Abstract
Our survival and wellness require a balance between optimism and pessimism. Undue pessimism makes life miserable; however, excessive optimism can lead to dangerously risky behaviors. A review and synthesis of the literature on the neurophysiology subserving these two worldviews suggests that optimism and pessimism are differentially associated with the two cerebral hemispheres. High self-esteem, a cheerful attitude that tends to look at the positive aspects of a given situation, as well as an optimistic belief in a bright future are associated with physiological activity in the left-hemisphere (LH). In contrast, a gloomy viewpoint, an inclination to focus on the negative part and exaggerate its significance, low self-esteem as well as a pessimistic view on what the future holds are interlinked with neurophysiological processes in the right-hemisphere (RH). This hemispheric asymmetry in mediating optimistic and pessimistic outlooks is rooted in several biological and functional differences between the two hemispheres. The RH mediation of a watchful and inhibitive mode weaves a sense of insecurity that generates and supports pessimistic thought patterns. Conversely, the LH mediation of an active mode and the positive feedback it receives through its motor dexterity breed a sense of confidence in one's ability to manage life's challenges, and optimism about the future.
Keywords: optimism, pessimism, depression, embodied cognition, hemispheric asymmetry, cerebral lateralization, laterality, handedness
INTRODUCTION
Optimism and pessimism - expecting a positive or negative future - are distinct modes of thinking that are best conceptualized, not rigidly and dichotomously but rather, as a continuum with many degrees of optimism and pessimism. A person can be optimistic in regard to a specific area of life (e.g. expecting his/her marriage/relationship to succeed) but pessimistic regarding other aspects (e.g. expecting financial difficulties ahead). People also may shift positions on the optimism-pessimism continuum as the timeline unfolds. We all have 'sunnier' days in which we wear the rosy and bright glasses, and 'rainier' days when the world is seen through the gray and dark glasses.
Some people, more than others, have a consistent tendency to think, feel and behave, regarding most aspects of their lives, in a way that is unbalanced and inclined toward one of the extremes on the optimism-pessimism continuum; we call them optimists and pessimists. An optimistic person sees good things everywhere, is generally confident and hopeful of what the future holds. From the optimist's point-of-view the world is full of potential opportunities. The pessimist, on the other hand, observes mainly the negative aspects of everything around. Thinking of all the potential dangers and pitfalls on the way, the pessimist is likely to have little hope for the future. Consequently, the pessimist tends to remain passive when encountered with a challenge, believing that his efforts are futile anyway.
As the main theme of this article is optimism and pessimism as experiential phenomena, whether they are expressed briefly as a mood, a temporary state of mind, or as a continual attitude, i.e. a stable personality trait, the terms 'optimism' and 'optimist' (as well as 'pessimism' and 'pessimist') will be referred to interchangeably.
Successful living requires a fine balance between optimism and pessimism. Over-optimism may encourage one to take uncalculated financial risks that will end up disastrously [1]. Similarly, over-confidence may lead to negligent and reckless behaviors - e.g. not taking the necessary precautions to prevent common health and fires hazards - which may result in a catastrophe. On the other hand, worrying too much about potential dangers and focusing one's energy on what might go wrong leads to avoidance behavior, passivity, exacerbation of low mood and an increase in the vulnerability to depression. Therefore, the optimal equilibrium is a cautious optimism which is firmly grounded in reality [2].
Is it possible to study optimism and pessimism in an objective, methodical and scientific manner? Indeed, although optimism and pessimism are uniquely and differentially applied in every individual's life, and despite the broadness of the subject matter, research on optimism is based on empirical and quantitative assessments. An operational definition of optimism and pessimism is anticipation of good or bad things to happen in the future, respectively [3]. Researchers have developed a set of scales that enable ranking people along the optimism-pessimism continuum in a fairly accurate manner. These instruments include the Unrealistic Optimism Scale [4], the Attributional Style Questionnaire [5], the Life Orientation Test [6, 7], the Hope Scale [8], the Generalized Expectancy for Success Scale [9, 10] and the Optimism-Pessimism Scale [11]. These self-report questionnaires comprise several statements about real or hypothetical events (e.g. 'I hardly ever expect things to go my way') and the person rates each of them (e.g. from 1 to 5) how much it describes his/her attitude. An individual's total score on such a scale provides a quantifiable measure of optimism/pessimism.
Like all other human experiences, these basic mental attitudes of optimism and pessimism are closely interlinked with distinct physiological processes. Converging evidence from psychology, physiology, psychiatry and neurology suggest that the two cerebral hemispheres are differentially involved in mediating the fundamental approach to life. Pessimistic views are generally mediated by the right-hemisphere (RH), whereas optimistic attitudes are mediated primarily by the left-hemisphere (LH). This article presents the variety of ways that this lateralization of optimism and pessimism is manifested in the thoughts, feelings, decisions and behaviors of healthy and unhealthy people. In addition, the article discusses the biological origins of this laterality.
Before proceeding further on the issue of brain lateralization, a clarification is needed to avoid simplifications such as 'the RH is doing X and the LH do Y' etc. Every human experience is mediated by both the RH and the LH. Nonetheless, the two hemispheres mediate different modes of experiencing the world and dealing with it. Under normal circumstances we are unaware of any asymmetry between the RH and LH, since the two brain hemispheres share information between themselves, via the corpus callosum, and the combined and integrated input results in a unified and coherent experience of ourselves and the environment. However, extreme conditions which amplify (or minify) the contribution of one hemisphere to the mental experience provide vivid manifestations of the two hemispheres' fundamental differences. For instance, in split-brain patients whose corpus callosum has been severed (usually in a surgical procedure aimed to prevent dangerous epileptic seizures) the inter-hemispheric flow of information is disrupted and each hemisphere functions as an independent brain within the same person. Similarly, patients with unilateral brain lesions perceive the world mainly through the intact hemisphere, i.e. the damaged hemisphere is the minor contributor, and the intact hemisphere the major contributor, to the patient's overall mental experience and impression of the world. Likewise, researchers try to create similar condition in the laboratory, with healthy people whose brains are fully intact (i.e. both hemispheres are assumed to contribute equally to the person's experiences), by manipulating various variables in order to produce a temporary change in the relative balance of activation between the two hemispheres, thereby creating optimal conditions for a particular hemisphere to manifest its unique processing style or advantage in a specific task. These techniques will be described shortly throughout the manuscript. All these unusual conditions reveal that our ordinary conscious experience is actually a synthesis of the output of both hemispheres, while at source each hemisphere mediates a different mode of experiencing the world [12-20].
Cognitive mechanisms of optimism and pessimism
Optimism and pessimism are associated with distinct perceptual and cognitive modes. The principal differences are: a) Selective attention and information processing. b) A belief (or lack thereof) that one has power to influence relevant situations, events and relationships (i.e. locus of control). c) The general schema one holds for interpreting personal events (i.e. attribution style).
Selective information processing
To use the common expression, the optimist focuses his attention on the glass half full; that is, an optimist selects the positive/reinforcing cues from the environment, and tends to filter and ignore information that does not match his brighter outlook. A pessimist tends to do the exact opposite. In general, a pessimist's attention is focused on the glass half empty; i.e. the pessimist allocates a disproportionately greater attention to the negative cues, while tending to forget the positive aspects of a situation. This conventional wisdom has been confirmed experimentally. Eye-tracking studies showed that optimists gazed at negative/unpleasant images less than pessimists [21, 22]. Similarly, optimism was found to be associated with a greater attentional bias toward positive stimuli relative to negative stimuli [23].
The two cerebral hemispheres are differentially attuned and receptive to environmental cues. The LH is more attuned to positive stimuli. In a split visual-field study, participants' attention was tested while they watched word pairs comprising of a positive and a negative word (e.g. 'vibrant' and 'failure'). When the positive word appeared on the right visual-field (RVF) and the negative word on the left visual-field (LVF) participants' attention shifted significantly toward the RVF, however, when the direction was reversed, so that the positive word appeared on the LVF and the negative word on the RVF, participants attended equally to both visual fields [24]. Since stimuli in the RVF are initially processed by the LH and vice versa, these findings suggest that the LH is preferentially attuned to positive information and shifts one's attention towards it.
In contrast, the RH is more attuned to negative information. When anxious individuals watched pairs of happy and fearful faces, there was a significant attentional bias toward the fearful face when it was presented in the LVF, but not in the RVF [25], implying that the RH allocates greater attention to negative emotional stimuli over positive emotional stimuli. Similarly, when participants watched words of likeable or unlikeable traits and rated how much each trait described themselves, likeable traits were more accepted as representing oneself when they were presented in the RVF (i.e. to the LH) and unlikeable traits were rejected more when they appeared in the LVF [26], suggesting that the left and right hemispheres are differentially attuned and receptive to positive and negative stimuli, respectively.
The same lateralization of processing positive and negative information applies to auditory stimuli. Participants in an experiment listened to a recorded message emphasizing the negative effects of being exposed to the sun without a sunscreen. The message was delivered via headphones only to one ear; the right or left ear. Analysis of participants' behavior showed that the group who received the distressing message through the left-ear (i.e. the RH) was more affected by the message and was more likely to use sun tan lotions at the beach [27].
When attention to the positive and brighter side of life does not come naturally, it can still be achieved through a conscious and mindful effort, and this process involves the LH. Cognitive re-appraisal is often used in psychotherapy where a person is trained to change his/her point-of-view on negative events by focusing on the positive aspects. In functional magnetic resonance imaging (fMRI) experiments designed to elucidate the brain areas involved in this mental process, participants watched a series of negative photographs and were asked to re-appraise them by imagining that the situation had a better outcome than the one suggested. For instance, in response to a picture of the car accident, the participants could imagine that the victims had only minor injuries and they survived and healed well. Analyses of the brain activity showed that while participants tried to apply the 'positive-thinking' strategy there was a higher physiological activity in regions within their LH [28-30].
The LH is associated not only with a temporary optimistic mental state but also with optimism as a stable personality characteristic. A positron emission tomography (PET) study showed that people who frequently use the cognitive re-appraisal strategy and try to attend to the positive aspects of a situation display a higher metabolic activity in the frontal part of their LH [31]. Similarly, when healthy participants are asked to mark the middle of a horizontal line, they tend to err and mark a point which is somewhat to the left or to the right of the true center. Individual differences in the direction and the degree of deviation can serve as a measure of hemispheric dominance. A systematic tendency to favor the left-side of space indicates a dominant RH and vice versa. It has been shown that people who deviate to the right - i.e. having a dominant LH - tend to be more optimistic [32].
Transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) are methods for temporarily stimulating or interfering with the physiological activity in selective brain regions by applying direct electrical current or magnetic pulses over the desired location [33]. In a study where participants rated a series of negative pictures, a tDCS enhancing the activity in their LH frontal regions influenced their ratings to be less negative than their own ratings without brain stimulation [34]. In contrast, TMS aimed to increase physiological activity in the frontal parts of the RH resulted in an impaired ability of the participants to ignore negative information [35]. Together, these studies demonstrate the existence of a fundamental difference between the two hemispheres; the LH mediates a brighter outlook on the world and ignores negative aspects in one's environment, while the RH mediates a tendency to concentrate on the glass half empty which leads to the anticipation of a dismal future.
Locus of control
A general belief about one's ability or inability to control important aspects of life is a crucial element that determines a person's attitude. Those who believe that events in their lives are controlled by outside forces (i.e. external locus-of-control) see themselves as relatively passive agents. Success and achievements occur, in their view, mainly due to luck, chance, affiliation with powerful people or institutions etc. The probability of a person succeeding by his own actions and efforts is very low, in their opinion. This fatalistic view is at the core of pessimism. In contrast, the optimists view themselves as active agents, feel that they are the masters of their destinies (i.e. internal locus-of-control), and trust their ability to influence their environment as well as social relationships [36].
Several studies suggest that a sense of inner locus-of-control is associated with LH activation, while an outer locus-of-control is linked with the RH. Comparisons between epileptic patients whose seizures originate from a sudden electrical discharge in their RH and those with LH epileptic foci, revealed that the latter group tended more to think of themselves as powerless and that their successes were caused by external factors rather than by their own efforts - an external locus-of-control mindset [37, 38]. Since in patients with LH epileptic foci the intact RH has a relatively greater influence on their mental experiences, these studies suggest a link between the RH and a sense of external locus-of-control.
Likewise, it was found that individuals with a perceived internal locus-of-control were better in semantic decision tasks engaging regions within the LH, whereas those with a perceived external locus-of-control performed better in a visuospatial navigation task that recruits RH neural systems [39-41]. These results suggest that inner control perception is associated with the LH - hence its superiority in LH tasks - and vice versa for outer sense of control and the RH. This link between perceived external locus-of-control and RH activation was associated with depressed mood [42, 43].
Again, not only a person's fundamental belief about the world and his/her stable personality traits and convictions about inner or outer sources of control are lateralized in the brain. Experiments have shown that manipulating one's perception about his ability or inability to control a specific situation results in physiological changes in the activation dynamics of the two hemispheres. In one study, electroencephalography (EEG) recorded the brain activity of students while they were exposed to a radio message about the university's new decision to increase their tuition payments. Half of the participants were told that the board's decision is final and the tuition increase would definitely take place. The other half was led to believe that they could reverse the decision by signing a petition against it. This manipulation created a group who believed they had no control over a specific negative situation, and another group who believed that their efforts could affect the outcome. The EEG data showed an increase in LH activation, selectively in the latter group [44].
Similarly, in other experiments, participants wrote an essay about a personal experience from their past in which they felt either having power over other individuals (i.e. a situation with internal locus-of-control) or that someone else had power over them (i.e. external locus-of-control). This writing assignment evoked corresponding feelings of being powerful or powerless, and the former group's EEG measurements revealed a greater activity in the left frontal cortex [45]. In contrast, the latter group showed a greater tendency to bisect horizontal lines to the left of the veridical center, as well as to bump into the right wall while walking through a narrow corridor - perceptual biases indicating a RH dominance [46]. Collectively, these studies demonstrate that a person's sense of having power or being powerless is associated with the LH and RH, respectively.
Attribution styles
Another difference between the optimist and the pessimist is their way of interpreting important events in their lives (i.e. attribution style), which has a momentous effect on their general attitude towards the world. Attributions of unsuccessful outcomes to internal, stable and global factors (e.g. I am incompetent and always fail in everything I do) lead to a pessimistic view since these factors are beyond one's control. Conversely, ascribing the outcome to external, temporary and local causes (e.g. the situation was very difficult and impossible, so that particular attempt was not very successful) enable one to hope for better results in the future. For successful results the reverse is true. Ascribing one's success to internal, stable and global factors (e.g. I am capable of succeeding in everything I chose to do) enhances one's good feelings and increases his/her confidence about future achievements. On the other hand, attributions of an accomplishment to external, temporary and local causes (e.g. this time I got lucky, it probably won't ever happen again) diminishes the positive reinforcement of a success [47-49].
In a dichotic listening experiment, participants heard a set of instructions through headphones either with their left or right ear. Following this task, the group that attended mainly to the right-ear and thereby employed mostly their contralateral LH attributed their successes to internal, stable and global causes, while failures were blamed on external, unstable and specific causes [50]. This study demonstrates that a positive thinking and attribution style which facilitates an optimistic view of the future is associated with the LH. Together with the aforementioned evidence, it seems that the RH and the LH are engaged in mediating different and opposite modes of thinking.
The optimism bias
In some areas of life, people tend to be slightly more optimistic than they ought to be. Studies show that many people believe that they are less at risk of experiencing negative events in the future compared to other people. In addition, many people feel that they have better chances than their peers to experience positive events [4]. This phenomenon of 'optimism bias' has long puzzled researchers because of its paradoxical nature; by definition the probabilities of random events (negative or positive) are equal for everyone. Nonetheless, the optimism bias is assumed to exist, despite its irrationality, due to its beneficial and self-serving properties - i.e. it helps individuals cope with an unknown future and it protects their self-esteem [51].
