuclear grade (p=0.007) and a lot of bladder recurrence (40.0%, p=0.034). Bcl-2 was overexpressed in 16 patients (30.1%), and there were no significant associations with any risk factors (p>0.05, respectively). In the multivariate analysis regarding bladder recurrence, the recurrence rate was higher with higher pT stage (p=0.048) and when GRP78 (p=0.033) was overexpressed. In the Kaplan-Meier survival analysis, although the survival rate was significantly lower in the group in which GRP78 was overexpressed (p=0.03), there was no correlation between Bcl-2 overexpression and survival rate (p=0.07).
Conclusions
Patients with ureter tumors who had overexpression of GRP78 had a high T stage and nuclear grade, a lot of bladder recurrence, and a low survival rate. Therefore, if GRP78 is overexpressed in ureter tumor patients, active postoperative follow-up should be carried out.
Keywords: Bcl-2, GRP78, Urothelial cell carcinoma
INTRODUCTION
Ureter tumors account for 5% of overall transitional cell carcinoma cases [1]. However, bladder recurrence is high after radical nephroureterectomy. According to recent studies, the incidence of bladder recurrence is reported to be 13% to approximately 47% [2,3]. To predict such postoperative bladder recurrence, various biomarkers such as Bcl-2, Ki-67, p53, and PTEN are used [4].
Glucose-regulated protein 78 (GRP78), which serves as a major molecular chaperone at the endoplasmic reticulum (ER), is involved in the folding and assembly of newly synthesized proteins within the ER. Accumulation of misfolded or unfolded protein aggregates in the ER lumen, a condition known as ER stress, leads to the activation of three unfolded protein response signaling pathways. This consequently promotes protein regulation. GRP78 overexpression can be induced by physiological stress, which perturbs ER function and homeostasis. Such overexpression protects against tissue or organ damage under pathological conditions. This suggests an important role for GRP78 overexpression in ureter tumor cell protection. Indeed, its overexpression has been associated with both tumor aggressiveness and an unfavorable prognosis [5,6].
In the present study, we compared pathophysiological characteristics and prognosis in patients who had undergone radical nephroureterectomy for ureter tumors on the basis of the extent of GRP78 and Bcl-2 immunohistochemical expression.
MATERIALS AND METHODS
1. Materials
This study was conducted on 53 patients who had undergone radical nephroureterectomy for ureter tumors from March 2002 to March 2012 at our institution. GRP78 and Bcl-2 immunohistochemistry was performed with use of the acquired specimens. Depending on the intensity of expression, the specimens were divided into two groups: a group with overexpression and a group with lower expression. We analyzed bladder recurrence and overall survival in the two groups. Time to overall survival was calculated from the date of radical nephroureterectomy to the date of death.
Urine cytology was performed in 3-month intervals in all postoperative patients. Recurrence of bladder and ureter tumors was checked by performing cystoscopy or abdomen computed tomography every 6 months.
2. Methods
After examining hematoxylin- and eosin-stained slides of the tissue samples, two to three most representative regions were indicated on the applicable paraffin block. A tissue microarray (TMA) was produced after sampling the indicated tissue by use of a punch measuring 3 mm in diameter and placing it on a new block. The TMA was cut into a thickness of 5 µm and a serial section was made. It was subsequently attached to a slide covered with poly-L-lysine (Sigma Chemical Co., St Louis, MO, USA) and then dried. Immunohistochemistry was performed by use of the iVIEW DAB Detection Kit reagent using the BenchMark XT (Ventana Medical Systems, Oro Valley, AZ, USA) device. GRP78 (×400; Catalog# 3158-1, Epitomics Inc., Burlingame, CA, USA) and Bcl-2 (×400; Zymed Laboratories Inc., San Francisco, CA, USA) were used as primary antibodies. All immunohistochemical staining was carried out in an identical environment, and one pathologist evaluated the extent of Bcl-2 and GRP78 expression. Immunostaining was used to grade the extent of immunoreactivity up to grade 4. Grading was scored as 0 when stained tumor cells were not observed at ×200 view, as grade 1 (mild) when stained cells were less than 25% of those observed, as grade 2 (moderate) when stained cells were between 26% and 50%, and as grade 3 (strong) when stained cells exceeded 50%. Among these, GRP78 was defined as overexpressed when graded as strong expression, whereas Bcl-2 was defined as overexpressed when expression was more than moderate (Fig. 1).
