Abstract
Mefenamic acid (MFA), a carboxylic acid–containing nonsteroidal anti-inflammatory drug, is metabolized into the chemically-reactive MFA-1-O-acyl-glucuronide (MFA-1-O-G), MFA-acyl-adenylate (MFA-AMP), and the MFA-S-acyl-coenzyme A (MFA-CoA), all of which are electrophilic and capable of acylating nucleophilic sites on biomolecules. In this study, we investigate the nonenzymatic ability of each MFA acyl-linked metabolite to transacylate amino and thiol functional groups on the acceptor biomolecules Gly, Tau, l-glutathione (GSH), and N-acetylcysteine (NAC). In vitro incubations with each of the MFA acyl-linked metabolites (1 μM) in buffer under physiologic conditions with Gly, Tau, GSH, or NAC (10 mM) revealed that MFA-CoA was 11.5- and 19.5-fold more reactive than MFA-AMP toward the acylation of cysteine-sulfhydryl groups of GSH and NAC, respectively. However, MFA-AMP was more reactive toward both Gly and Tau, 17.5-fold more reactive toward the N-acyl-amidation of taurine than its corresponding CoA thioester, while MFA-CoA displayed little reactivity toward glycine. Additionally, mefenamic acid-S-acyl-glutathione (MFA-GSH) was 5.6- and 108-fold more reactive toward NAC than MFA-CoA and MFA-AMP, respectively. In comparison with MFA-AMP and MFA-CoA, MFA-1-O-G was not significantly reactive toward all four bionucleophiles. MFA-AMP, MFA-CoA, MFA-1-O-G, MFA-GSH, and mefenamic acid-taurine were also detected in rat in vitro hepatocyte MFA (100 μM) incubations, while mefenamic acid-glycine was not. These results demonstrate that MFA-AMP selectively reacts with the amino functional groups of glycine and lysine nonenzymatically, MFA-CoA selectively reacts nonenzymatically with the thiol functional groups of GSH and NAC, and MFA-GSH reacts with the thiol functional group of GSH nonenzymatically, all of which may potentially elicit an idiosyncratic toxicity in vivo.
Introduction
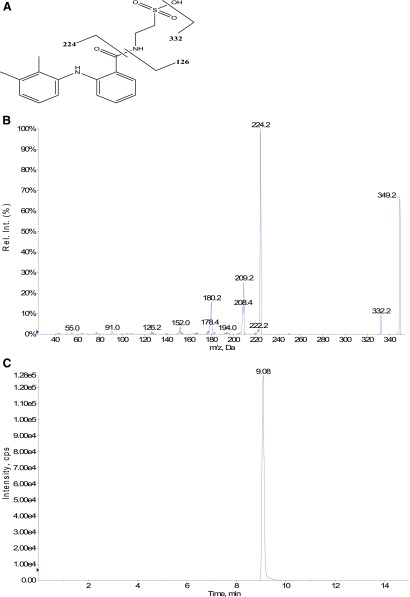
Mefenamic acid (MFA), (2′,3′)-dimethyl-N-phenyl-anthranilic acid, is a carboxylic acid-containing nonsterodial anti-inflammatory drug associated with a rare, but sometimes serious idiosyncratic nephrotoxicity (Robertson et al., 1980; Drury et al., 1981; Woods and Michael, 1981; Taha et al., 1985) and possibly hepatotoxicity (Somchit et al., 2004). A proposed mechanism for the occurrence of these MFA-induced toxicities suggests that MFA undergoes bioactivation into chemically-reactive acyl-linked metabolites that ultimately become covalently bound to tissue proteins, resulting in adverse immunologic responses (Fig. 1). MFA is metabolized to 3-hydroxy-MFA (Glazko, 1966) and 3-carboxy-MFA (Sato et al., 1993) via CYP 2C9. MFA also undergoes glucuronidation via uridine 5′-diphospho-glucuronosyltransferase (UGT) into the unstable, reactive acyl glucuronide metabolite MFA-1-O-acyl-glucuronide (MFA-1-O-G) (McGurk et al., 1996; Somchit et al., 2004). Acyl glucuronides of acidic drugs are proposed to bind covalently to protein via a direct transacylation reaction in which protein nucleophiles react with the facile carbonyl-carbon of the acyl glucuronide, resulting in the liberation of the glucuronic acid, and via the formation of a drug-protein conjugate or a glycation mechanism involving prior acyl migration of the drug on the glucuronic