This unrealistic optimism is enabled by selective information processing. It was demonstrated in experiments where participants estimated their probabilities of experiencing a range of positive and negative events (e.g. having a happy marriage, winning the lottery, or suffering from cancer, Alzheimer's disease etc.). Later, they were informed about the real probabilities of these events occurring to them, based on actual statistical records segmented by socio-demographic, location and other characteristics. When asked to give a second estimate about their chances of experiencing the same events, the participants tended to update their knowledge mainly when the new information was in their favor (i.e. when the positive events were statistically more likely to occur, or that the probabilities of negative events were lower, than previously estimated). However, when the newly learned facts were not in their favor, the participants tended to ignore it, and at the second round they forgot to correct and update their estimations [52, 53].
Analysis of participants' brain activity during that assignment revealed that the right inferior frontal gyrus was activated when the new information called for pessimistic adjustments but not when it called for optimistic adjustments. Furthermore, this brain region which selectively coded pessimistic information showed differential activation within the group; it was less active among the optimistic participants and more active in the pessimistic participants [52, 53]. This study demonstrates clearly how selective information processing which focuses on the positive side and ignores negative information leads to optimism, and that this process involves attenuation of activity in a certain region of the RH.
Optimistic and pessimistic attitudes can also be induced by manipulating the brain's physiological activity in a way that selectively enhances activity in one hemisphere. Participants in an experiment were randomly assigned into a task requiring them to turn their heads and eyes rightward or leftward of their body orientation. Both groups estimated the likelihood of several specific positive and negative events happening to them and to other people in the near future. The results indicated that those who were induced to orient their head and gaze rightward, thereby enhancing neural activation in their contralateral LH, had a greater optimism bias, i.e. they believed that the positive events were more likely to occur to them than to other people [12]. The same effect of optimism bias occurred when participants listened to audio stimuli monaurally with the right ear, which temporarily shifted their inter-hemispheric balance into a relative LH dominance [54].
Caloric vestibular stimulation (CVS) is a standard neurological technique, in which cold water are irrigated into the ear canal, for diagnosing vestibulo-ocular reflexes. A side-effect of this procedure is a temporary enhancement of activity in the contralateral hemisphere. Experiments with healthy participants estimating their chances of suffering from various diseases while they underwent left-ear CVS, right-ear CVS, or no CVS, showed that left-ear CVS, which momentarily boosts activity in the RH, made participants significantly less optimistic and more likely to believe that they were vulnerable to health misfortunes in the future [55, 56].
A study on the optimism bias found that a TMS interference to the activity of the right inferior frontal gyrus caused healthy participants to be more optimistic; they judged the probabilities that good things will happen to them in the future higher than their own estimations without TMS [57]. Furthermore, when the stimulation site was reversed and the TMS was applied to the left inferior frontal gyrus (i.e. temporarily interfering with the activity in the LH) their optimism bias disappeared and their estimations resembled an overly pessimistic thinking [57, 58].
Hypochondriasis
Hypochondriasis is a mental disorder with excessive worry of having a serious illness that has not been diagnosed [59]. A typical hypochondriac will interpret minor physical symptoms as signs of a serious disease, will undertake many unnecessary medical checks and will express doubts and disbelief at a physician's assurance that he/she is perfectly OK. In a way, hypochondriasis can be viewed as the opposite of an optimism bias, i.e. a 'pessimism bias', in regard to health issues. A comparison of the clinical profiles of patients complaining about chronic pain in their left-side of the body with patients with chronic pain in their right-side of the body found greater proportions of hypochondriacs among the left-side pain patients [60]. Since the left-side of the body is innervated by the RH, it implies a strong link between hypochondriasis - i.e. a pessimistic view about one's health - and the RH. Similarly, a comparison between epileptic patients with a predominant RH-lesion and those with a LH-lesion found more manifestations of hypochondriasis in the latter group, whose psychological profile is presumably dominated by their intact RH [61].
Anosognosia
Some patients with motor paralysis after stroke lack awareness of their deficits or simply deny them - a neurological condition called anosognosia [62]. A typical patient with anosognosia for hemiplegia cannot move his paralyzed limb but still insists that nothing is wrong. Based on several lines of evidence, some scholars argue that anosognosia and denial of one's illness or motor dysfunctions is not merely a manifestation of the loss of sensorimotor feedback which results in a cognitive impairment, rather anosognosia includes a significant motivational element and therefore it can be viewed as a delusion, i.e. the patient creates a self-serving wishful representation of reality [63-66]. Given the consensus in the literature that the frequency of anosognosia in unilateral lesions is far greater after RH, compared to LH, stroke [e.g. 67, 68], this phenomenon further suggests the relatively greater involvement of the LH (that is intact in RH stroke patients and therefore it dominates their mental experiences) in painting a nicer and more optimistic picture of one's condition.
Furthermore, some stroke patients (even those who are not officially diagnosed with anosognosia) underestimate their limitations and despite the nurses' warnings they try to stand up and walk unassisted which often end up in falling accidents. Neurologists, noting this ungrounded optimism, devised a questionnaire that measures a patient's risk of falling accidents. It is based on comparing the patient's self-rating of his ability to perform a range of daily activities with a more objective source - the caregiver's ratings. The difference between these two ratings indicates how over-optimistic a patient is and how strictly he should be monitored. Studies comparing patients with a RH stroke to those with a LH stroke found that the former group (whose brain is dominated by the undamaged LH) overestimated their abilities to perform daily activities significantly more than the latter group [69, 70]. This is yet another demonstration of the LH mediation of an over-optimistic and unrealistic assessment of one's physical ability.
The RH, in contrast, mediates a more critical and less biased judgment about oneself. In a morphometric study, patients with various neurodegenerative diseases underwent a set of neuropsychological tests and the researchers asked them, after the assessment, to rate how well they think they did on the tests compared to other people. Analysis of the correlation between the patients' estimates of their own cognitive performance and their brain scan images found that reduced gray matter volume in the right ventromedial prefrontal cortex (vmPFC) was associated with greater overestimation of one's abilities, and that the accuracy of self-appraisal correlated positively with the tissue size in the RH vmPFC [71]. Similarly, a study on individuals with traumatic brain injuries (mostly resulting from automobile accidents) found that a more accurate insight into one's post-injury cognitive deficits was associated with the functioning of specific neural networks in the RH [72]. These studies underscore the RH involvement in mediating a more realistic and less exaggerated evaluation of oneself.
Self-esteem
Self-esteem is a person's evaluation and appraisal of him/herself. It is one of the factors leading to a sense of happiness and a general success in life [73]. Self-esteem is closely linked with one's basic outlook on life and the motivation to deal with challenges. Those with a high self-esteem feel confident, capable, worthy and tend to be optimistic, whereas people with a low self-esteem are typically more critical of themselves, somewhat insecure, often feel incapable of dealing with life's challenges, and are generally pessimistic [74, 75].
Psychological studies show that high self-esteem is associated with the LH. In a dichotic listening experiment, words describing positive or negative traits were presented either to the right-ear (i.e. LH) or to the left-ear (i.e. RH) and participants' task was to attribute each of these traits either to themselves or to other people. Faster response times for linking good qualities to themselves and negative traits to others were observed when the words were presented through the right-ear [76]. Similarly, rightward errors in the line bisection test, which indicate a relative LH hyperfunctioning, were associated with a tendency of participants to describe themselves as active, dominant, mighty, powerful and strong [77].
EEG studies revealed that a greater physiological activity in the frontal parts of the LH, relative to the RH, is associated with a higher self-esteem [78, 79], a general sense of happiness and well-being [80, 81], as well as a better resilience and recovery from an abusive childhood [82]. In contrast, greater physiological activity in the frontal parts of the RH is associated with an increased risk of feeling hopeless about negative events in one's life, and a higher likelihood of falling into depression [83]. Likewise, neurological reports suggest that LH stroke patients, whose emotional and psychological profile presumably reflect the intact RH, have a relatively lower self-esteem [84], and their caregivers suffer from greater stress [85], as compared to RH stroke patients. These studies demonstrate how a high self-esteem and good appreciation of oneself are associated with the LH, while a low self-esteem and a negative attitude are linked with the RH.
Body image
A person's body image is an important factor of his/her self-esteem. Accordingly, the RH involvement in negative-thinking and low self-esteem is reflected also in body image perceptions. Healthy women viewed size-distorted pictures of themselves and had to decide for each image whether it was fatter or thinner than their real body. The results indicated that when the pictures were presented in the left visual-field (i.e. to the RH) they were judged as 'fatter' [86]. In a rare psychiatric disorder, called 'body integrity identity disorder' (BIID), able-bodied individuals feel that their body form is inappropriate and desire an amputation of their own limb, which they describe as not belonging to them [87]. Interestingly, several cortical abnormalities were found in the RH of individuals with BIID [88]. Furthermore, the majority of these individuals desire a removal of their left limb [89, 90] which is innervated by the RH. These findings imply that dissatisfaction with one's body shape is associated with neurophysiological processes in the RH.
The RH also mediates a more critical and harsher judgement about other people's faces and bodies. When adults and children judged facial images of boys and girls with strabismus, a squinting right-eye was rated as more disturbing than a squinting left-eye [91]. Given that the right-eyes in the photographs were within the perceiver's left visual-field and processed initially by his RH, and vice versa for the left-eyes, these findings suggest that the RH is involved, more than the LH, in mediating an unfavourable look at bodily imperfections.1) Likewise, when patients with anorexia or bulimia watched drawings or pictures of female bodies and rated their attractiveness, increased activity in several RH regions were observed when the drawings and pictures were disliked by the patients [93-95]. Together, these studies link the RH with a relatively less favorable viewpoint. That is, the tendency to see more body imperfections (real or imagined) and exaggerate their significance is associated primarily with the RH.
Persistence
When faced with obstacles on the way toward a desired goal, the pessimist will usually give-up quite fast. An optimist, on the other hand, tends to be more determined and persistent in his/her efforts to achieve a goal [3]. Volunteers in an experiment squeezed a rubber ball either with their right or left hand. This physical exercise, which its purpose was hidden from the participants, was aimed to selectively activate the contralateral hemisphere that executed the muscle activations. Then, participants received a cognitive challenge; to trace some figure drawings without backtracking or removing the pencil from the paper. Two of these puzzles were easily soluble but the other two were insoluble. Measuring how many attempts participant made to solve the insoluble puzzles revealed that the group which previously stimulated the LH, by activating their right-hand muscles, made about 60% more attempts to solve the problems as compared with the RH-stimulated group [96]. In another study, the hemispheres were unilaterally stimulated by a passive sensory stimulation - tactile vibrations applied to the contralateral hand - instead of active ball squeezing. Similar results were obtained; the LH-stimulated group showed greater persistence in trying to solve the (insoluble) puzzles [97].
Risk-taking
Personal growth and success - achievement of meaningful things and desired states - require courage and readiness to take some risks, i.e. getting out of one's 'comfort zone', stepping into the unknown and doing things that initially may seem to have some elements of risk/threat [98-100]. Optimism leads one to take more reasonable risks, whereas pessimism is associated with a reduced tolerance for risks [101, 102]. Apart from extreme conditions where risk-taking can be motivated by hopelessness and a desperate sense of 'there is nothing to lose anymore' [e.g. 103], in moderate and normal situations risking something important is not done with the intention to lose. Rather, taking a risk reflects the person's optimistic forecast that the outcome will be favorable. In other words, risk-taking implies an underestimation of the chances to lose and/or an overestimation of the chances to win.
There is evidence in the literature that risk-taking is associated with the LH and risk-avoidance with the RH. Induction of a rightward attentional orientation (thereby activating the LH) can increase the propensity for risk-taking [104]. Similarly, LH dominance, as indicated by the line bisection test, is associated with a higher preference for risks [32]. Risk aversion, on the other hand, is positively correlated with baseline cortical activity in the right prefrontal cortex [105]. Likewise, brief interruptions of inter-hemispheric balance with tDCS or TMS can affect a person's choice in decisions that involve risk-taking. Enhancement of the RH activity resulted in less risky decisions in a gambling session [106], while disruption of the RH activity or enhancement of the LH activity is capable of inducing more risky behaviors [107, 108]. When patients with unilateral lesions in the prefrontal cortex played the Iowa Gambling Task (a measure for risk-taking), those with the RH-lesion preferred the riskier decks, compared to LH-lesion patients and controls, and this preference correlated with the lesion volume [109, 110]. Collectively, these studies suggest that risk-taking is mediated by the LH and that the RH mediates the temperance and suppression of risk-taking tendencies. At the broader context of hemispheric asymmetry in optimism and pessimism, risk-taking and risk-avoidance are behavioral manifestations of these attitudes, respectively.
Depression
Perhaps, the most striking evidence, for the two hemispheres asymmetric role in mediating optimism and pessimism, comes from the association between the RH physiological activity and depression - a pathological state of pessimism. Depression is characterized by overly pessimistic thoughts, a negative-thinking style and a tendency to focus and ruminate on what is wrong and magnify it, while ignoring the good things in one's life. A variety of physiological and neurological studies have shown that depression is associated with a hyperactive RH and/or a hypoactive LH [111-118]. Furthermore, the severity of the illness correlates with the relative imbalance of the physiological activity in the two hemispheres [119, 120].
Accordingly, recovery from depression is associated with a restoration of the inter-hemispheric balance. Studies of different treatment methods (e.g. pharmacological, meditation and mindfulness techniques, massage or music therapy) have found that the post-depression stage is characterized by a reduced imbalance in the physiological activity of the two hemispheres [121-124]. In addition, some novel treatments for depression are based on the principle of directly manipulating the brain's physiological activity, in an attempt to restore the inter-hemispheric balance - i.e. increasing activity in the frontal regions of the LH and/or attenuating activity in the RH. Three of these methods, EEG neurofeedback, TMS and tDCS have been shown to alleviate depressive symptoms while reinstating a balanced neurophysiological activity [125-138]. Similarly, right-ear CVS which temporarily increases activity in the contarlateral LH (thereby minimizing the inter-hemispheric imbalance in depression) has been proposed as an alternative or complementary treatment for alleviating depression [139, 140].
Mania
While the extreme pessimism of depression is associated with a hyperfunctioning RH, mania - elevated mood, euphoria and unjustified optimism - occurs when the LH is hyperactivated. Neurological reports indicate that whilst patients with LH stroke (i.e. having a relatively undamaged RH) tend to be pessimistic and depressed, RH stroke often leads to euphoric and manic states [141-157].
Similarly, Parkinson's disease typically starts with asymmetrical motor deficits; the side with the most severe motor symptoms is contralateral to the hemisphere with the worst dopamine denervation. A study found that patients with motor impairments predominantly on the left-hand - indicating a damaged RH and a relatively less-damaged LH - have higher frequencies of hypomania [158]. Likewise, injection of a sedative drug (sodium amobarbital) to the left carotid artery - thereby anesthetizing the LH - resulted in crying, pessimistic statements and worries about the future, whereas sedation of the RH produced smiling, laughing, elated mood and euphoric reactions [159-164]. In the same line, left-ear CVS had been used as an effective technique to reduce manic symptoms; presumably, activating the contralateral RH that mediates pessimism re-balances the inter-hemispheric dynamics [165, 166].
Suicide
Suicide attempts reflect the ultimate pessimistic state and extreme hopelessness. An EEG comparison between healthy girls and those who tried to commit suicide but failed, found a greater physiological activity in the RH among the suicide-attempters group [167]. Further analysis revealed that this greater RH activation was more notable among the individuals who really intended to die (i.e. complete hopelessness) compared to those who did not intend to die and their suicide attempt was 'a cry for help' (i.e. still having a tiny bit of hope) [167]. Similarly, suicidal patients were reported to have a larger right amygdala than non-suicidal patients [168]. In addition, a postmortem histological analysis revealed a greater density of von Economo neurons in the right anterior cingulate cortex of suicide victims, compared to individuals who died from other causes [169]. Together, these studies further demonstrate that pessimistic mood is strongly interlinked with the RH at the physiological level (i.e. a relative hyperactivation of the RH), as well as at the anatomical level (i.e. having larger and denser neural systems in the RH to subserve this mental state).