FIG. 1.
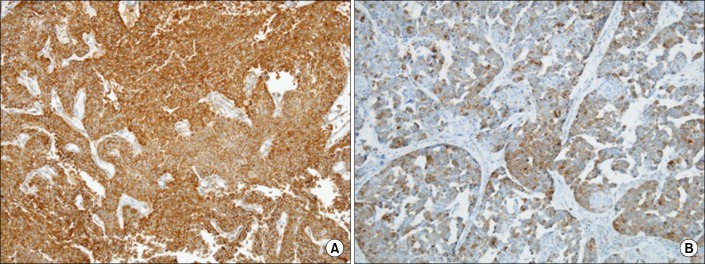
Immunohistochemical stainings for glucose-regulated protein 78 (A) and Bcl-2 (B) show positivity in cytoplasm of tumor cells (×200).
3. Statistical analysis
The groups were compared by using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The correlation between the overexpression group and the lower expression group in terms of age, sex, T stage, and nuclear grade was determined by using the chi-square test and linear by linear association method. Multivariate analysis was conducted between the risk factors of recurrence through use of a Cox proportional hazard model. The correlation between the overexpression group and survival rate followed the Kaplan-Meier method. The comparison of survival curve between the two groups was evaluated by using the Wilcoxon and the log-rank test. It was deemed statistically significant when the p value was below 0.05.
RESULTS
The average age of the patients in this study was 65.9 years (range, 41 to 81 years). There were 33 men and 20 women, and the mean postoperative follow-up period was 38 months (range, 13 to 112 months). Fourteen patients (26.4%) experienced bladder recurrence, and there were 4 (7.5%) deaths. All 4 patients died because of their tumor (Table 1).
TABLE 1.
Characteristics of patients with upper urinary tract (UUT) tumors
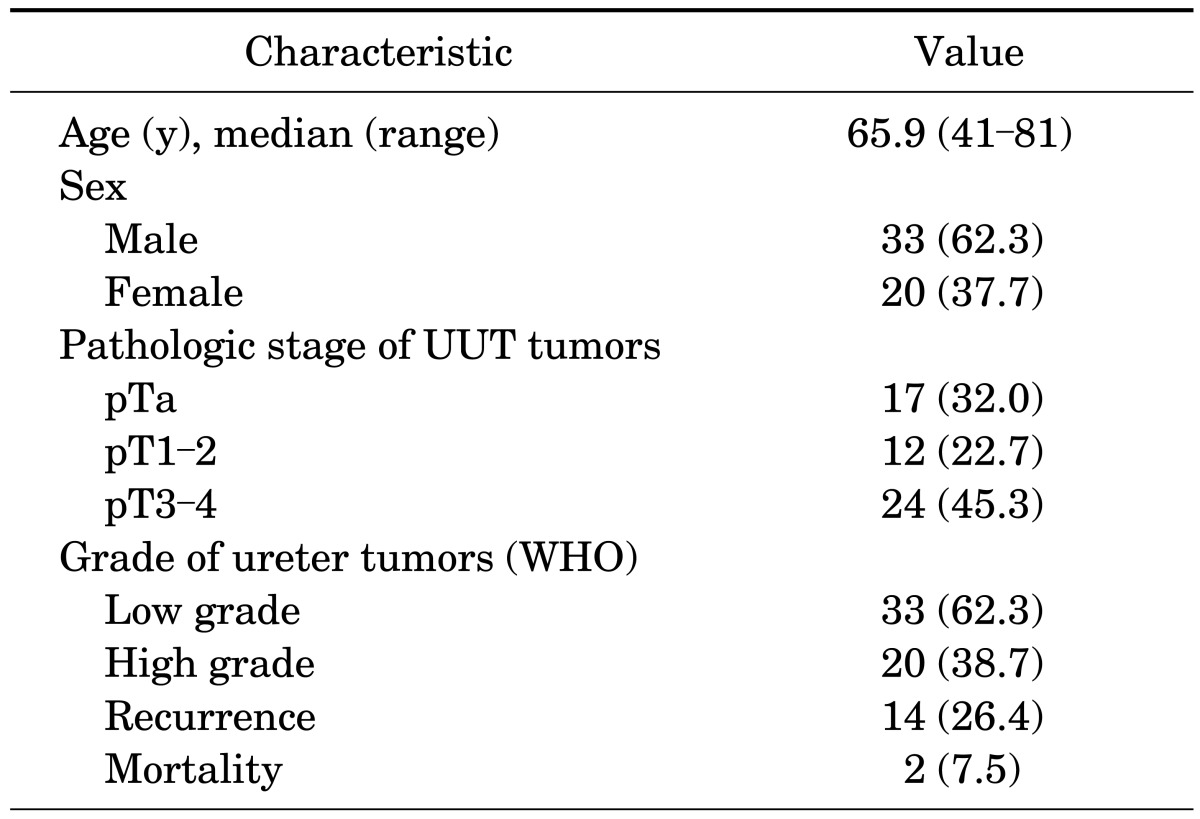
Values are presented as number (%) unless otherwise indicated. WHO, World Health Organization.