acid moiety permitting ring opening of the sugar resulting in an exposed reactive aldehyde group that reversibly forms an imine (Schiff base) with an amine group on proteins. Subsequent Amadori rearrangement results in a stable ketoamine derivative in which both the drug and the glucuronic acid moiety become covalently bound onto the protein (Benet et al., 1993). MFA is also metabolized into the reactive MFA-acyl-adenylate (MFA-AMP) and MFA-S-acyl-coenzyme A (MFA-CoA) via acyl-CoA synthetase (ACS), both of which have been demonstrated to form l-glutathione (GSH)-adducts and are proposed to play a role in MFA-mediated idiosyncratic toxicity (Grillo et al., 2012; Horng and Benet, 2013). This pathway occurs when the AMP moiety of ATP is covalently transferred to the carboxyl group of MFA to form MFA-AMP, followed by the displacement of the AMP with coenzyme A (CoA) to form MFA-CoA thioesters (Kelley and Vessey, 1994; Mano et al., 2001). Upon their formation, the carbonyl carbons of both MFA-AMP and MFA-CoA increase in electrophilicity, enabling them to transacylate the biologic nucleophile GSH (Horng and Benet, 2013). It is proposed that these drug-protein adducts could act as haptens and are recognized by the immune system as foreign, eliciting an autoimmune-type response resulting in the associated idiosyncratic toxicity (Uetrecht, 2007). Previous in vitro incubations with the model nucleophile GSH under physiologic conditions showed MFA-AMP to be reactive toward GSH, but 11-fold less reactive than MFA-CoA, while MFA-1-O-G exhibited little GSH reactivity (Horng and Benet, 2013). In vitro rat hepatocyte incubations have also resulted in the detection of MFA-AMP, MFA-CoA, MFA-1-O-G, and mefenamic acid-S-acyl-glutathione (MFA-GSH) (Horng and Benet, 2013), all of which could be more reactive than MFA per se and potentially involved in the formation of drug-protein adducts. Additionally, studies involving the acyl-linked metabolites of the bile acid cholic acid (CA) revealed that CA-AMP selectively reacts nonenzymatically with amino functional groups while CA-CoA preferentially reacts nonenzymatically with thiol functional groups (Mitamura et al., 2011).
Fig. 1.
Proposed conjugative bioactivation pathways of mefenamic acid.
The following experiments were designed to characterize the nonenzymatic acylation of the nucleophilic biomolecules containing the amino functional groups of Gly and Tau and the thiol functional groups of GSH and N-acetylcysteine (NAC) by MFA-AMP, MFA-CoA, MFA-1-O-G, and MFA-GSH (NAC only), as well as the detection of these adducts in rat hepatocyte in vitro incubations. We propose that MFA-AMP, MFA-CoA, and MFA-1-O-G are all selective toward their acylation of nucleophilic functional groups on biologic molecules, all of which can contribute to the formation of MFA adducts with amino acids, peptides, and proteins. We also hypothesize that MFA-GSH is reactive in its own right due to its structural similarity to MFA-CoA via the thioester bond. If this proposal is correct, the reactive acyl-linked metabolites of MFA would be anticipated to be selective in their formation of drug-protein adducts in vivo, which may potentially mediate the idiosyncratic toxicities associated with MFA and other carboxylic acid-containing drugs.