Plausible biological mechanisms
The aforementioned studies demonstrate clearly that optimistic and pessimistic thoughts, attitudes, moods and behaviors are lateralized in the brain. Optimism is associated with physiological activity in the LH, whilst pessimism is linked with the RH. This leads to the question: what are the underlying biological mechanisms that can explain this difference between the two hemispheres? More specifically, is there something in the RH neurophysiology and functions that make it more suitable to mediate and sustain pessimistic thinking patterns? Similarly, what is it in the LH physiology that enables it to mediate a less vulnerable and more resilient optimistic attitude?
Pessimistic thoughts are stained with fear and stress
Various studies suggest that the RH mediates a relatively more watchful and fearful mode, than the LH; a mental state which seems to be the major factor in the link between the RH and pessimistic thought patterns. The peripheral autonomic nervous system regulates visceral functions via two branches; the sympathetic and parasympathetic nervous systems (SNS and PSNS, respectively). The SNS prepares the body to respond to a potential threat or danger by increasing heartbeats in order to accelerate blood supply to the motor organs. The SNS also increases the sweating rate to keep the body temperature cool within the optimal range, it tightens the blood vessels of the gastro-intestinal tract so digestion is slowed and energy is saved, and it dilates the pupils to allow more light to come in and increase far vision. When the threat situation is over and the potential 'fight or flight' response is no longer required, the PSNS functions as a dampener of the SNS effects, thereby restoring physiological regularity and calmness. This is achieved through the PSNS reversal of the SNS actions: i.e. the PSNS slows heartbeats and respiration rate back to normal levels, expands the gastro-intestinal blood vessels to facilitate the digestion process, stops the sweating, and contracts the pupils back to their normal size to allow closer vision [170-173].
Evidence from anatomy, physiology and neurology suggest that the RH plays a major role in mediating the SNS processes, whereas the LH is associated mainly with the PSNS [172, 174-181]. For instance, electrical stimulation of the left insular cortex or vibrotactile stimulation applied to the right-hand (i.e. LH) slows heartbeats (i.e. activates the PSNS), while the opposite effect can be induced with right insula electrical stimulation or left-hand vibrotactile stimulation [182-184]. Similarly, left insular stroke often shifts cardiovascular functioning toward SNS hyperactivation, presumably due to the loss of the PSNS balancing effects [185, 186]. Likewise, pharmacologically induced anesthesia of regions within the LH (thereby letting the RH to take control) resulted in accelerated heartbeats, while sedation of the RH slowed heartbeats [179, 187, 188].
This principle - that the RH is biologically designed to handle potential threats, more than the LH - is manifested also in the central nervous system. Sub-threshold or masked frightening stimuli are better detected and trigger a greater response in skin conductivity when they are presented in the LVF (initially processed by the RH), compared to identical presentations in the RVF [189-191]. Similarly, an increased neural activity in the amygdala, pulvinar and superior colliculus was found when participant viewed fearful facial expressions, compared to neutral expressions, selectively for LVF, but not for RVF, presentations [192, 193]. Individuals with fear of spiders (arachnophobia) detect spiders in their LVF faster than their RVF [194].
In addition, the startle reflex - the behavioral response of humans and many animals to a sudden fearful stimulus - can be potentiated during perception of negative emotional stimuli and attenuated during perception of positive emotional stimuli [195]. Experiments showed that the differences in the reflex magnitude between pleasant and unpleasant stimuli were consistently large and reliable for reflexes elicited by left-ear probes (i.e. RH processing) but weak and inconsistent for reflexes elicited by right-ear probes [196], suggesting that startle modulation is mediated primarily by neural structures within the RH. Studies on the amygdala - the neural system with the most robust activity during fear - found that the right amygdala reaction to fear conditioned stimuli is faster and longer in duration than the left amygdala [197]. Likewise, lesions in the right amygdala result in a significantly lower emotional response to fearful images, compared to left amygdala lesions [198, 199].
The RH greater involvement in mediating fearful experiences is reflected also in its significant role in regulating the stress hormone cortisol. Higher levels of cortisol induced by stress and anxiety correlated with a greater RH activation [200-204]. There is evidence that post-traumatic stress disorder (PTSD) - where the original mental trauma is re-experienced through flashbacks and nightmares that re-activate the fear and stress responses - is coupled with an increase in RH activation [205-209]. Similarly, panic disorder and social phobia are associated with a RH hyperactivity [210-216].
This division of labor between the hemispheres is also reflected in the RH relatively greater involvement in automatic orientation of spatial attention [217-220], and in the RH advantage in detecting a change in the environment [221-224]. These superior attentional functions of the RH sustain and complement its role of mediating the SNS processes by maintaining higher alertness and a vigilant mode. The LH, on the contrary, is relatively more involved in routine, well-rehearsed and repetitive responses [15, 16, 225].
This fundamental difference in the physiological and psychological functions of the two hemispheres, in which the LH primary role is to maintain homeostasis and relaxation by keeping physiological activity within normal levels, whereas the RH functions as the 'alarm system' that identifies potential threats and prepares the body to deal with dangerous situations (via the SNS), explains very well why pessimistic tendencies are associated with the RH. Firstly, the RH major role in the detection of potential threats and possible conflicts effectively means that the RH is biologically geared to focus attention on what might go wrong, rather than on what is good. Pessimistic thoughts, worries about the future, a preoccupation with the negative instead of the positive aspects of a stimulus/situation/event are therefore naturally associated with physiological activity in neural networks within the RH, which are designed to process exactly these sorts of mental activities - i.e. checking for potential perils. Secondly, pessimism in regard to the unknown that lies ahead is essentially looking at the world through lenses that are stained with fear, anxiety and stress. Experiments show that even neutral/ambiguous stimuli are more likely to be perceived and interpreted negatively and pessimistically when a person is under anxiety and stress [226-228]. Therefore, a pessimistic thinking style that anticipates negative outcomes in the future is naturally associated with the RH physiological activity, since pessimism and worry about the uncertain future contain significant elements of fear, anxiety and stress - emotions that are mediated primarily by neural circuits within the RH. The LH, on the contrary, is biologically designed to keep the body's physiology within normalcy (via the PSNS) and promote relaxation and balance - mental states that enable positive thinking patterns and optimistic thoughts to thrive and flourish.
Optimism is interlinked with a proactive attitude
A somewhat related functional distinction between the two hemispheres, that can explain their different involvement in processing optimistic and pessimistic attitudes, is Jeffrey Gray's concept of behavioral activation and inhibition systems. Based on a variety of experimental observations and the principles of behavioral learning theories, Gray [229] proposed that our interaction with the environment is controlled by two neural systems; the behavioral activation system (BAS) and the behavioral inhibition system (BIS). The BAS regulates appetitive, positive-incentive motivation, and is manifested in approach behaviors toward potentially rewarding stimuli. The BIS, on the other hand, is sensitive to aversive cues, and regulates avoidance/withdrawal behaviors from potentially harmful/pain-inducing stimuli. By correlating participants' EEG patterns with their BAS/BIS measurements it has been shown that the BAS and BIS are hemispherically lateralized in the brain; the LH is primarily associated with motivating one to act and approach positive/appealing stimuli, while the RH is involved in avoiding dangers and inhibiting actions that may lead to painful consequences [230, 231].
This model of LH/activation and RH/inhibition, although originally conceptualized around motor behaviors, extends across the whole range of mental phenomena. Initiation of a verbal response activates regions within the LH, whereas withholding speech engages RH regions [232]. The LH is involved in approach-related emotions, while the RH is associated with withdrawal-related emotions [19, 233]. Cognitive processes show the same hemispheric asymmetry. For instance, a commitment to do a specific act is associated with an increased left frontal activation [234], presumably by stimulating an 'action mode'. Likewise, an increased physiological activation in the LH was detected when participants were presented publically with a bogus negative assessment of their personality test, thereby prompting them into an 'active mode' for defending their good reputation [235]. Similarly, making or avoiding a decision is associated with left and right frontal activations, respectively [105, 236].
This functional organization of the two cerebral hemispheres means that the right and left hemispheres subserve different modes of dealing with the environment. The LH mediates an active mode for achieving one's goals which is reflected in the motor/behavioral, emotional and cognitive domains; i.e. being physically active, and also in a proactive state-of-mind - taking the initiative to improve one's conditions. The RH, on the other hand, mediates a cautious mode which balances the drive for actions, and promotes a relatively passive state-of-mind.
A major distinctive characteristic between optimistic and pessimistic people is their coping strategies. Optimism is associated with taking an active approach for both maximizing one's well-being and minimizing stressors. Pessimism, on the contrary, is associated with using mostly escape and avoidance strategies when dealing with distress, as well as with hesitations and a passive attitude when faced with an opportunity. Furthermore, having confidence about eventual success prompts the optimist to continue trying even when the going gets tough, while doubts about the future discourage the pessimist from persisting [3, 237].
The link between passivity and pessimism is well exemplified in depression. A prominent symptom of major depression is psychomotor retardation - an overall slowness and reduction of physical movements [59]. In severe depression, the process is intensified and the patient may have difficulties in doing mundane and simple motor activities such as taking a shower, dressing, self-grooming, or even getting out of bed. Mental activity and verbal speech are also slowed in depression [238, 239]. The passivity of depressed patients is reflected also in excessive hesitations and difficulties/slowness in making decisions [240, 241]. Accordingly, a unique treatment program for depression - the behavioural activation therapy - attempts to help patients reengage in their lives through focused activation strategies; creating daily schedules of activity, seeking pleasurable experiences and sources of positive reinforcements, as well as breaking the patterns of avoidance, withdrawal and inactivity [242-245].
Similarly, a regular habit of exercise and physical activity can prevent and alleviate depression [246-249]. Comparisons among healthy people found that athletes and highly active individuals were significantly more optimistic than inactive/low-active individuals [250, 251]. From a neurochemistry perspective, the positive mood induced by physical exercise is attributed to the release of β-endorphin, melatonin, serotonin and other substances [e.g. 252-254]. Nevertheless, experimental studies show that during active physical exercise there is an increased neural activation in several LH regions and/or a reduction in RH activation [255-257]. Similarly, a comparison of athletes and non-athletes in the line bisection test, found that while the latter group tended to deviate leftward from the veridical center, indicating a RH dominance, the athletes deviated rightward [258], suggesting a link between high levels of physical activity and a LH dominance. Thus, from an inter-hemispheric dynamics perspective, the positive mood that comes with exercise and physical activity is achieved, at least partly, through the increased neural activation in the LH [255, 256].
The relationship between activity and optimism is bi-directional. Optimistic thoughts lead to active coping strategies and the rewarding results weave a sense of self-efficacy and mastery over one's environment (internal locus-of-control), which further reinforces the proactive attitude. In contrast, pessimism facilitates a passive attitude which hinders and minimizes positive feedback, thereby further exacerbating a 'learned helplessness' thinking pattern and depressed mood [259-263].
Thus, the LH association with optimism seems to be related also to its mediation of an active approach mode, which enables the positive sense of accomplishment following successful experiences to further promote and reinforce an 'I can do it' attitude and optimism. The RH association with pessimism, on the contrary, is facilitated by its mediation of a relatively passive mode, escape and avoidance coping style, which minimizes and prevents potential positive experiences and their reinforcing effects on one's attitude.
Dexterity breeds confidence
Another account for the lateralization of optimism and pessimism is related to the notion that perceptual and cognitive processes are fundamentally intertwined with actions. According to ecological perception theorists James and Eleanor Gibson, perception is action oriented and essentially it is an adaptive process, sensitive to the feedback an organism receives from its particular environment and its affordances. That is, perception is a reciprocal interaction between a person's (potential and actual) motor actions and locomotion in the environment and the actionable properties and opportunities that the environment provides for the person. Therefore, learning occurs mainly through acting in the environment [264-266]. Furthermore, cognitive development theorists Jean Piaget and Lev Vygotsky assert that almost all higher mental functions originally arise from actions, and that, from an evolutionary perspective, the main function of cognition is to guide actions and to contribute to situation-appropriate behaviors [267-271]. Thus, the process of concept formation is essentially a regulatory process of adaptation to the environment. Schemas - the organized patterns of reasoning, emotions and behaviors - begin to form in early infancy at the initial sensory-motor (pre-linguistic) stage through primitive symbols. Every encounter with something unfamiliar (a new object, environment or situation) is perceived and understood by reference to previous knowledge. When the new information fits into one's pre-existing schema it is integrated and assimilated into the schema, and when it cannot fit in, the schema is altered to accommodate the new information [272, 273].
Typical infant behaviors exemplify this. An infant comes into the world with an inborn reflex of sucking. As this action satisfies the baby's basic needs it is further reinforced. As it grows a bit, the infant starts to suck everything within its reach. By realizing that some objects do not satisfy when sucked, the infant learns to distinguish between eatable and non-eatable objects. From a cognitive perspective, the infant is engaged in trying to assimilate its new experiences to its existing knowledge (that sucking leads to satisfaction) and through the interaction with objects that do not confirm that expectation the schema is modified. Cognitive development and intelligence, in children and adults, are therefore a progressive reorganization of knowledge into larger, more elaborated and sophisticated frameworks, a process that results from biological maturation and environmental experiences [272, 273]. More examples of how such schemas and constructs of knowledge guide our cognition, and that we essentially think and reason through conceptual metaphors, can be found in the works of Lakoff & Johnson [274, 275] and Lakoff & Núñez [276]. Crucially, however, schemas are not abstract concepts detached from the nervous system; rather they are based on concrete sensory and motor experiences [267, 272-277].
Indeed, recent research demonstrates that perceptual, cognitive and emotional processes are inherently embodied. That is, mental processes are grounded in bodily states - i.e. sensory-motor, somato-visceral and proprioceptive experiences - and simulations of actions [277-280]. The following experiments illustrate this point vividly. Participants in a study were asked to hold a pen in their mouth, and simultaneously read several cartoons and rate their funniness. Two groups were compared; one group was asked to hold the pen only with their teeth but not with their lips, a condition that caused them to open their lips widely thereby unconsciously activating the smiling muscles which draw the angle of the mouth upward and backward, whereas the other group was asked to hold the pen only with their lips, a condition that caused them to hold their lips closed thereby inhibiting the production of a smiling facial expression (pictures related to this experiment can be found in [280]). It was found that the former group rated the cartoons as funnier than the latter group [280, 281]. Presumably, the activation of the facial muscles associated with smiling evoked the mental representation and some degree of the mood associated with that physiomuscular state and influenced participants' judgments of the humor in the cartoons.
Similarly, in another study, participants either pulled a lever toward themselves or pushed it away from themselves while simultaneously watching neutral stimuli and rating them. The researchers found that the pulling and pushing which imitate arm flexion and extension movements associated in everyday life with liking and disliking something, affected participants' preferences of the neutral stimuli, respectively. That is, in the pulling condition the participants reported higher preferences for the neutral stimuli than in the pushing condition [282, 283].
Likewise, experiments in psychology have shown that manipulating participants into experiences of physical warmth, e.g. by asking them to hold a cup of hot beverage for a moment or by raising the laboratory's ambient temperature, increased their feelings of closeness, trust and generosity toward newly met people, i.e. 'physical warmth' affected their perception of 'social warmth' [284, 285]. On the other hand, experiences of social exclusion and loneliness, i.e. 'social coldness' are often associated with increased feelings of physical coldness [286-288]. This effect presumably occurs because our mind often relies on scaffolding processes whereby newer concepts are built on and make use of older structures; in this case, mental experiences are conceptualized by analogy and linkage to physiological experiences [272, 273, 289-291]. Accordingly, early childhood experiences of being cradled and cuddled in the bosom of a loving caregiver form the semantic association between warm sensation and affection (more evidence of this semantic link between physical and psychological warmth can be found in the works of John Bowlby [292] and Mary Ainsworth [293] with human infants, and in the works of Harry Harlow [294] with monkey infants). This concept of 'warmth = affection' is subserved by interconnections between the related neural networks in a way that an experience in one realm (physical or psychological) evokes the other.