GRP78 was overexpressed in 25 patients (47.2%). When GRP78 was overexpressed, there was a high T stage (p=0.001) and nuclear grade (p=0.007), and a considerable number of cases of bladder recurrence (40.0%, p=0.034). There were no significant differences in age, sex, or tumor location according to GRP78 overexpression (p>0.05, respectively). Bcl-2 was overexpressed in 16 patients (30.1%), and there were no significant associations in terms of age, sex, T stage, nuclear grade, or bladder recurrence (p>0.05, respectively) (Table 2).
TABLE 2.
GRP78 and Bcl-2 overexpression and tumor characteristics
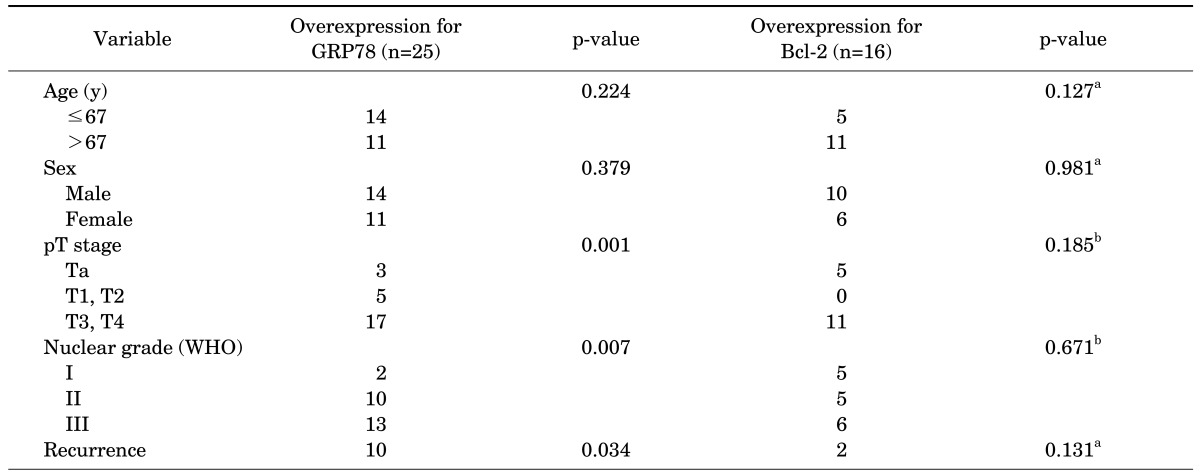
GRP78, glucose-regulated protein 78; WHO, World Health Organization.
a:Chi-square test. b:Linear by linear test.
In the multivariate analysis between each risk factor regarding bladder recurrence, the recurrence rate was shown to be higher (p=0.033) as pT stage was higher (p=0.048) and when GRP78 was overexpressed (p=0.033). There was no statistical correlation between age, sex, WHO grade, and Bcl-2 (Table 3). When Bcl-2 was overexpressed, there was no correlation between age, sex, T stage, nuclear grade, and bladder recurrence. There was also no correlation in the multivariate analysis. In the Kaplan-Meier survival analysis, the survival rate was significantly lower in the GRP78 overexpression group (p=0.03), but there was no correlation between the overexpression of Bcl-2 and the survival rate (p=0.07) (Fig. 2).