Materials and Methods
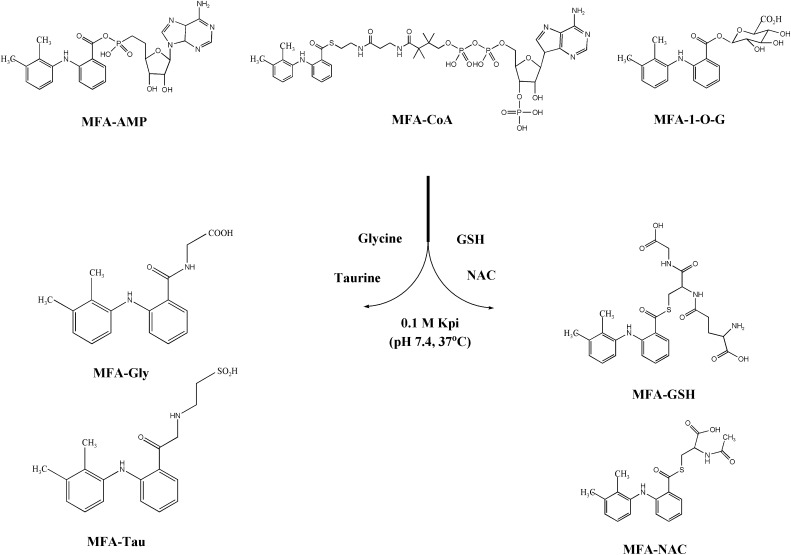
MFA, AMP, CoA, anhydrous tetrahydrofuran (THF), triethylamine, ethyl chloroformate (ECF), N,N′-dicyclohexylcarbodiimide, pyridine, potassium phosphate, potassium carbonate, dimethylsulfoxide (DMSO), carbamazepine (CBZ), GSH, Gly, NAC, and Tau were all purchased from Sigma-Aldrich (St. Louis, MO). Acetonitrile (ACN), methanol, acetone, ammonium acetate, and ethyl acetate were all purchased from Fisher Scientific (Pittsburgh, PA). MFA-1-O-G was purchased from Toronto Research Chemicals (TRC), Inc. (North York, ON, Canada). All solvents used for high-performance liquid chromatography (HPLC) and liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis were of chromatographic grade. Williams’ Medium E and l-glutamine were purchased from Gibco (Grand Island, NY). Male Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). Stock solutions of MFA-AMP, MFA-CoA, MFA-GSH, MFA-1-O-G, mefenamic acid-glycine (MFA-Gly), mefenamic acid-taurine (MFA-Tau), and mefenamic acid-N-acetylcysteine (MFA-NAC) were all prepared as 1 mM solutions in DMSO.
Instrumentation and Analytical Methods.
HPLC/UV analysis was performed on a Hewlett Packard 1100 series binary pump HPLC system (Santa Clara, CA) coupled to a Hewlett Packard 1100 UV-Vis detector, utilizing HP Chemstation software for the acquisition of all HPLC/UV data. LC-MS/MS analyses of synthetic standards and in vitro samples were performed on a Shimadzu LC-20AD (Kyoto, Japan) HPLC system coupled to an Applied Biosystem/MDS Sciex API (Framingham, MA) 4000 triple quadrupole mass spectrometer outfitted with a Turbo V ion source using positive ionization mode. All LC-MS/MS analyses were performed on a reverse phase column (XTerra C-18, 5.0 μm, 4.6 × 150 mm; Waters, Milford, MA). The detection of MFA, MFA-AMP, MFA-CoA, MFA-1-O-G, MFA-GSH, MFA-Gly, MFA-Tau, and MFA-NAC were obtained using electrospray ionization (ESI) in positive mode, and a gradient system of either aqueous ammonium acetate (10 mM, pH 5.6) and acetonitrile (MFA-CoA) or aqueous solution (0.1% formic acid) and acetonitrile (0.1% formic acid) (MFA, MFA-AMP, MFA-1-O-G, MFA-GSH, MFA-Gly, MFA-Tau, and MFA-NAC), 5% ACN to 100%, over 15 minutes at a flow rate of 0.5 ml/min. The high pH and ion strength afforded by the aqueous ammonium acetate is necessary to elute MFA-CoA from the column. Electrospray positive ionization was employed with a needle potential held at 5.5 kV. Tandem mass spectrometry conditions used 2 mTorr argon collision gas and a collision potential of 89 eV. Mass spectral data were acquired with Analyst software (version 1.5.2; AB Sciex, Framingham, MA).