Building on the aforementioned ideas that (a) mental processes involve operations of schemas - structured networks of symbols, knowledge and feelings linked by conceptual metaphors - and (b) that schemas are intrinsically shaped by the sensory feedback received through motor and muscular activation, it seems plausible that the laterality in handedness may also have its contribution to the lateralization of optimism and pessimism. The overwhelming majority of the population (~90%) is more dexterous in the right-hand, i.e. they perform motor skills better with their right-hand than with their left-hand. Anatomically, the right-hand is controlled by the LH, and the right-hand dexterity is subserved by a superior neural connectivity in LH motor regions, compared to the homotopic RH areas [295]. Perhaps, the dexterity associated with the LH and the relative clumsiness associated with the RH, breed a sense of potency and a sense of incompetence, respectively.
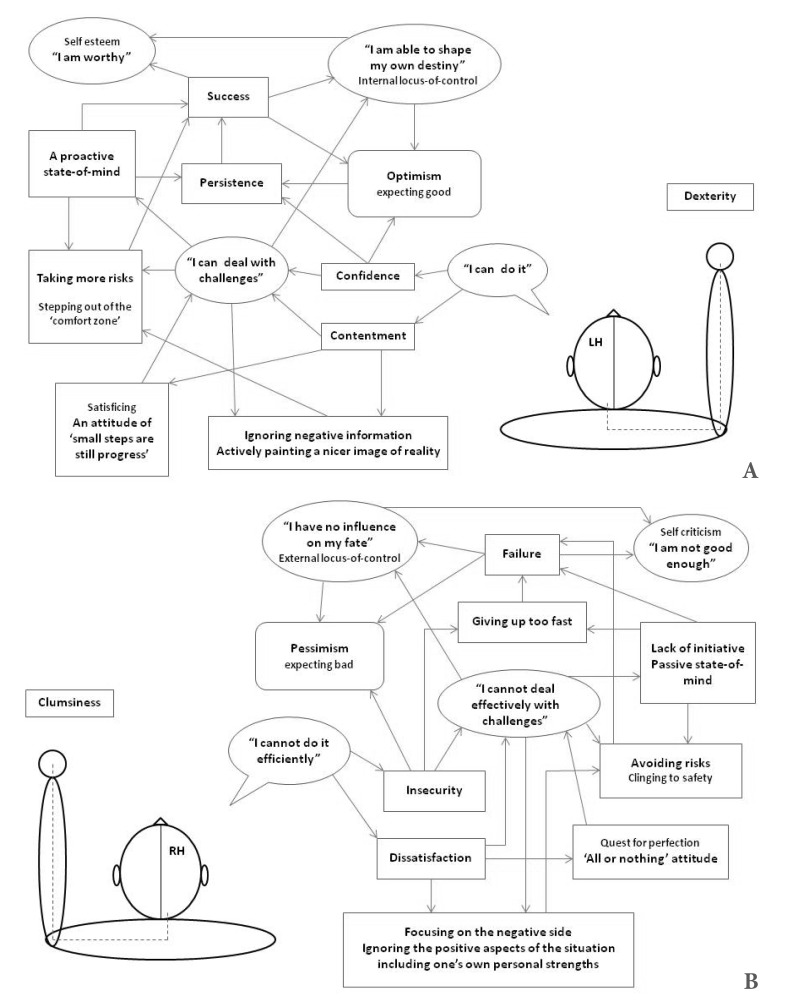
The early sensory-motor experiences of a child interacting with its environment provide both, positive feedback through the dexterous, smooth and fluent motor actions of the right limbs, as well as negative feedback from the left limbs clumsiness and ineptness. These primitive sensorimotor experiences of concrete strength and weakness, which are encoded into the LH and RH respectively, constitute the kernel of the child schemas about its abilities and limitations in dealing with environmental challenges. That is, even in the pre-linguistic stage and before the child has a sufficient cognitive capability and conceptual frameworks to comprehend the full meaning of skillfulness and ineptitude, these feelings exist in an intuitive and crude form of a tacit knowledge (i.e. something that is known and felt deeply although it cannot be reasoned consciously or expressed verbally). During development, these schemas evolve further so that experiences of success and failure, confidence and insecurity, as well as more elaborated concepts such as self-esteem (i.e. feeling pride or humility), body image (i.e. acceptance or criticism of oneself), a general worldview (i.e. optimism or pessimism), and related behaviors - persistence in spite of difficulty or surrender to hardships, taking risks to achieve a desired goal or avoiding risks etc. - are all being assimilated and integrated into these fundamental schemas, respectively. Fig. 1 present a sketch of these schemas.
Fig. 1.
Schematic presentation of the neural basis of optimism and pessimism. The primitive right and left sensorimotor experiences of concrete strength and weakness, which are encoded into the LH and RH respectively, are the basis of optimism (A) and pessimism (B).
Indeed, studies show a positive correlation between the sense of power - i.e. being able to influence one's environment and overcome its challenges - and optimism; people with a higher sense of power are more optimistic than those with a lower sense of power, presumably because power shifts one's attentional focus from the potential pitfalls into the potential payoffs [296, 297]. Similarly, confidence is robustly associated with hope, optimism and resilience [298, 299]. In addition, a sense of power increases active and approach behaviors, while it reduces inhibition and withdrawal tendencies [297, 300, 301] - behavioral patterns that are associated with optimism and pessimism, respectively, as outlined in the previous section.
These cognitive schemas about one's abilities and limitations are ingrained in the activation patterns of their respective neural networks, and as the aforementioned studies suggest they are relatively lateralized in the two cerebral hemispheres. The optimistic schema is scaffolded and assimilated into neural structures and systems within the LH, while the pessimistic schema is primarily associated with, and integrated into, neural circuits and networks in the RH.2)
Perfectionism
Concentration on the shortcomings of a person's environment and conditions, or when the focus on the glass half empty is directed inward in the form of the thought that 'I am not good enough' etc. has a positive role in our lives; it motivates the quest for perfection, self-improvement and personal development. However, when taken extremely, it can lead to perfectionism - i.e. unacceptance of the need for satisficing in some areas of life - which ultimately leads to even more disappointments with one's imperfect existential condition and personal flaws. Constant assessments of oneself or comparisons with unattainable high standards are doomed to find flaws and failures. Repeated failures reinforce one's belief in his/her incompetence, personal inadequacy and inferiority. This vicious cycle further exacerbate negative thinking patterns and may lead to a sense of hopelessness, self-blame and eventually depression. Indeed, studies showed that unhappiness, low self-esteem, pessimism and depression are all linked to the chase after perfectness [302-305]. Accordingly, therapeutic methods for overcoming pessimism and unhappiness concentrate on setting realistically achievable goals for oneself, cultivating a non-judgmental attitude and practicing unconditional self-acceptance - applying compassion, generosity and love to oneself [306, 307].
From a neurobiological perspective, it is not a mere coincidence that the RH: (a) receives a relatively poorer motor feedback and an overall sense of clumsiness and awkwardness, (b) mediates negative thought patterns (i.e. low self-esteem, pessimism, depression etc.), and (c) is more sensitive to one's body image and other flaws in general. Negative thinking, a focus on what's not good, a poor self-image and the strive for perfection are all parts of the same pessimistic schema that is mediated by associated neural networks of the same cerebral hemisphere that registers the sensorimotor feedback and evaluates its meaning (i.e. weakness). The early childhood realization of one's relative motor ineptness and physical weakness of the left limbs is the conceptual skeleton on which all subsequent thematically related experiences (i.e. awareness of one's limitations, flaws, unfitness, failures etc.) are built on.
Since the body-mind relationship is bi-directional - mental states affect body states and body states affect mental states (e.g. understanding the comical aspect of a situation produces laughter, and re-enactment of a smiling facial expression can facilitate the appreciation of humor [e.g. 278-281] - and given that the schema of pessimism is encoded into, and mediated by, physiological activity of neural networks within the RH, conditions in which the RH has a relatively leading role in dictating the mental experience (e.g. LH brain lesions or laboratory induced manipulations of the inter-hemispheric dynamics which provide the RH with a temporary advantage) activate the pessimistic schema with its ensuing set of behaviors. The opposite applies to the LH and the optimistic schema.3)
SUMMARY
Optimism and pessimism are both necessary for our survival and wellness. As in many areas of life, the 'golden mean' - the middle between the two extremes of excess and deficiency - is the desirable optimum. This classic notion is widely accepted in both Western and Eastern cultures which were traditionally influenced by the ideas of Aristotle and Confucius. These philosophers discussed the concepts of symmetry, proportion, harmony, balance and stability, and emphasized the benefits of applying these principles in personal life and social relationships. Nonetheless, the empirical literature suggests that, in regard to one's general attitude, being in the middle of the optimism-pessimism continuum (i.e. practicing 'realism') is not necessarily the best. A moderate dose of optimism, although it distorts one's perception of reality to some extent, can be advantageous. Studies that investigated the correlation between optimism and health suggest that optimists generally have better physical health [317], less cardiovascular diseases [318] and improved immunological functioning [319]. Furthermore, optimists and their romantic partners indicated greater satisfaction in their relationships [320].
A review of the literature on the neurophysiology associated with these two fundamental approaches to life suggests that optimism and pessimism are differentially associated with the two cerebral hemispheres. Attentiveness to the positive aspects of a given situation, a high self-esteem and a belief that the future will be bright are all mediated by the LH. The RH, in contrast, is generally involved in mediating a focus on the negative side, a low self-esteem and a gloomy view about the future.
The biological sources of this hemispheric asymmetry in thinking styles and attitudes are severalfold. The RH is relatively more involved in mediating fear and stress, and it serves as the brain's 'alarm system'. Physiologically, the RH is primed to detect perils and prepare the body for dealing with threats (by activating the SNS). This special sensitivity of the RH is necessary for survival, yet it has a negative side; it entails focusing attention on what can potentially go wrong. Furthermore, a preoccupation with menace is accompanied with fear, anxiety and stress - emotions which further taint and bias one's perception to see the world through negative lenses. In addition, the RH mediation of a fearful mode sustains a relative passivity - inhibition, escape and avoidance strategies - when dealing with obstacles along the way. These primal biological functions of the RH shape its mode of perceiving the world, and therefore the RH 'sees' more potential troubles than potential opportunities. This biased perceptual mode gives rise to a general tense feeling of uncertainty and insecurity which breed pessimistic thoughts and attitudes. Consequently, negative views as well as experiences that may strengthen a pessimistic outlook on life are mediated by, and become assimilated into, neural networks in the RH which support and facilitate fear, stress, negative thinking patterns and their ensuing sets of actions.
The LH, on the contrary, is biologically designed to keep the body in physiological equilibrium (via the PSNS) - a relaxed state that enables optimistic thoughts to thrive and flourish. In addition, the LH has a more active role in dealing with the environment. This fosters and encourages a proactive mindset of taking the initiative in dealing with life's challenges - a basic component of optimism - and the rewarding results further reinforce an optimistic attitude. Furthermore, the LH receives, from the early formative years of infancy, a relatively more positive sensory-motor feedback, due to its dexterity and smoothness of actions, compared to the RH. This motor fluency breeds a sense of confidence and high self-esteem - a feeling that one has a fair degree of control in life and capability to overcome potential obstacles on the way. Accordingly, optimistic thinking patterns and positive experiences that reinforce them are primarily connected with, and integrated into, neural systems within the LH.
Footnotes
1)An alternative explanation is that something in the facial musculature of the poser, rather than the observer's preference, elicits this bias of favoring the left-side or disliking the right-side of a face in a photograph [92]. However, Mojon-Azzi et al. [91] used identical pictures with the same facial musculature and (neutral) expression for both, right-eye and left-eye strabismus, and the squint was made by using digital software to crop the eye and replace it in a squint angle. Therefore, the findings of Mojon-Azzi et al. [91] cannot be explained by the alternative account. It seems that the bias had arisen, not from the physiological properties and the facial expression in the photograph, but rather within the eye of the beholder and through his/her perceptual/cognitive processes (i.e. the hemispheric differences in filtering data and focusing on the positive or on the negative aspects).
2)Although the concrete experiences of strength and weakness are encoded primarily into the motor cortex and therefore it is the neural substrate on which these schemas are formed on, other brain regions within each hemisphere are also involved in the planning and evaluation of actions, as well as in processing their emotional meaning (e.g. the joy of a child when it plays with a toy, or its pain when clumsiness results in falling). Therefore, all these neural networks, which are spread throughout each hemisphere, are integral parts of these schemas.
3)Most experimental studies in psychology which test brain lateralization issues selectively recruit right-handed participants. This is done to enable inferences from the tested sample to the general population which is ~90% right-handed, as well as for other methodological considerations. Therefore, most of our knowledge on issues of functional laterality, including the current topic of optimism and pessimism, is relevant mainly to right-handed people. As for the left-handers, theoretically their optimism and pessimism schemas should be lateralized in reverse, i.e. optimism associated with their RH and pessimism with their LH, according to their respective sensory-motor feedback of strength and weakness. However, the research on left-handers is inconclusive on this issue. Some studies show that left-handers have a symmetrically reversed functional lateralization. For instance, right-handers perceive positive facial expressions better when they are presented in the RVF (i.e. LH) and negative faces better when they appear in the LVF (i.e. RH), while the reverse is true for some left-handers [308, 309]. Likewise, right-handers reported more positive and less negative affect for music played to their right-ear (i.e. LH), while the same affective experience was obtained for music played to the left-ear (i.e. RH) of left-handers [310]. Similarly, approach motivation tendencies are associated with LH activation in right-handers and with RH activation in left-handers [311, 312]. However, some studies on left-handers' emotional lateralization show that it is not a symmetric reverse of right-handers [e.g. 313, 314]. In addition, some inborn left-handers were 'converted' and forced, since childhood, to use their right non-dominant hand for writing and other tasks; an intervention which may have shaped their neural development (i.e. the formation of neural pathways, networks and connectivity patterns) in a way that altered their hemispheric specialization in unknown directions [315]. Furthermore, even non-converted left-handers may not necessarily have a perfect symmetrical reverse of functional laterality for optimism and pessimism, since our world is significantly biased in favor of right-handers. For instance, most manual tools, equipments, machines and environments are ergonomically designed for the use of right-handers. Consequently, the sensory-motor feedback that the RH of left-handers receive, when interacting with the environment, may not always be substantially better than the feedback that their LH receives, since many tools and environments can be equally (or better) handled and accessed by left-handers with their (weak) right-hand/LH. Besides, although motor dominance and its sensory-motor feedback are hemispherically reversed in left-handers, there is no evidence for a reversal of the association between the SNS and PSNS with RH and LH processes, respectively, in left-handers, though the magnitude of these links, may not be similar in right- and left-handers [e.g. 316]. Therefore, even though the RH of left-handers receives a motor feedback of relative strengths, this hemisphere is still involved in SNS processes which bias perception and cognition toward a pessimistic mode. All these reasons - the inconclusive evidence about the lateralization patterns of left-handers, as well as the major cultural/environmental biases which surely affect the neural development and lateralization of left-handers in unknown ways - further complicate the possibility to investigate whether and to what extent the optimism and pessimism schemas are hemispherically reversed in left-handers.