TABLE 3.
Cox proportional hazard model for estimating recurrence
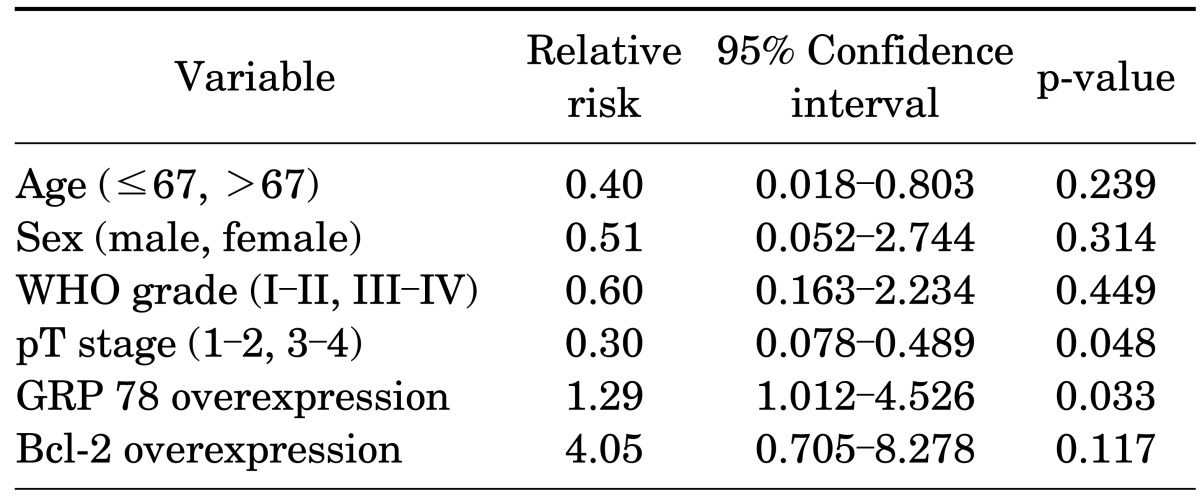
WHO, World Health Organization.
FIG. 2.
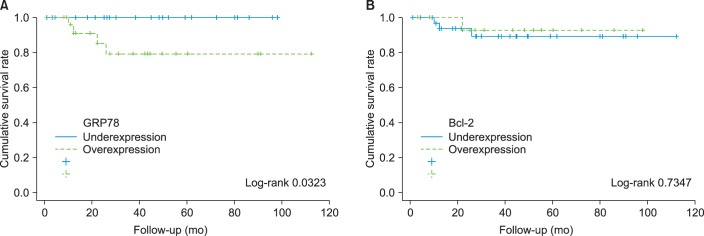
Effect of GRP78 (A) and Bcl-2 (B) overexpression with survival of upper ureter tumor patients from original cohorts. GRP78, glucose-regulated protein 78.
DISCUSSION
Among ureter tumor patients, 13% to 47% exhibit recurrence of transitional cell carcinoma in the bladder after radical nephroureterectomy. The first recurrence is usually 5 to 25 months after radical nephroureterectomy [7]. For ureter tumor patients, postoperative follow-up is a very important factor in successful treatment. Various biomarkers are currently being developed to predict recurrence, but a clinically useful biomarker has yet to be discovered.
GRP78, which serves as a major molecular chaperone bound at the ER, is involved in the folding and synthesis of newly synthesized proteins within the ER. Under normal conditions, GRP78 expression is maintained at a low level in various organs such as the brain, lungs, and heart [5,6,8]. However, GRP78 overexpression can be induced by physiological stress that perturbs ER function and homeostasis. Such overexpression protects against tissue or organ damage owing to pathological conditions such as neurotoxic stress, myocardial infarction, and atherosclerosis [5,9].