Synthesis of MFA-AMP, MFA-Gly, MFA-NAC, and MFA-Tau Derivatives.
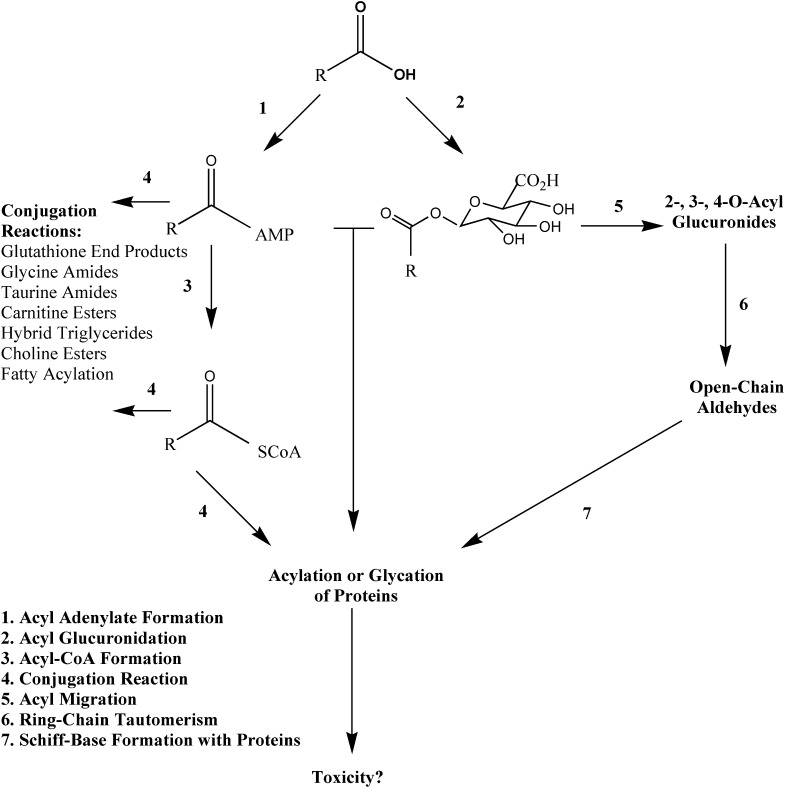
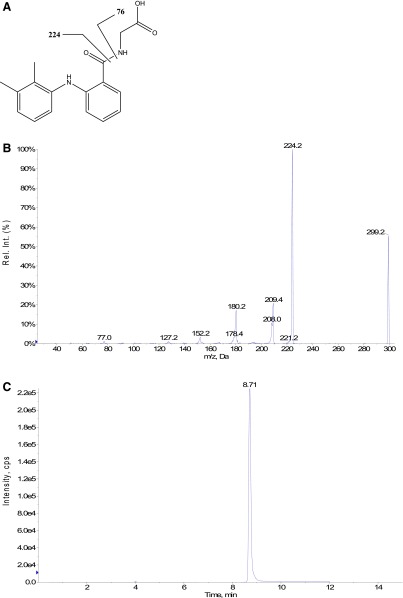
The synthesis of MFA-AMP, MFA-Gly, MFA-NAC, and MFA-Tau was carried out with a solution consisting of 110 mg N,N′-dicyclohexylcarbodiimide in 0.4 ml pyridine (Ikegawa et al., 1999; Horng and Benet, 2013). Briefly, an N,N′-dicyclohexylcarbodiimide solution was added to a solution containing MFA (0.49 mmol), and either AMP, Gly, Tau, or NAC (0.49 mmol) separately in 75% pyridine/25% water. The reaction mixture was stirred at 4°C for 7 hours and then centrifuged at 3000g for 5 minutes to remove any N-acylurea derivatives. The supernatant was transferred to another culture tube for precipitation by the addition of acetone (10 ml). The resulting precipitate was isolated by centrifugation at 3000g for 5 minutes followed by further washes with acetone (10 × 10 ml) and acidified water (pH 4–5) (10 × 10 ml). For MFA-AMP, the precipitate was dissolved in 0.1 M potassium phosphate buffer (pH 6) and underwent continued liquid-liquid washes with ethyl acetate (10 × 10 ml). Following precipitation via 1 M HCl, the MFA-AMP was further washed with acetone (10 × 10 ml). The MFA-AMP precipitate was -down to dryness using N2 gas and weighed out for preparation of a 1 mM MFA-AMP solution in DMSO. For MFA-Gly, MFA-NAC, and MFA-Tau, the initial acetone-derived precipitate was dissolved in DMSO and subjected to purification via HPLC/UV-mass spectrometry. The correct HPLC eluent fractions, as determined by UV-MS, of each acyl-linked metabolite were collected, blown down to dryness, weighed, and then prepared as 1-mM solutions in DMSO. MFA-AMP eluted at a retention time of 7.6 minutes and showed no impurities when analyzed by HPLC/UV (wavelengths: 220, 254, 262, and 280 nm) and LC-MS via reverse-phase gradient elution (as described above), and 1H-NMR (Horng and Benet, 2013). LC-MS/MS analysis of MFA-AMP revealed collision-induced dissociation (CID) of MH+ ion at m/z 571, m/z (%) yielded: m/z 224 ([M + H – AMP]+, 100%), m/z 207 ([M + H – 364]+, 25%), and m/z 136 ([M + H – adenine]+, 28%). MFA-Gly eluted at a retention time of 8.7 minutes (Fig. 2C) and showed no impurities when analyzed by HPLC/UV (wavelengths: 220, 254, 262, and 280 nm) and LC-MS via reverse-phase gradient elution (as described above). LC-MS/MS analysis of MFA-Gly (CID of MH+ ion at m/z 299), m/z (%): m/z 224 ([M + H – Gly]+, 99%), m/z 209 ([M + H – 90]+, 20%), m/z 180 ([M + H – 119]+, 18%), m/z 152 ([M + H – 147]+, 4%), m/z 127 ([M + H – 172]+, 2%), m/z 77 ([Gly + H]+, 1%) (Fig. 2, A and B). MFA-Tau eluted at a retention time of 9.1 minutes (Fig. 3C) and showed no impurities when analyzed by HPLC/UV (wavelengths: 220, 254, 262, and 280 nm) and LC-MS via reverse-phase gradient elution (as described above). LC-MS/MS analysis of MFA-Tau (CID of MH+ ion at m/z 349), m/z (%): m/z 332 ([M + H – H2O]+, 10%), m/z 224 ([M + H – Tau]+, 99%), m/z 209 ([M + H – 140]+, 25%), m/z 180 ([M + H – 169]+, 16%), m/z 152 ([M + H – 197]+, 4%), and m/z 126 ([Tau + H+]+, 2%) (Fig. 3, A and B). MFA-NAC eluted at a retention time of 9.3 minutes (Fig. 4C) and showed no impurities when analyzed by HPLC/UV (wavelengths: 220, 254, 262, and 280 nm) and LC-MS via reverse-phase gradient elution (as described above). LC-MS/MS analysis of MFA-NAC (CID of MH+ ion at m/z 387), m/z (%): m/z 309 ([M + H – 78]+, 30%), m/z 224 ([M + H – NAC]+, 99%), m/z 209 ([M + H – 178]+, 18%), m/z 180 ([M + H – 207]+, 13%), and m/z 165 ([NAC + H]+, 3%) (Fig. 4, A and B).
Fig. 2.