References
- 1.Kahneman D. Thinking, fast and slow. New York, NY: Farrar, Straus and Giroux; 2011. [Google Scholar]
- 2.Wallston KA. Cautious optimism vs. cockeyed optimism. Psychol Health. 1994;9:201–203. [Google Scholar]
- 3.Carver CS, Scheier MF, Segerstrom SC. Optimism. Clin Psychol Rev. 2010;30:879–889. doi: 10.1016/j.cpr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein ND. Unrealistic optimism about future life events. J Pers Soc Psychol. 1980;39:806–820. [Google Scholar]
- 5.Peterson C, Semmel A, von Baeyer C, Abramson LY, Metalsky GI, Seligman ME. The attributional style questionnaire. Cognit Ther Res. 1982;6:287–299. [Google Scholar]
- 6.Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 7.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 8.Snyder CR, Harris C, Anderson JR, Holleran SA, Irving LM, Sigmon ST, Yoshinobu L, Gibb J, Langelle C, Harney P. The will and the ways: development and validation of an individual-differences measure of hope. J Pers Soc Psychol. 1991;60:570–585. doi: 10.1037//0022-3514.60.4.570. [DOI] [PubMed] [Google Scholar]
- 9.Fibel B, Hale WD. The Generalized Expectancy for Success Scale: a new measure. J Consult Clin Psychol. 1978;46:924–931. [Google Scholar]
- 10.Hale WD, Fiedler LR, Cochran CD. The revised Generalized Expectancy for Success Scale: a validity and reliability study. J Clin Psychol. 1992;48:517–521. doi: 10.1002/1097-4679(199207)48:4<517::aid-jclp2270480413>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Dember WN, Martin SH, Hummer MK, Howe SR, Melton RS. The measurement of optimism and pessimism. Curr Psychol. 1989;8:102–119. [Google Scholar]
- 12.Drake RA. Lateral asymmetry of personal optimism. J Res Pers. 1984;18:497–507. [Google Scholar]
- 13.Drake RA. Effects of gaze manipulation on aesthetic judgments: hemisphere priming of affect. Acta Psychol (Amst) 1987;65:91–99. doi: 10.1016/0001-6918(87)90020-5. [DOI] [PubMed] [Google Scholar]
- 14.Hellige JB. Hemispheric asymmetry for visual information processing. Acta Neurobiol Exp (Wars) 1996;56:485–497. doi: 10.55782/ane-1996-1151. [DOI] [PubMed] [Google Scholar]
- 15.McGilchrist I. The master and his emissary: the divided brain and the making of the western world. New Haven, CT: Yale University Press; 2009. [Google Scholar]
- 16.McGilchrist I. Reciprocal organization of the cerebral hemispheres. Dialogues Clin Neurosci. 2010;12:503–515. doi: 10.31887/DCNS.2010.12.4/imcgilchrist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran VS, Blakeslee S. Phantoms in the brain: human nature and the architecture of the mind. London: Harper Collins; 2005. [Google Scholar]
- 19.Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 20.Zaidel E, Iacoboni M. The parallel brain: the cognitive neuroscience of the corpus callosum. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- 21.Isaacowitz DM. The gaze of the optimist. Pers Soc Psychol Bull. 2005;31:407–415. doi: 10.1177/0146167204271599. [DOI] [PubMed] [Google Scholar]
- 22.Isaacowitz DM. Motivated gaze: the view from the gazer. Curr Dir Psychol Sci. 2006;15:68–72. [Google Scholar]
- 23.Segerstrom SC. Optimism and attentional bias for negative and positive stimuli. Pers Soc Psychol Bull. 2001;27:1334–1343. [Google Scholar]
- 24.Kakolewski KE, Crowson JJ, Jr, Sewell KW, Cromwell RL. Laterality, word valence, and visual attention: a comparison of depressed and non-depressed individuals. Int J Psychophysiol. 1999;34:283–292. doi: 10.1016/s0167-8760(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 25.Fox E. Processing emotional facial expressions: the role of anxiety and awareness. Cogn Affect Behav Neurosci. 2002;2:52–63. doi: 10.3758/cabn.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsolek CJ, DeYoung CG, Domansky WS, Deason RG. Hemispheric asymmetries in motivation neurally dissociate self-description processes. Emotion. 2013;13:462–467. doi: 10.1037/a0030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick M, Seta JJ. Lateralized goal framing: how selective presentation impacts message effectiveness. J Health Psychol. 2012;17:1099–1109. doi: 10.1177/1359105311435944. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 30.McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SH, Cornwell B, Kim SE. Individual differences in emotion regulation and hemispheric metabolic asymmetry. Biol Psychol. 2012;89:382–386. doi: 10.1016/j.biopsycho.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Drake RA, Ulrich G. Line bisecting as a predictor of personal optimism and desirability of risky behaviors. Acta Psychol (Amst) 1992;79:219–226. doi: 10.1016/0001-6918(92)90058-l. [DOI] [PubMed] [Google Scholar]
- 33.Vallar G, Bolognini N. Behavioural facilitation following brain stimulation: implications for neurorehabilitation. Neuropsychol Rehabil. 2011;21:618–649. doi: 10.1080/09602011.2011.574050. [DOI] [PubMed] [Google Scholar]
- 34.Peña-Gómez C, Vidal-Piñeiro D, Clemente IC, Pascual-Leone Á, Bartrés-Faz D. Down-regulation of negative emotional processing by transcranial direct current stimulation: effects of personality characteristics. PLoS One. 2011;6:e22812. doi: 10.1371/journal.pone.0022812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leyman L, De Raedt R, Vanderhasselt MA, Baeken C. Influence of high-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex on the inhibition of emotional information in healthy volunteers. Psychol Med. 2009;39:1019–1028. doi: 10.1017/S0033291708004431. [DOI] [PubMed] [Google Scholar]
- 36.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80:1–28. [PubMed] [Google Scholar]
- 37.Feddersen B, Herzer R, Hartmann U, Gaab MR, Runge U. On the psychopathology of unilateral temporal lobe epilepsy. Epilepsy Behav. 2005;6:43–49. doi: 10.1016/j.yebeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Mathiak KA, Mathiak K, Wolańczyk T, Ostaszewski P. Psychosocial impairments in children with epilepsy depend on the side of the focus. Epilepsy Behav. 2009;16:603–608. doi: 10.1016/j.yebeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 39.De Brabander B, Gerits P, Boone C. Relationships among locus of control, type of task (visuospatial versus semantic), and arousal or activation by an unexpected preparatory signal. Percept Mot Skills. 1990;71:1091–1098. doi: 10.2466/pms.1990.71.3f.1091. [DOI] [PubMed] [Google Scholar]
- 40.De Brabander B, Boone C, Gerits P. Locus of control and cerebral asymmetry. Percept Mot Skills. 1992;75:131–143. doi: 10.2466/pms.1992.75.1.131. [DOI] [PubMed] [Google Scholar]
- 41.De Brabander B, Hellemans J. Locus of control, latent inhibition and reaction times in a semantic (left-hemisphere) and visuospatial (right-hemisphere) stimulus discrimination task. Percept Mot Skills. 1997;84:239–242. doi: 10.2466/pms.1997.84.1.239. [DOI] [PubMed] [Google Scholar]
- 42.De Brabander B, Gerits P, Hellemans J. Self-reported locus of control is based on feelings of depression as well as on asymmetry in activation of cerebral hemispheres. Psychol Rep. 1997;80:1227–1232. doi: 10.2466/pr0.1997.80.3c.1227. [DOI] [PubMed] [Google Scholar]
- 43.Declerck CH, Boone C, De Brabander B. On feeling in control: a biological theory for individual differences in control perception. Brain Cogn. 2006;62:143–176. doi: 10.1016/j.bandc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Harmon-Jones E, Sigelman JD, Bohlig A, Harmon-Jones C. Anger, coping, and frontal cortical activity: the effect of coping potential on anger-induced left frontal activity. Cogn Emot. 2003;17:1–24. doi: 10.1080/02699930302278. [DOI] [PubMed] [Google Scholar]
- 45.Boksem MA, Smolders R, De Cremer D. Social power and approach-related neural activity. Soc Cogn Affect Neurosci. 2012;7:516–520. doi: 10.1093/scan/nsp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson D, Guinote A, Weick M, Molinari R, Graham K. Feeling socially powerless makes you more prone to bumping into things on the right and induces leftward line bisection error. Psychon Bull Rev. 2010;17:910–914. doi: 10.3758/PBR.17.6.910. [DOI] [PubMed] [Google Scholar]
- 47.Abramson LY, Seligman ME, Teasdale JD. Learned helplessness in humans: critique and reformulation. J Abnorm Psychol. 1978;87:49–74. [PubMed] [Google Scholar]
- 48.Mezulis AH, Abramson LY, Hyde JS, Hankin BL. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychol Bull. 2004;130:711–747. doi: 10.1037/0033-2909.130.5.711. [DOI] [PubMed] [Google Scholar]
- 49.Seligman ME, Nolen-Hoeksema S. Explanatory style and depression. In: Magnusson D, Öhman A, editors. Psychopathology: an interactional perspective. New York, NY: Academic Press; 1987. pp. 125–139. [Google Scholar]
- 50.Drake RA, Seligman ME. Self-serving biases in causal attributions as a function of altered activation asymmetry. Int J Neurosci. 1989;45:199–204. doi: 10.3109/00207458908986232. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein ND, Klein WM. Unrealistic optimism: present and future. J Soc Clin Psychol. 1996;15:1–8. [Google Scholar]
- 52.Sharot T, Korn CW, Dolan RJ. How unrealistic optimism is maintained in the face of reality. Nat Neurosci. 2011;14:1475–1479. doi: 10.1038/nn.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharot T. The optimism bias. Curr Biol. 2011;21:R941–R945. doi: 10.1016/j.cub.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 54.Drake RA. Conceptions of own versus others' outcomes: manipulation by monaural attentional orientation. Eur J Soc Psychol. 1987;17:373–375. [Google Scholar]
- 55.Tamagni C, Palla A, Krummenacher P, Vitacco D, Huberle E, Straumann D, Hegemann SC, McKay R, Brugger P. Vestibular stimulation reduces unrealistic optimism. Nat Preced [Internet] 2010. [cited]. Available from: http://precedings.nature.com/documents/4519/version/1.
- 56.McKay R, Tamagni C, Palla A, Krummenacher P, Hegemann SC, Straumann D, Brugger P. Vestibular stimulation attenuates unrealistic optimism. Cortex. 2013;49:2272–2275. doi: 10.1016/j.cortex.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Sharot T. The optimism bias. A TED talk on February 2012 [Internet] New York, NY: TED conferences LLC; 2012. [cited]. Available from: http://www.ted.com/talks/tali_sharot_the_optimism_bias.html. [Google Scholar]
- 58.Sharot T, Kanai R, Marston D, Korn CW, Rees G, Dolan RJ. Selectively altering belief formation in the human brain. Proc Natl Acad Sci U S A. 2012;109:17058–17062. doi: 10.1073/pnas.1205828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 60.Gagliese L, Schiff BB, Taylor A. Differential consequences of left- and right-sided chronic pain. Clin J Pain. 1995;11:201–207. [PubMed] [Google Scholar]
- 61.Belaia II, Torba VA. Perceptive behavior of epileptic patients with a predominant lesion in the right or left hemisphere. Zh Nevropatol Psikhiatr Im S S Korsakova. 1978;78:570–575. [PubMed] [Google Scholar]
- 62.Babinski J. Contribution to the study of mental disorders in organic cerebral hemiplegia (anosognosia) Rev Neurol (Paris) 1914;27:845–848. [Google Scholar]
- 63.Ramachandran VS. Anosognosia in parietal lobe syndrome. Conscious Cogn. 1995;4:22–51. doi: 10.1006/ccog.1995.1002. [DOI] [PubMed] [Google Scholar]
- 64.Davies M, Aimola Davies A, Coltheart M. Anosognosia and the two-factor theory of delusions. Mind Lang. 2005;20:209–236. [Google Scholar]
- 65.Fotopoulou A. The affective neuropsychology of confabulation and delusion. Cogn Neuropsychiatry. 2010;15:38–63. doi: 10.1080/13546800903250949. [DOI] [PubMed] [Google Scholar]
- 66.McKay R, Kinsbourne M. Confabulation, delusion, and anosognosia: motivational factors and false claims. Cogn Neuropsychiatry. 2010;15:288–318. doi: 10.1080/13546800903374871. [DOI] [PubMed] [Google Scholar]
- 67.Pia L, Neppi-Modona M, Ricci R, Berti A. The anatomy of anosognosia for hemiplegia: a meta-analysis. Cortex. 2004;40:367–377. doi: 10.1016/s0010-9452(08)70131-x. [DOI] [PubMed] [Google Scholar]
- 68.Azouvi P, Peskine A. Anosognosia and denial after right hemisphere stroke. In: Godefroy O, editor. The behavioral and cognitive neurology of stroke. Cambridge: Cambridge University Press; 2013. pp. 158–169. Section III, Chapter 13. [Google Scholar]
- 69.Taylor-Cooke PA, Ricci R, Baños JH, Zhou X, Woods AJ, Mennemeier MS. Perception of motor strength and stimulus magnitude are correlated in stroke patients. Neurology. 2006;66:1444–1456. doi: 10.1212/01.wnl.0000210489.92317.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tezuka K, Meguro K, Akanuma K, Tanaka N, Ishii H, Yamaguchi S. Overestimation of self-reported activities of daily living in vascular dementia patients with a right hemisphere lesion. J Stroke Cerebrovasc Dis. 2013;22:9–14. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Rosen HJ, Alcantar O, Rothlind J, Sturm V, Kramer JH, Weiner M, Miller BL. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage. 2010;49:3358–3364. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmitz TW, Rowley HA, Kawahara TN, Johnson SC. Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia. 2006;44:762–773. doi: 10.1016/j.neuropsychologia.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Judge TA, Hurst C. How the rich (and happy) get richer (and happier): relationship of core self-evaluations to trajectories in attaining work success. J Appl Psychol. 2008;93:849–863. doi: 10.1037/0021-9010.93.4.849. [DOI] [PubMed] [Google Scholar]
- 74.Heinonen K, Räikkönen K, Keltikangas-Järvinen L. Self-esteem in early and late adolescence predicts dispositional optimism-pessimism in adulthood: a 21-year longitudinal study. Pers Individ Dif. 2005;39:511–521. [Google Scholar]
- 75.Neff KD, Vonk R. Self-compassion versus global self-esteem: two different ways of relating to oneself. J Pers. 2009;77:23–50. doi: 10.1111/j.1467-6494.2008.00537.x. [DOI] [PubMed] [Google Scholar]
- 76.McKay R, Arciuli J, Atkinson A, Bennett E, Pheils E. Lateralisation of self-esteem: an investigation using a dichotically presented auditory adaptation of the Implicit Association Test. Cortex. 2010;46:367–373. doi: 10.1016/j.cortex.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Drake RA, Myers LR. Visual attention, emotion, and action tendency: feeling active or passive. Cogn Emot. 2006;20:608–622. [Google Scholar]
- 78.De Raedt R, Franck E, Fannes K, Verstraeten E. Is the relationship between frontal EEG alpha asymmetry and depression mediated by implicit or explicit self-esteem? Biol Psychol. 2008;77:89–92. doi: 10.1016/j.biopsycho.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Putnam KM, McSweeney LB. Depressive symptoms and baseline prefrontal EEG alpha activity: a study utilizing Ecological Momentary Assessment. Biol Psychol. 2008;77:237–240. doi: 10.1016/j.biopsycho.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Davidson RJ. Well-being and affective style: neural substrates and biobehavioural correlates. Philos Trans R Soc Lond B Biol Sci. 2004;359:1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, Rosenkranz MA, Ryff CD, Singer BH, Davidson RJ. Making a life worth living: neural correlates of well-being. Psychol Sci. 2004;15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 82.Curtis WJ, Cicchetti D. Emotion and resilience: a multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Dev Psychopathol. 2007;19:811–840. doi: 10.1017/S0954579407000405. [DOI] [PubMed] [Google Scholar]
- 83.Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. J Abnorm Psychol. 2011;120:497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vickery CD, Evans CC, Lee JE, Sepehri A, Jabeen LN. Multilevel modeling of self-esteem change during acute inpatient stroke rehabilitation. Rehabil Psychol. 2009;54:372–380. doi: 10.1037/a0017854. [DOI] [PubMed] [Google Scholar]