On the basis of a multivariate analysis involving lung cancer and neuroblastoma, the mortality risk of lack of expression of GRP is 2.3 times and 3.1 times higher compared with overexpression for each disease, respectively. Hence, GRP78 overexpression is reported to be a favorable prognostic factor [10,11]. Uematsu et al. [12] reported that in patients with upper ureter tumors, the group with GRP78 overexpression tended to have higher disease-free survival than the group with lower expression. A tumor with a lower nuclear grade shows higher GRP78 expression than does a tumor with a higher grade.
However, GRP78 tends to be overexpressed compared with the expression in normal cells in most tumors, and diseases tend to progress as its expression becomes higher. Dong et al. [13] examined a genetic model of breast cancer in GRP78-heterozygous mice and showed that GRP78 acted via three major mechanisms: 1) enhancement of tumor cell proliferation, 2) protection against apoptosis, and 3) promotion of tumor. GRP78 overexpression in cancer cells induces the progression and recurrence of diseases by protecting the tumor itself. Matsuo et al. [14] reported that GRP78 expression increases from visceral adipocytes in endometrial cancer, and the higher the expression level, the higher the probability of disease progression and lower the rate of disease-free survival. Furthermore, Daneshmand et al. [15] claimed that in a study of 153 patients who had undergone radical prostatectomy among hormone-refractory prostate cancer patients, the group with strong GRP78 expression manifested a recurrence rate that was about 2 times higher than that in the group without GRP78 expression. The mortality rate was also 1.8 times higher. With regard to the recurrence in gastric cancer and color cancer (another adenocarcinoma form), Winder et al. [16] reported that the group with a specific GRP78 genotype expression had a 2.6 times higher risk of recurrence and a 3.2 times higher mortality risk than did the group without. Various other reports support these claims [9,10,17-21].
Even in the present study, the GRP78 overexpression group showed a statistically higher T stage and high nuclear grade. In the multivariate analysis, GRP78 overexpression showed a positive correlation with the recurrence of the tumor in the bladder. Furthermore, according to the Kaplan-Meier analysis, the GRP78 overexpression group had a statistically significantly lower survival rate than did the lower expression group. This conflicted with results of studies by Uematsu et al. [12] regarding the prognosis of ureter tumors mentioned earlier, but the results were enough to support the cell protection mechanism of the overexpression of GRP78 in tumor cells, which Dong et al. [13] suggested in an animal experiment.
Bcl-2 is a protein measuring 25 kDa and consisting of 239 amino acids that has been used in the prognostic evaluation of ureter tumors. It is induced by heat, radiation, various chemicals, and steroids and is vital in suppressing apoptosis. It is specifically associated with regulating the progression of apoptosis.
However, Bcl-2 expression exhibits various prognoses depending on the tumor. Bcl-2 overexpression in prostate cancer, leukemia, neuroblastoma, and high-grade lymphoma is considered an unfavorable prognosis factor but it becomes a favorable factor in lung cancer and breast cancer patients [22]. Joung et al. [4] reported that although Bcl-2 overexpression in 65 ureter tumor patients tended to be associated with a high risk of bladder recurrence, this finding was not statistically significant. Similarly, Masuda et al. [23] reported that according to the univariate analysis of ureter patients, Bcl-2 expression was evaluated as a prognosis factor along with the apoptotic labeling index and mitotic index. However, in the multivariate analysis, Bcl-2 expression tended to be associated with a poor survival rate, but this association was not statistically significant. In the present study of ureter tumors, there was no statistical significance in the multivariate analysis or the Kaplan-Meier analysis regarding bladder recurrence in the Bcl-2 overexpression group and the lower expression group.
Bladder cancer that recurred after radical nephroureterectomy can be detected by repetitive follow-ups with the use of abdomen computed tomography or cystoscopy. However, the problem is that patient compliance drops because the procedure is invasive and considerably expensive. If follow-up is concentrated on patients with GRP78 overexpression as shown by postoperative immunohistochemical staining, the efficiency of the test and patients' compliance can be enhanced.
CONCLUSIONS
Patients with ureter tumors who have overexpression of GRP78 showed a high T stage and nuclear grade, a considerable frequency of bladder recurrence, and a low survival rate. Therefore, if GRP78 is overexpressed in ureter tumor patients, more aggressive follow-up should be performed after surgery.