- 85.Blonder LX, Langer SL, Pettigrew LC, Garrity TF. The effects of stroke disability on spousal caregivers. NeuroRehabilitation. 2007;22:85–92. [PubMed] [Google Scholar]
- 86.Mohr C, Porter G, Benton CP. Psychophysics reveals a right hemispheric contribution to body image distortions in women but not men. Neuropsychologia. 2007;45:2942–2950. doi: 10.1016/j.neuropsychologia.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Giummarra MJ, Bradshaw JL, Nicholls ME, Hilti LM, Brugger P. Body integrity identity disorder: deranged body processing, right fronto-parietal dysfunction, and phenomenological experience of body incongruity. Neuropsychol Rev. 2011;21:320–333. doi: 10.1007/s11065-011-9184-8. [DOI] [PubMed] [Google Scholar]
- 88.Hilti LM, Hänggi J, Vitacco DA, Kraemer B, Palla A, Luechinger R, Jäncke L, Brugger P. The desire for healthy limb amputation: structural brain correlates and clinical features of xenomelia. Brain. 2013;136:318–329. doi: 10.1093/brain/aws316. [DOI] [PubMed] [Google Scholar]
- 89.Blanke O, Morgenthaler FD, Brugger P, Overney LS. Preliminary evidence for a fronto-parietal dysfunction in able-bodied participants with a desire for limb amputation. J Neuropsychol. 2009;3:181–200. doi: 10.1348/174866408X318653. [DOI] [PubMed] [Google Scholar]
- 90.First MB. Desire for amputation of a limb: paraphilia, psychosis, or a new type of identity disorder. Psychol Med. 2005;35:919–928. doi: 10.1017/s0033291704003320. [DOI] [PubMed] [Google Scholar]
- 91.Mojon-Azzi SM, Kunz A, Mojon DS. The perception of strabismus by children and adults. Graefes Arch Clin Exp Ophthalmol. 2011;249:753–757. doi: 10.1007/s00417-010-1555-y. [DOI] [PubMed] [Google Scholar]
- 92.Blackburn K, Schirillo J. Emotive hemispheric differences measured in real-life portraits using pupil diameter and subjective aesthetic preferences. Exp Brain Res. 2012;219:447–455. doi: 10.1007/s00221-012-3091-y. [DOI] [PubMed] [Google Scholar]
- 93.Friederich HC, Brooks S, Uher R, Campbell IC, Giampietro V, Brammer M, Williams SC, Herzog W, Treasure J. Neural correlates of body dissatisfaction in anorexia nervosa. Neuropsychologia. 2010;48:2878–2885. doi: 10.1016/j.neuropsychologia.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 94.Seeger G, Braus DF, Ruf M, Goldberger U, Schmidt MH. Body image distortion reveals amygdala activation in patients with anorexia nervosa -- a functional magnetic resonance imaging study. Neurosci Lett. 2002;326:25–28. doi: 10.1016/s0304-3940(02)00312-9. [DOI] [PubMed] [Google Scholar]
- 95.Uher R, Murphy T, Friederich HC, Dalgleish T, Brammer MJ, Giampietro V, Phillips ML, Andrew CM, Ng VW, Williams SC, Campbell IC, Treasure J. Functional neuroanatomy of body shape perception in healthy and eating-disordered women. Biol Psychiatry. 2005;58:990–997. doi: 10.1016/j.biopsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Schiff BB, Guirguis M, Kenwood C, Herman CP. Asymmetrical hemispheric activation and behavioral persistence: effects of unilateral muscle contractions. Neuropsychology. 1998;12:526–532. doi: 10.1037//0894-4105.12.4.526. [DOI] [PubMed] [Google Scholar]
- 97.Bassel C, Schiff BB. Unilateral vibrotactile stimulation induces emotional biases in cognition and performance. Neuropsychologia. 2001;39:282–287. doi: 10.1016/s0028-3932(00)00116-0. [DOI] [PubMed] [Google Scholar]
- 98.Maslow AH. Toward a psychology of being. 2nd ed. New York, NY: Van Nostrand Reinhold; 1968. [Google Scholar]
- 99.Rogers CR. On becoming a person: a therapist's view of psychotherapy. London: Constable; 1961. [Google Scholar]
- 100.Rogers CR. A way of being. Boston, MA: Houghton Mifflin Harcourt; 1980. [Google Scholar]
- 101.Gibson B, Sanbonmatsu DM. Optimism, pessimism, and gambling: the downside of optimism. Pers Soc Psychol Bull. 2004;30:149–160. doi: 10.1177/0146167203259929. [DOI] [PubMed] [Google Scholar]
- 102.Houghton JD, Johnson TD. Aggression, risk taking, and leadership effectiveness: leadership lessons from the explanatory styles of civil war generals. Int J Leadersh Stud. 2009;5:51–68. [Google Scholar]
- 103.Testa CR, Steinberg L. Depressive symptoms and health-related risk-taking in adolescence. Suicide Life Threat Behav. 2010;40:298–305. doi: 10.1521/suli.2010.40.3.298. [DOI] [PubMed] [Google Scholar]
- 104.Drake RA. Lateral asymmetry of risky recommendations. Pers Soc Psychol Bull. 1985;11:409–417. [Google Scholar]
- 105.Gianotti LR, Knoch D, Faber PL, Lehmann D, Pascual-Marqui RD, Diezi C, Schoch C, Eisenegger C, Fehr E. Tonic activity level in the right prefrontal cortex predicts individuals' risk taking. Psychol Sci. 2009;20:33–38. doi: 10.1111/j.1467-9280.2008.02260.x. [DOI] [PubMed] [Google Scholar]
- 106.Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boggio PS, Campanhã C, Valasek CA, Fecteau S, Pascual-Leone A, Fregni F. Modulation of decision-making in a gambling task in older adults with transcranial direct current stimulation. Eur J Neurosci. 2010;31:593–597. doi: 10.1111/j.1460-9568.2010.07080.x. [DOI] [PubMed] [Google Scholar]
- 108.Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41:1474–1483. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 110.Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- 111.Deslandes AC, de Moraes H, Pompeu FA, Ribeiro P, Cagy M, Capitão C, Alves H, Piedade RA, Laks J. Electroencephalographic frontal asymmetry and depressive symptoms in the elderly. Biol Psychol. 2008;79:317–322. doi: 10.1016/j.biopsycho.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 112.Fingelkurts AA, Fingelkurts AA, Rytsälä H, Suominen K, Isometsä E, Kähkönen S. Composition of brain oscillations in ongoing EEG during major depression disorder. Neurosci Res. 2006;56:133–144. doi: 10.1016/j.neures.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 113.Flor-Henry P, Lind JC, Koles ZJ. A source-imaging (low-resolution electromagnetic tomography) study of the EEGs from unmedicated males with depression. Psychiatry Res. 2004;130:191–207. doi: 10.1016/j.pscychresns.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 114.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- 115.Janocha A, Pilecki W, Bolanowski M, Małyszczak K, Salomon E, Laszki-Szczachor K, Kałka D, Sebzda T, Sobieszczańska M. Interhemispheric cerebral asymmetry detected by VEPS in diabetic patients with recognized depression. Neuro Endocrinol Lett. 2009;30:119–124. [PubMed] [Google Scholar]
- 116.Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJ. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stewart JL, Coan JA, Towers DN, Allen JJ. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J Affect Disord. 2011;129:167–174. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rashid N, Clarke C, Rogish M. Post-stroke depression and expressed emotion. Brain Inj. 2013;27:223–238. doi: 10.3109/02699052.2012.729287. [DOI] [PubMed] [Google Scholar]
- 119.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 120.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barnhofer T, Chittka T, Nightingale H, Visser C, Crane C. State effects of two forms of meditation on prefrontal EEG asymmetry in previously depressed individuals. Mindfulness (N Y) 2010;1:21–27. doi: 10.1007/s12671-010-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biol Psychiatry. 2008;63:1171–1177. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deslandes AC, Moraes H, Alves H, Pompeu FA, Silveira H, Mouta R, Arcoverde C, Ribeiro P, Cagy M, Piedade RA, Laks J, Coutinho ES. Effect of aerobic training on EEG alpha asymmetry and depressive symptoms in the elderly: a 1-year follow-up study. Braz J Med Biol Res. 2010;43:585–592. doi: 10.1590/s0100-879x2010007500041. [DOI] [PubMed] [Google Scholar]
- 124.Jones NA, Field T. Massage and music therapies attenuate frontal EEG asymmetry in depressed adolescents. Adolescence. 1999;34:529–534. [PubMed] [Google Scholar]
- 125.Choi SW, Chi SE, Chung SY, Kim JW, Ahn CY, Kim HT. Is alpha wave neurofeedback effective with randomized clinical trials in depression? A pilot study. Neuropsychobiology. 2011;63:43–51. doi: 10.1159/000322290. [DOI] [PubMed] [Google Scholar]
- 126.Dell'Osso B, Zanoni S, Ferrucci R, Vergari M, Castellano F, D'Urso N, Dobrea C, Benatti B, Arici C, Priori A, Altamura AC. Transcranial direct current stimulation for the outpatient treatment of poor-responder depressed patients. Eur Psychiatry. 2012;27:513–517. doi: 10.1016/j.eurpsy.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 127.Dias AM, van Deusen A. A new neurofeedback protocol for depression. Span J Psychol. 2011;14:374–384. doi: 10.5209/rev_sjop.2011.v14.n1.34. [DOI] [PubMed] [Google Scholar]
- 128.Eche J, Mondino M, Haesebaert F, Saoud M, Poulet E, Brunelin J. Low- vs. high-frequency repetitive transcranial magnetic stimulation as an add-on treatment for refractory depression. Front Psychiatry. 2012;3:13. doi: 10.3389/fpsyt.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eschweiler GW, Wegerer C, Schlotter W, Spandl C, Stevens A, Bartels M, Buchkremer G. Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Res. 2000;99:161–172. doi: 10.1016/s0925-4927(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 130.Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009;118:215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 131.Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26:229–234. doi: 10.1002/da.20454. [DOI] [PubMed] [Google Scholar]
- 132.Fitzgerald PB, Hoy KE, Herring SE, McQueen S, Peachey AV, Segrave RA, Maller J, Hall P, Daskalakis ZJ. A double blind randomized trial of unilateral left and bilateral prefrontal cortex transcranial magnetic stimulation in treatment resistant major depression. J Affect Disord. 2012;139:193–198. doi: 10.1016/j.jad.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 133.George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, Arana GW, Risch SC, Ballenger JC. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- 134.Hernández-Ribas R, Deus J, Pujol J, Segalàs C, Vallejo J, Menchón JM, Cardoner N, Soriano-Mas C. Identifying brain imaging correlates of clinical response to repetitive transcranial magnetic stimulation (rTMS) in major depression. Brain Stimul. 2013;6:54–61. doi: 10.1016/j.brs.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 135.Jorge RE, Moser DJ, Acion L, Robinson RG. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65:268–276. doi: 10.1001/archgenpsychiatry.2007.45. [DOI] [PubMed] [Google Scholar]
- 136.Lam RW, Chan P, Wilkins-Ho M, Yatham LN. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53:621–631. doi: 10.1177/070674370805300909. [DOI] [PubMed] [Google Scholar]
- 137.Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry. 2012;200:52–59. doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- 138.Martin DM, Alonzo A, Mitchell PB, Sachdev P, Gálvez V, Loo CK. Fronto-extracephalic transcranial direct current stimulation as a treatment for major depression: an open-label pilot study. J Affect Disord. 2011;134:459–463. doi: 10.1016/j.jad.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 139.Miller SM, Ngo TT. Studies of caloric vestibular stimulation: implications for the cognitive neurosciences, the clinical neurosciences and neurophilosophy. Acta Neuropsychiatr. 2007;19:183–203. doi: 10.1111/j.1601-5215.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- 140.Pettigrew JD, Miller SM. A 'sticky' interhemispheric switch in bipolar disorder? Proc Biol Sci. 1998;265:2141–2148. doi: 10.1098/rspb.1998.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barker-Collo SL. Depression and anxiety 3 months post stroke: prevalence and correlates. Arch Clin Neuropsychol. 2007;22:519–531. doi: 10.1016/j.acn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 142.Belyi BI. Mental impairment in unilateral frontal tumours: role of the laterality of the lesion. Int J Neurosci. 1987;32:799–810. doi: 10.3109/00207458709043334. [DOI] [PubMed] [Google Scholar]
- 143.Braun CM, Larocque C, Daigneault S, Montour-Proulx I. Mania, pseudomania, depression, and pseudo-depression resulting from focal unilateral cortical lesions. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:35–51. [PubMed] [Google Scholar]
- 144.Braun CM, Daigneault R, Gaudelet S, Guimond A. Diagnostic and statistical manual of mental disorders, fourth edition symptoms of mania: which one(s) result(s) more often from right than left hemisphere lesions? Compr Psychiatry. 2008;49:441–459. doi: 10.1016/j.comppsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 145.Burton LA, Labar D. Emotional status after right vs. left temporal lobectomy. Seizure. 1999;8:116–119. doi: 10.1053/seiz.1999.0271. [DOI] [PubMed] [Google Scholar]
- 146.Carran MA, Kohler CG, O'Connor MJ, Bilker WB, Sperling MR. Mania following temporal lobectomy. Neurology. 2003;61:770–774. doi: 10.1212/01.wnl.0000086378.74539.85. [DOI] [PubMed] [Google Scholar]
- 147.Hama S, Yamashita H, Shigenobu M, Watanabe A, Kurisu K, Yamawaki S, Kitaoka T. Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. 2007;257:149–152. doi: 10.1007/s00406-006-0698-7. [DOI] [PubMed] [Google Scholar]
- 148.Katayama Y, Usuda K, Nishiyama Y, Katsura K. Post-stroke depression. Nihon Ronen Igakkai Zasshi. 2003;40:127–129. doi: 10.3143/geriatrics.40.127. [DOI] [PubMed] [Google Scholar]
- 149.Koreki A, Takahata K, Tabuchi H, Kato M. Increased left anterior insular and inferior prefrontal activity in post-stroke mania. BMC Neurol. 2012;12:68. doi: 10.1186/1471-2377-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morris PL, Robinson RG, de Carvalho ML, Albert P, Wells JC, Samuels JF, Eden-Fetzer D, Price TR. Lesion characteristics and depressed mood in the stroke data bank study. J Neuropsychiatry Clin Neurosci. 1996;8:153–159. doi: 10.1176/jnp.8.2.153. [DOI] [PubMed] [Google Scholar]
- 151.Nishiyama Y, Komaba Y, Ueda M, Nagayama H, Amemiya S, Katayama Y. Early depressive symptoms after ischemic stroke are associated with a left lenticulocapsular area lesion. J Stroke Cerebrovasc Dis. 2010;19:184–189. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 152.Regard M, Landis T. The 'smiley': a graphical expression of mood in right anterior cerebral lesions. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:303–307. [Google Scholar]
- 153.Robinson RG, Boston JD, Starkstein SE, Price TR. Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry. 1988;145:172–178. doi: 10.1176/ajp.145.2.172. [DOI] [PubMed] [Google Scholar]
- 154.Robinson RG, Starkstein SE. Mood disorders following stroke: new findings and future directions. J Geriatr Psychiatry. 1989;22:1–15. [PubMed] [Google Scholar]
- 155.Santos CO, Caeiro L, Ferro JM, Figueira ML. Mania and stroke: a systematic review. Cerebrovasc Dis. 2011;32:11–21. doi: 10.1159/000327032. [DOI] [PubMed] [Google Scholar]
- 156.Vataja R, Pohjasvaara T, Leppävuori A, Mäntylä R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. 2001;58:925–931. doi: 10.1001/archpsyc.58.10.925. [DOI] [PubMed] [Google Scholar]
- 157.Wongwandee M, Tangwongchai S, Phanthumchinda K. Relationship between poststroke depression and ischemic lesion location. J Med Assoc Thai. 2012;95:330–336. [PubMed] [Google Scholar]
- 158.Piacentini S, Versaci R, Romito L, Ferré F, Albanese A. Behavioral and personality features in patients with lateralized Parkinson's disease. Eur J Neurol. 2011;18:772–777. doi: 10.1111/j.1468-1331.2010.03279.x. [DOI] [PubMed] [Google Scholar]
- 159.Ahern GL, Herring AM, Tackenberg JN, Schwartz GE, Seeger JF, Labiner DM, Weinand ME, Oommen KJ. Affective self-report during the intracarotid sodium amobarbital test. J Clin Exp Neuropsychol. 1994;16:372–376. doi: 10.1080/01688639408402647. [DOI] [PubMed] [Google Scholar]
- 160.Ahern GL, Herring AM, Labiner DM, Weinand ME, Hutzler R. Affective self-report during the intracarotid sodium amobarbital test: group differences. J Int Neuropsychol Soc. 2000;6:659–667. doi: 10.1017/s1355617700666031. [DOI] [PubMed] [Google Scholar]
- 161.Christianson SA, Säisä J, Garvill J, Silfvenius H. Hemisphere inactivation and mood-state changes. Brain Cogn. 1993;23:127–144. doi: 10.1006/brcg.1993.1051. [DOI] [PubMed] [Google Scholar]
- 162.Lee GP, Loring DW, Meader KJ, Brooks BB. Hemispheric specialization for emotional expression: a reexamination of results from intracarotid administration of sodium amobarbital. Brain Cogn. 1990;12:267–280. doi: 10.1016/0278-2626(90)90019-k. [DOI] [PubMed] [Google Scholar]
- 163.Lee GP, Loring DW, Meador KJ. Influence of premorbid personality and location of lesion on emotional expression. Int J Neurosci. 1993;72:157–165. doi: 10.3109/00207459309024104. [DOI] [PubMed] [Google Scholar]
- 164.Trimble M. Sodium amytal testing and the laterality of emotion. Clin EEG Neurosci. 2010;41:211–213. doi: 10.1177/155005941004100408. [DOI] [PubMed] [Google Scholar]
- 165.Dodson MJ. Vestibular stimulation in mania: a case report. J Neurol Neurosurg Psychiatry. 2004;75:168–169. [PMC free article] [PubMed] [Google Scholar]
- 166.Levine J, Toder D, Geller V, Kraus M, Gauchman T, Puterman M, Grisaru N. Beneficial effects of caloric vestibular stimulation on denial of illness and manic delusions in schizoaffective disorder: a case report. Brain Stimul. 2012;5:267–273. doi: 10.1016/j.brs.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 167.Graae F, Tenke C, Bruder G, Rotheram MJ, Piacentini J, Castro-Blanco D, Leite P, Towey J. Abnormality of EEG alpha asymmetry in female adolescent suicide attempters. Biol Psychiatry. 1996;40:706–713. doi: 10.1016/0006-3223(95)00493-9. [DOI] [PubMed] [Google Scholar]
- 168.Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2007;12:360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 169.Brüne M, Schöbel A, Karau R, Faustmann PM, Dermietzel R, Juckel G, Petrasch-Parwez E. Neuroanatomical correlates of suicide in psychosis: the possible role of von Economo neurons. PLoS One. 2011;6:e20936. doi: 10.1371/journal.pone.0020936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cannon WB. Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. New York, NY: D. Appleton and Company; 1929. [Google Scholar]
- 171.Cerqueira JJ, Almeida OF, Sousa N. The stressed prefrontal cortex. Left? Right! Brain Behav Immun. 2008;22:630–638. doi: 10.1016/j.bbi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 172.Pereira VH, Cerqueira JJ, Palha JA, Sousa N. Stressed brain, diseased heart: a review on the pathophysiologic mechanisms of neurocardiology. Int J Cardiol. 2013;166:30–37. doi: 10.1016/j.ijcard.2012.03.165. [DOI] [PubMed] [Google Scholar]
- 173.Taggart P, Boyett MR, Logantha S, Lambiase PD. Anger, emotion, and arrhythmias: from brain to heart. Front Physiol. 2011;2:67. doi: 10.3389/fphys.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Avnon Y, Nitzan M, Sprecher E, Rogowski Z, Yarnitsky D. Autonomic asymmetry in migraine: augmented parasympathetic activation in left unilateral migraineurs. Brain. 2004;127:2099–2108. doi: 10.1093/brain/awh236. [DOI] [PubMed] [Google Scholar]
- 175.Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 176.Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci. 2009;364:1933–1942. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Critchley HD, Taggart P, Sutton PM, Holdright DR, Batchvarov V, Hnatkova K, Malik M, Dolan RJ. Mental stress and sudden cardiac death: asymmetric midbrain activity as a linking mechanism. Brain. 2005;128:75–85. doi: 10.1093/brain/awh324. [DOI] [PubMed] [Google Scholar]
- 178.Lane RD, Jennings JR. Hemispheric asymmetry, autonomic asymmetry, and the problem of sudden cardiac death. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge, MA: MIT Press; 1995. pp. 271–304. [Google Scholar]
- 179.Rogers MC, Battit G, McPeek B, Todd D. Lateralization of sympathetic control of the human sinus node: ECG changes of stellate ganglion block. Anesthesiology. 1978;48:139–141. doi: 10.1097/00000542-197802000-00009. [DOI] [PubMed] [Google Scholar]
- 180.Taggart P. Brain-heart interactions and cardiac ventricular arrhythmias. Neth Heart J. 2013;21:78–81. doi: 10.1007/s12471-012-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wittling W, Block A, Genzel S, Schweiger E. Hemisphere asymmetry in parasympathetic control of the heart. Neuropsychologia. 1998;36:461–468. doi: 10.1016/s0028-3932(97)00129-2. [DOI] [PubMed] [Google Scholar]
- 182.Foster PS, Hubbard T, Yung RC, Ferguson BJ, Drago V, Harrison DW. Cerebral asymmetry in the control of cardiovascular functioning: evidence from lateral vibrotactile stimulation. Laterality. 2013;18:108–119. doi: 10.1080/1357650X.2011.631545. [DOI] [PubMed] [Google Scholar]
- 183.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 184.Swartz CM, Abrams R, Lane RD, DuBois MA, Srinivasaraghavan J. Heart rate differences between right and left unilateral electroconvulsive therapy. J Neurol Neurosurg Psychiatry. 1994;57:97–99. doi: 10.1136/jnnp.57.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66:477–483. doi: 10.1212/01.wnl.0000202684.29640.60. [DOI] [PubMed] [Google Scholar]
- 186.Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6:131–140. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- 187.Heilman KM. Emotional experience: a neurological model. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York, NY: Oxford University Press; 2000. pp. 328–344. [Google Scholar]
- 188.Yoon BW, Morillo CA, Cechetto DF, Hachinski V. Cerebral hemispheric lateralization in cardiac autonomic control. Arch Neurol. 1997;54:741–744. doi: 10.1001/archneur.1997.00550180055012. [DOI] [PubMed] [Google Scholar]
- 189.Forscher EC, Li W. Hemispheric asymmetry and visuo-olfactory integration in perceiving subthreshold (micro) fearful expressions. J Neurosci. 2012;32:2159–2165. doi: 10.1523/JNEUROSCI.5094-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kimura Y, Yoshino A, Takahashi Y, Nomura S. Interhemispheric difference in emotional response without awareness. Physiol Behav. 2004;82:727–731. doi: 10.1016/j.physbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 191.Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 192.Noesselt T, Driver J, Heinze HJ, Dolan R. Asymmetrical activation in the human brain during processing of fearful faces. Curr Biol. 2005;15:424–429. doi: 10.1016/j.cub.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 193.Siman-Tov T, Papo D, Gadoth N, Schonberg T, Mendelsohn A, Perry D, Hendler T. Mind your left: spatial bias in subcortical fear processing. J Cogn Neurosci. 2009;21:1782–1789. doi: 10.1162/jocn.2009.21120. [DOI] [PubMed] [Google Scholar]
- 194.Brailsford R, Catherwood D, Tyson PJ, Edgar G. Noticing spiders on the left: Evidence on attentional bias and spider fear in the inattentional blindness paradigm. Laterality. 2013 doi: 10.1080/1357650X.2013.791306. (in press) [DOI] [PubMed] [Google Scholar]
- 195.Bradley MM, Cuthbert BN, Lang PJ. Startle reflex modification: emotion or attention? Psychophysiology. 1990;27:513–522. doi: 10.1111/j.1469-8986.1990.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 196.Bradley MM, Cuthbert BN, Lang PJ. Lateralized startle probes in the study of emotion. Psychophysiology. 1996;33:156–161. doi: 10.1111/j.1469-8986.1996.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 197.Moses SN, Houck JM, Martin T, Hanlon FM, Ryan JD, Thoma RJ, Weisend MP, Jackson EM, Pekkonen E, Tesche CD. Dynamic neural activity recorded from human amygdala during fear conditioning using magnetoencephalography. Brain Res Bull. 2007;71:452–460. doi: 10.1016/j.brainresbull.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 198.Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, di Paola F. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119:1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- 199.Gläscher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hewig J, Schlotz W, Gerhards F, Breitenstein C, Lürken A, Naumann E. Associations of the cortisol awakening response (CAR) with cortical activation asymmetry during the course of an exam stress period. Psychoneuroendocrinology. 2008;33:83–91. doi: 10.1016/j.psyneuen.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 201.Lueken U, Leisse M, Mattes K, Naumann D, Wittling W, Schweiger E. Altered tonic and phasic cortisol secretion following unilateral stroke. Psychoneuroendocrinology. 2009;34:402–412. doi: 10.1016/j.psyneuen.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 202.Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wittling W, Pflüger M. Neuroendocrine hemisphere asymmetries: salivary cortisol secretion during lateralized viewing of emotion-related and neutral films. Brain Cogn. 1990;14:243–265. doi: 10.1016/0278-2626(90)90032-j. [DOI] [PubMed] [Google Scholar]
- 204.Wittling W. The right hemisphere and the human stress response. Acta Physiol Scand Suppl. 1997;640:55–59. [PubMed] [Google Scholar]
- 205.Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. Am J Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- 206.Looi JC, Maller JJ, Pagani M, Högberg G, Lindberg O, Liberg B, Botes L, Engman EL, Zhang Y, Svensson L, Wahlund LO. Caudate volumes in public transportation workers exposed to trauma in the Stockholm train system. Psychiatry Res. 2009;171:138–143. doi: 10.1016/j.pscychresns.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 207.Rabe S, Beauducel A, Zöllner T, Maercker A, Karl A. Regional brain electrical activity in posttraumatic stress disorder after motor vehicle accident. J Abnorm Psychol. 2006;115:687–698. doi: 10.1037/0021-843X.115.4.687. [DOI] [PubMed] [Google Scholar]
- 208.Rabe S, Zoellner T, Beauducel A, Maercker A, Karl A. Changes in brain electrical activity after cognitive behavioral therapy for posttraumatic stress disorder in patients injured in motor vehicle accidents. Psychosom Med. 2008;70:13–19. doi: 10.1097/PSY.0b013e31815aa325. [DOI] [PubMed] [Google Scholar]
- 209.Tillman GD, Kimbrell TA, Calley CS, Kraut MA, Freeman TW, Hart J., Jr Repetitive transcranial magnetic stimulation and threat memory: selective reduction of combat threat memory p300 response after right frontal-lobe stimulation. J Neuropsychiatry Clin Neurosci. 2011;23:40–47. doi: 10.1176/jnp.23.1.jnp40. [DOI] [PubMed] [Google Scholar]
- 210.Akiyoshi J, Hieda K, Aoki Y, Nagayama H. Frontal brain hypoactivity as a biological substrate of anxiety in patients with panic disorders. Neuropsychobiology. 2003;47:165–170. doi: 10.1159/000070587. [DOI] [PubMed] [Google Scholar]
- 211.Bruder GE, Schneier FR, Stewart JW, McGrath PJ, Quitkin F. Left hemisphere dysfunction during verbal dichotic listening tests in patients who have social phobia with or without comorbid depressive disorder. Am J Psychiatry. 2004;161:72–78. doi: 10.1176/appi.ajp.161.1.72. [DOI] [PubMed] [Google Scholar]
- 212.Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biol Psychiatry. 2000;47:85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- 213.Meyer JH, Swinson R, Kennedy SH, Houle S, Brown GM. Increased left posterior parietal-temporal cortex activation after D-fenfluramine in women with panic disorder. Psychiatry Res. 2000;98:133–143. doi: 10.1016/s0925-4927(00)00048-2. [DOI] [PubMed] [Google Scholar]
- 214.Moscovitch DA, Santesso DL, Miskovic V, McCabe RE, Antony MM, Schmidt LA. Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biol Psychol. 2011;87:379–385. doi: 10.1016/j.biopsycho.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 215.Nordahl TE, Semple WE, Gross M, Mellman TA, Stein MB, Goyer P, King AC, Uhde TW, Cohen RM. Cerebral glucose metabolic differences in patients with panic disorder. Neuropsychopharmacology. 1990;3:261–272. [PubMed] [Google Scholar]
- 216.Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch Gen Psychiatry. 1999;56:78–84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]
- 217.Gainotti G. Lateralization of brain mechanisms underlying automatic and controlled forms of spatial orienting of attention. Neurosci Biobehav Rev. 1996;20:617–622. doi: 10.1016/0149-7634(95)00074-7. [DOI] [PubMed] [Google Scholar]
- 218.Mapstone M, Weintraub S, Nowinski C, Kaptanoglu G, Gitelman DR, Mesulam MM. Cerebral hemispheric specialization for spatial attention: spatial distribution of search-related eye fixations in the absence of neglect. Neuropsychologia. 2003;41:1396–1409. doi: 10.1016/s0028-3932(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 219.Verleger R, Sprenger A, Gebauer S, Fritzmannova M, Friedrich M, Kraft S, Jaśkowski P. On why left events are the right ones: neural mechanisms underlying the left-hemifield advantage in rapid serial visual presentation. J Cogn Neurosci. 2009;21:474–488. doi: 10.1162/jocn.2009.21038. [DOI] [PubMed] [Google Scholar]
- 220.Okada T, Sato W, Kubota Y, Toichi M, Murai T. Right hemispheric dominance and interhemispheric cooperation in gaze-triggered reflexive shift of attention. Psychiatry Clin Neurosci. 2012;66:97–104. doi: 10.1111/j.1440-1819.2011.02302.x. [DOI] [PubMed] [Google Scholar]
- 221.Iyilikci O, Becker C, Güntürkün O, Amado S. Visual processing asymmetries in change detection. Perception. 2010;39:761–769. doi: 10.1068/p6623. [DOI] [PubMed] [Google Scholar]
- 222.Spotorno S, Faure S. Change detection in complex scenes: hemispheric contribution and the role of perceptual and semantic factors. Perception. 2011;40:5–22. doi: 10.1068/p6524. [DOI] [PubMed] [Google Scholar]
- 223.Spotorno S, Faure S. The right hemisphere advantage in visual change detection depends on temporal factors. Brain Cogn. 2011;77:365–371. doi: 10.1016/j.bandc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 224.Vos L, Whitman D. Maintaining perceptual constancy while remaining vigilant: left hemisphere change blindness and right hemisphere vigilance. Laterality. 2013 doi: 10.1080/1357650X.2013.778274. (in press) [DOI] [PubMed] [Google Scholar]
- 225.Ferrè ER, Vagnoni E, Haggard P. Galvanic vestibular stimulation influences randomness of number generation. Exp Brain Res. 2013;224:233–241. doi: 10.1007/s00221-012-3302-6. [DOI] [PubMed] [Google Scholar]
- 226.Eysenck MW, Mogg K, May J, Richards A, Mathews A. Bias in interpretation of ambiguous sentences related to threat in anxiety. J Abnorm Psychol. 1991;100:144–150. doi: 10.1037//0021-843x.100.2.144. [DOI] [PubMed] [Google Scholar]
- 227.MacLeod C, Cohen IL. Anxiety and the interpretation of ambiguity: a text comprehension study. J Abnorm Psychol. 1993;102:238–247. doi: 10.1037//0021-843x.102.2.238. [DOI] [PubMed] [Google Scholar]
- 228.Mogg K, Baldwin DS, Brodrick P, Bradley BP. Effect of short-term SSRI treatment on cognitive bias in generalised anxiety disorder. Psychopharmacology (Berl) 2004;176:466–470. doi: 10.1007/s00213-004-1902-y. [DOI] [PubMed] [Google Scholar]
- 229.Gray JA. A critique of Eysenck's theory of personality. In: Eysenck HJ, editor. A model for personality. New York, NY: Springer-Verlag; 1981. pp. 246–276. [Google Scholar]
- 230.Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- 231.Sutton SK, Davidson RJ. Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychol Sci. 1997;8:204–210. [Google Scholar]
- 232.Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- 233.Harmon-Jones E. Early Career Award. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–848. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- 234.Harmon-Jones E, Harmon-Jones C, Serra R, Gable PA. The effect of commitment on relative left frontal cortical activity: tests of the action-based model of dissonance. Pers Soc Psychol Bull. 2011;37:395–408. doi: 10.1177/0146167210397059. [DOI] [PubMed] [Google Scholar]