Footnotes
The authors have nothing to disclose.
References
- 1.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 2.Matsui Y, Utsunomiya N, Ichioka K, Ueda N, Yoshimura K, Terai A, et al. Risk factors for subsequent development of bladder cancer after primary transitional cell carcinoma of the upper urinary tract. Urology. 2005;65:279–283. doi: 10.1016/j.urology.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Palou J, Rodriguez-Rubio F, Huguet J, Segarra J, Ribal MJ, Alcaraz A, et al. Multivariate analysis of clinical parameters of synchronous primary superficial bladder cancer and upper urinary tract tumor. J Urol. 2005;174:859–861. doi: 10.1097/01.ju.0000169424.79702.6d. [DOI] [PubMed] [Google Scholar]
- 4.Joung JY, Yang SO, Jeong IG, Han KS, Seo HK, Chung J, et al. Identification of immunohistochemical factors that predict the synchronous or metachronous development of bladder tumors in patients with upper urinary tract tumors. Urol Int. 2008;81:306–311. doi: 10.1159/000151409. [DOI] [PubMed] [Google Scholar]
- 5.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 7.Chen MK, Ye YL, Zhou FJ, Liu JY, Lu KS, Han H, et al. Clipping the extremity of ureter prior to nephroureterectomy is effective in preventing subsequent bladder recurrence after upper urinary tract urothelial carcinoma. Chin Med J (Engl) 2012;125:3821–3826. [PubMed] [Google Scholar]
- 8.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 9.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 10.Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Yoshimastu T, et al. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Hsu WM, Hsieh FJ, Jeng YM, Kuo ML, Tsao PN, Lee H, et al. GRP78 expression correlates with histologic differentiation and favorable prognosis in neuroblastic tumors. Int J Cancer. 2005;113:920–927. doi: 10.1002/ijc.20693. [DOI] [PubMed] [Google Scholar]
- 12.Uematsu K, Ogata S, Nakanishi K, Hiroi S, Tominaga S, Aida S, et al. Glucose-regulated protein 78 expression in urothelial carcinoma of the upper urinary tract. BJU Int. 2010;106:873–878. doi: 10.1111/j.1464-410X.2009.09144.x. [DOI] [PubMed] [Google Scholar]
- 13.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo K, Gray MJ, Yang DY, Srivastava SA, Tripathi PB, Sonoda LA, et al. The endoplasmic reticulum stress marker, glucose-regulated protein-78 (GRP78) in visceral adipocytes predicts endometrial cancer progression and patient survival. Gynecol Oncol. 2013;128:552–559. doi: 10.1016/j.ygyno.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Winder T, Bohanes P, Zhang W, Yang D, Power DG, Ning Y, et al. GRP78 promoter polymorphism rs391957 as potential predictor for clinical outcome in gastric and colorectal cancer patients. Ann Oncol. 2011;22:2431–2439. doi: 10.1093/annonc/mdq771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez PM, Tabbara SO, Jacobs LK, Manning FC, Tsangaris TN, Schwartz AM, et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 18.Song MS, Park YK, Lee JH, Park K. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumor cells through a protein kinase C-epsilon/ERK/AP-1 signaling cascade. Cancer Res. 2001;61:8322–8330. [PubMed] [Google Scholar]
- 19.Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG, et al. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12(20 Pt 1):5987–5993. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 20.Xing X, Lai M, Wang Y, Xu E, Huang Q. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta. 2006;364:308–315. doi: 10.1016/j.cca.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 22.Lu QL, Abel P, Foster CS, Lalani EN. bcl-2: role in epithelial differentiation and oncogenesis. Hum Pathol. 1996;27:102–110. doi: 10.1016/s0046-8177(96)90362-7. [DOI] [PubMed] [Google Scholar]
- 23.Masuda M, Takano Y, Iki M, Asakura T, Hashiba T, Noguchi S, et al. Apoptosis in transitional cell carcinoma of the renal pelvis and ureter: association with proliferative activity, bcl-2 expression and prognosis. J Urol. 1997;158(3 Pt 1):750–753. doi: 10.1097/00005392-199709000-00014. [DOI] [PubMed] [Google Scholar]