- 235.Crost NW, Pauls CA, Wacker J. Defensiveness and anxiety predict frontal EEG asymmetry only in specific situational contexts. Biol Psychol. 2008;78:43–52. doi: 10.1016/j.biopsycho.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 236.Lieberman M. The brain's braking system (and how to 'use your words' to tap into it) Neuroleadership. 2009;2:9–14. [Google Scholar]
- 237.Ironson G, Balbin E, Stuetzle R, Fletcher MA, O'Cleirigh C, Laurenceau JP, Schneiderman N, Solomon G. Dispositional optimism and the mechanisms by which it predicts slower disease progression in HIV: proactive behavior, avoidant coping, and depression. Int J Behav Med. 2005;12:86–97. doi: 10.1207/s15327558ijbm1202_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brébion G, Smith MJ, Allilaire JF. Psychometric characteristics of ideational retardation in depressives. Br J Clin Psychol. 1995;34:371–381. doi: 10.1111/j.2044-8260.1995.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 239.Widlöcher DJ. Psychomotor retardation: clinical, theoretical, and psychometric aspects. Psychiatr Clin North Am. 1983;6:27–40. [PubMed] [Google Scholar]
- 240.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 241.Ghozlan A, Widlöcher D. Decision time and movement time in depression: differential effects of practice before and after clinical improvement. Percept Mot Skills. 1989;68:187–192. doi: 10.2466/pms.1989.68.1.187. [DOI] [PubMed] [Google Scholar]
- 242.Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: returning to contextual roots. Clin Psychol (New York) 2001;8:255–270. [Google Scholar]
- 243.Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Gallop R, McGlinchey JB, Markley DK, Gollan JK, Atkins DC, Dunner DL, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 244.Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, Rizvi SL, Gollan JK, Dunner DL, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dimidjian S, Barrera M, Jr, Martell C, Muñoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- 246.Herring MP, Puetz TW, O'Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:101–111. doi: 10.1001/archinternmed.2011.696. [DOI] [PubMed] [Google Scholar]
- 247.Sarris J. Clinical depression: an evidence-based integrative complementary medicine treatment model. Altern Ther Health Med. 2011;17:26–37. [PubMed] [Google Scholar]
- 248.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46:397–411. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 249.Tyson P, Wilson K, Crone D, Brailsford R, Laws K. Physical activity and mental health in a student population. J Ment Health. 2010;19:492–499. doi: 10.3109/09638230902968308. [DOI] [PubMed] [Google Scholar]
- 250.Kavussanu M, McAuley E. Exercise and optimism: are highly active individuals more optimistic? J Sport Exerc Psychol. 1995;17:246–258. [Google Scholar]
- 251.Lipowski M. Level of optimism and health behavior in athletes. Med Sci Monit. 2012;18:CR39–CR43. doi: 10.12659/MSM.882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Escames G, Ozturk G, Baño-Otálora B, Pozo MJ, Madrid JA, Reiter RJ, Serrano E, Concepción M, Acuña-Castroviejo D. Exercise and melatonin in humans: reciprocal benefits. J Pineal Res. 2012;52:1–11. doi: 10.1111/j.1600-079X.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- 253.Hegadoren KM, O'Donnell T, Lanius R, Coupland NJ, Lacaze-Masmonteil N. The role of β-endorphin in the pathophysiology of major depression. Neuropeptides. 2009;43:341–353. doi: 10.1016/j.npep.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 254.Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Petruzzello SJ, Landers DM. State anxiety reduction and exercise: does hemispheric activation reflect such changes? Med Sci Sports Exerc. 1994;26:1028–1035. [PubMed] [Google Scholar]
- 256.Vogt T, Schneider S, Brümmer V, Strüder HK. Frontal EEG asymmetry: the effects of sustained walking in the elderly. Neurosci Lett. 2010;485:134–137. doi: 10.1016/j.neulet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 257.Williamson JW, Nobrega AC, McColl R, Mathews D, Winchester P, Friberg L, Mitchell JH. Activation of the insular cortex during dynamic exercise in humans. J Physiol. 1997;503:277–283. doi: 10.1111/j.1469-7793.1997.277bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Carlstedt RA. Line-bisecting performance in highly skilled athletes: Does preponderance of rightward error reflect unique cortical organization and functioning? Brain Cogn. 2004;54:52–57. doi: 10.1016/s0278-2626(03)00259-8. [DOI] [PubMed] [Google Scholar]
- 259.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 260.Bargiel-Matusiewicz K, Krzyszkowska A. Dispositional optimism and coping with pain. Eur J Med Res. 2009;14(Suppl 4):271–274. doi: 10.1186/2047-783X-14-S4-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lin EH, Peterson C. Pessimistic explanatory style and response to illness. Behav Res Ther. 1990;28:243–248. doi: 10.1016/0005-7967(90)90007-6. [DOI] [PubMed] [Google Scholar]
- 262.Lopes MP, Cunha MP. Who is more proactive, the optimist or the pessimist? Exploring the role of hope as a moderator. J Posit Psychol. 2008;3:100–109. [Google Scholar]
- 263.Ramírez-Maestre C, Esteve R, López AE. The role of optimism and pessimism in chronic pain patients adjustment. Span J Psychol. 2012;15:286–294. doi: 10.5209/rev_sjop.2012.v15.n1.37335. [DOI] [PubMed] [Google Scholar]
- 264.Gibson JJ. The senses considered as perceptual systems. Boston, MA: Houghton Mifflin; 1966. [Google Scholar]
- 265.Gibson JJ. The Ecological Approach to Visual Perception. Boston, MA: Houghton Mifflin; 1979. [Google Scholar]
- 266.Gibson EJ, Pick AD. An ecological approach to perceptual learning and development. Oxford: Oxford University Press; 2000. [Google Scholar]
- 267.Piaget J. The grasp of consciousness: action and concept in the young child. Cambridge, MA: Harvard University Press; 1976. [Google Scholar]
- 268.Vygotskiĭ LS. Mind in society: the development of higher psychological processes. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- 269.Hesslow G. Conscious thought as simulation of behaviour and perception. Trends Cogn Sci. 2002;6:242–247. doi: 10.1016/s1364-6613(02)01913-7. [DOI] [PubMed] [Google Scholar]
- 270.Hesslow G. The current status of the simulation theory of cognition. Brain Res. 2012;1428:71–79. doi: 10.1016/j.brainres.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 271.Wilson M. Six views of embodied cognition. Psychon Bull Rev. 2002;9:625–636. doi: 10.3758/bf03196322. [DOI] [PubMed] [Google Scholar]
- 272.Piaget J. The origins of intelligence in children. New York, NY: International Universities Press; 1952. [Google Scholar]
- 273.Piaget J. Biology and knowledge: an essay on the relations between organic regulations and cognitive processes. Chicago, IL: University of Chicago Press; 1974. [Google Scholar]
- 274.Lakoff G, Johnson M. Metaphors we live by. Chicago, IL: University of Chicago Press; 1980. [Google Scholar]
- 275.Lakoff G, Johnson M. Philosophy in the flesh: the embodied mind and its challenge to western thought. New York, NY: Basic Books; 1999. [Google Scholar]
- 276.Lakoff G, Núñez RE. Where mathematics comes from: how the embodied mind brings mathematics into being. New York, NY: Basic Books; 2000. [Google Scholar]
- 277.Varela FJ, Thompson E, Rosch E. The embodied mind: cognitive science and human experience. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- 278.Barsalou LW. Grounded cognition. Annu Rev Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- 279.Damasio A, Damasio H. Minding the body. Daedalus. 2006;135:15–22. [Google Scholar]
- 280.Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- 281.Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54:768–777. doi: 10.1037//0022-3514.54.5.768. [DOI] [PubMed] [Google Scholar]
- 282.Cacioppo JT, Priester JR, Berntson GG. Rudimentary determinants of attitudes. II: Arm flexion and extension have differential effects on attitudes. J Pers Soc Psychol. 1993;65:5–17. doi: 10.1037//0022-3514.65.1.5. [DOI] [PubMed] [Google Scholar]
- 283.Ping RM, Dhillon S, Beilock SL. Reach for what you like: the body's role in shaping preferences. Emot Rev. 2009;1:140–150. [Google Scholar]
- 284.Williams LE, Bargh JA. Experiencing physical warmth promotes interpersonal warmth. Science. 2008;322:606–607. doi: 10.1126/science.1162548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ijzerman H, Semin GR. The thermometer of social relations: mapping social proximity on temperature. Psychol Sci. 2009;20:1214–1220. doi: 10.1111/j.1467-9280.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- 286.Zhong CB, Leonardelli GJ. Cold and lonely: does social exclusion literally feel cold? Psychol Sci. 2008;19:838–842. doi: 10.1111/j.1467-9280.2008.02165.x. [DOI] [PubMed] [Google Scholar]
- 287.Ijzerman H, Gallucci M, Pouw WT, Weiβgerber SC, Van Doesum NJ, Williams KD. Cold-blooded loneliness: social exclusion leads to lower skin temperatures. Acta Psychol (Amst) 2012;140:283–288. doi: 10.1016/j.actpsy.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 288.Bargh JA, Shalev I. The substitutability of physical and social warmth in daily life. Emotion. 2012;12:154–162. doi: 10.1037/a0023527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cosmides L, Tooby J. Cognitive adaptations for social exchange. In: Barkow JH, Cosmides L, Tooby J, editors. The adapted mind: evolutionary psychology and the generation of culture. New York, NY: Oxford University Press; 1992. pp. 163–228. [Google Scholar]
- 290.Vygotskiĭ LS. In: Thought and language. Kozulin A, editor. Cambridge, MA: MIT Press; 1986. [Google Scholar]
- 291.Williams LE, Huang JY, Bargh JA. The Scaffolded Mind: Higher mental processes are grounded in early experience of the physical world. Eur J Soc Psychol. 2009;39:1257–1267. doi: 10.1002/ejsp.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bowlby J. Attachment and loss: Vol. 1. Attachment. New York, NY: Basic Books; 1969. [Google Scholar]
- 293.Ainsworth MD. Infancy in Uganda: infant care and the growth of love. Baltimore, MD: Johns Hopkins Press; 1967. [Google Scholar]
- 294.Harlow HF. The nature of love. Am Psychol. 1958;13:673–685. [Google Scholar]
- 295.Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. Motor "dexterity"?: evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 2012;22:51–59. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Anderson C, Galinsky AD. Power, optimism, and risk-taking. Eur J Soc Psychol. 2006;36:511–536. [Google Scholar]
- 297.Anderson C, Jennifer BL. The experience of power: examining the effects of power on approach and inhibition tendencies. J Pers Soc Psychol. 2002;83:1362–1377. [PubMed] [Google Scholar]
- 298.Fast NJ, Gruenfeld DH, Sivanathan N, Galinsky AD. Illusory control: a generative force behind power's far-reaching effects. Psychol Sci. 2009;20:502–508. doi: 10.1111/j.1467-9280.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 299.Stajkovic AD. Development of a core confidence-higher order construct. J Appl Psychol. 2006;91:1208–1224. doi: 10.1037/0021-9010.91.6.1208. [DOI] [PubMed] [Google Scholar]
- 300.Keltner D, Gruenfeld DH, Anderson C. Power, approach, and inhibition. Psychol Rev. 2003;110:265–284. doi: 10.1037/0033-295x.110.2.265. [DOI] [PubMed] [Google Scholar]
- 301.Smith PK, Bargh JA. Nonconscious effects of power on basic approach and avoidance tendencies. Soc Cogn. 2008;26:1–24. doi: 10.1521/soco.2008.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schwartz B, Ward A, Monterosso J, Lyubomirsky S, White K, Lehman DR. Maximizing versus satisficing: happiness is a matter of choice. J Pers Soc Psychol. 2002;83:1178–1197. doi: 10.1037//0022-3514.83.5.1178. [DOI] [PubMed] [Google Scholar]
- 303.Flett GL, Besser A, Davis RA, Hewitt PL. Dimensions of perfectionism, unconditional self-acceptance, and depression. J Ration Emot Cogn Behav Ther. 2003;21:119–138. [Google Scholar]
- 304.Scott J. The effect of perfectionism and unconditional self-acceptance on depression. J Ration Emot Cogn Behav Ther. 2007;25:35–64. [Google Scholar]
- 305.Ben-Shahar T. The pursuit of perfect: how to stop chasing perfection and start living a richer, happier life. New York, NY: McGraw-Hill; 2009. [Google Scholar]
- 306.Ellis A. How I learned to help clients feel better and get better. Psychotherapy (Chic) 1996;33:149–151. [Google Scholar]
- 307.Ryan RM, Brown KW. Why we don't need self-esteem: on fundamental needs, contingent love, and mindfulness. Psychol Inq. 2003;14(1):71–76. [Google Scholar]
- 308.Everhart DE, Harrison DW, Crews WD., Jr Hemispheric asymmetry as a function of handedness: perception of facial affect stimuli. Percept Mot Skills. 1996;82:264–266. doi: 10.2466/pms.1996.82.1.264. [DOI] [PubMed] [Google Scholar]
- 309.Reuter-Lorenz PA, Givis RP, Moscovitch M. Hemispheric specialization and the perception of emotion: evidence from right-handers and from inverted and non-inverted left-handers. Neuropsychologia. 1983;21:687–692. doi: 10.1016/0028-3932(83)90068-4. [DOI] [PubMed] [Google Scholar]
- 310.McFarland RA, Kennison R. Handedness affects emotional valence asymmetry. Percept Mot Skills. 1989;68:435–441. doi: 10.2466/pms.1989.68.2.435. [DOI] [PubMed] [Google Scholar]
- 311.Brookshire G, Casasanto D. Motivation and motor control: hemispheric specialization for approach motivation reverses with handedness. PLoS One. 2012;7:e36036. doi: 10.1371/journal.pone.0036036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Maxwell JS, Davidson RJ. Emotion as motion: asymmetries in approach and avoidant actions. Psychol Sci. 2007;18:1113–1119. doi: 10.1111/j.1467-9280.2007.02033.x. [DOI] [PubMed] [Google Scholar]
- 313.Perry RJ, Rosen HR, Kramer JH, Beer JS, Levenson RL, Miller BL. Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–160. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- 314.Smith BD, Kline R, Meyers M. The differential hemispheric processing of emotion: a comparative analysis in strongly-lateralized sinistrals and dextrals. Int J Neurosci. 1990;50:59–71. doi: 10.3109/00207459008987157. [DOI] [PubMed] [Google Scholar]
- 315.Siebner HR, Limmer C, Peinemann A, Drzezga A, Bloem BR, Schwaiger M, Conrad B. Long-term consequences of switching handedness: a positron emission tomography study on handwriting in "converted" left-handers. J Neurosci. 2002;22:2816–2825. doi: 10.1523/JNEUROSCI.22-07-02816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jaju DS, Dikshit MB, Purandare VR, Raje S. Heart rate and blood pressure responses of left-handers and right-handers to autonomic stressors. Indian J Physiol Pharmacol. 2004;48:31–40. [PubMed] [Google Scholar]
- 317.Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: a meta-analytic review. Ann Behav Med. 2009;37:239–256. doi: 10.1007/s12160-009-9111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Boehm JK, Kubzansky LD. The heart's content: the association between positive psychological well-being and cardiovascular health. Psychol Bull. 2012;138:655–691. doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- 319.Segerstrom SC, Sephton SE. Optimistic expectancies and cell-mediated immunity: the role of positive affect. Psychol Sci. 2010;21:448–455. doi: 10.1177/0956797610362061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Srivastava S, McGonigal KM, Richards JM, Butler EA, Gross JJ. Optimism in close relationships: how seeing things in a positive light makes them so. J Pers Soc Psychol. 2006;91:143–153. doi: 10.1037/0022-3514.91.1.143. [DOI] [PubMed] [Google Scholar]