Proposed identities of the fragment ions of MFA-Gly (A), tandem mass spectrum (B), and representative reverse-phase gradient LC-MS/MS SRM (m/z 299 to m/z 224) (C) of MFA-Gly authentic standard.
Fig. 3.
Proposed identities of the fragment ion of MFA-Tau (A), tandem mass spectrum (B), and representative reverse-phase gradient LC-MS/MS SRM (m/z 349 to m/z 224) (C) of MFA-Tau authentic standard.
Fig. 4.
Proposed identities of the fragment ions of MFA-NAC (A), tandem mass spectrum (B), and representative reverse-phase gradient LC-MS/MS SRM (m/z 387 to m/z 224) (C) of MFA-NAC authentic standard.
Synthesis of MFA-CoA and MFA-GSH Thioester Derivatives.
The synthesis of MFA-CoA and MFA-GSH thioesters was accomplished by a method employing ECF as described previously (Stadtman and Elliott, 1957; Grillo and Benet, 2002; Horng and Benet, 2013). Briefly, MFA (1.6 mmol) was dissolved in anhydrous THF (25 ml). While stirring at room temperature, triethylamine (1.6 mmol) was added to the solution followed by the addition of ECF (1.6 mmol). After 30 minutes, the resulting triethylamine hydrochloride was removed by passing the reaction mixture through a glass funnel fitted with a glass wool plug. The filtered solution was then added to a solution containing CoA (0.13 mmol, 100 mg) or GSH (1 g) and KHCO3 (1.6 mmol) in nanopure water (10 ml) and THF (15 ml). The solution was stirred continuously at room temperature for 2 hours, after which the reaction was terminated by acidification (pH 4–5) through the addition of 1 M HCl. THF was then removed by evaporation under N2 gas, followed by further solvent washes: acidified water (pH 5) (3 × 10 ml) and ethyl acetate (3 × 10 ml) for MFA-CoA or acetone (3 × 10 ml) for MFA-GSH. MFA-CoA and MFA-GSH precipitate was blown down to dryness using N2 gas and then weighed out for preparation of a 1-mM MFA-CoA or 1-mM MFA-GSH solution in DMSO. HPLC analysis of MFA-CoA thioester resulted in an elution time of 7.3 minutes and showed no impurities when analyzed by HPLC/UV (wavelengths: 220, 254, 262, and 280 nm) and LC-MS via reverse-phase gradient elution (as described above). LC-MS/MS analysis of MFA-CoA standard yielded (CID of MH+ ion at m/z 991), m/z (%): m/z 582 ([M + H – adenosine diphosphate – H2O]+, 20%), m/z 484 ([M + H – adenosine triphosphate]+, 94%), m/z 428 ([adenosine diphosphate + H+]+, 40%), m/z 382 ([M + H – 609]+, 25%), m/z 330 ([adenosine monophosphate + H – H2O]+, 3%), m/z 224 ([M + H – CoA]+, 99%). Synthetic MFA-GSH eluted at a retention time of 7.7 minutes and showed no detectable impurities when analyzed by HPLC/UV (wavelengths: 220, 254, 262, and 280 nm) and LC-MS via reverse-phase gradient elution (as described above). LC-MS/MS analysis of MFA-GSH standard yielded product in mass spectrum under CID of the protonated molecular ion at MH+ m/z 531, m/z (%): m/z 456 ([M + H – GSH]+, 10%), m/z 384 ([M + H – pyroglutamic acid – water]+, 82%), m/z 224 ([MFA + H – H2O]+, 73%).
Stability and Reactivity Incubation Conditions and Quantitative Analysis of Reaction Products.
Chemical stability was assessed by incubating MFA-AMP, MFA-CoA, MFA-GSH, MFA-Gly, MFA-Tau, and MFA-NAC (1 μM) with CBZ (internal standard) in 0.1 M potassium phosphate buffer (Kpi) (pH 7.4) in 2-ml HPLC vials (n = 3). Each solution was then placed into an HPLC autosampler warmed to 37°C and injections were taken every 15 minutes for 3 or 24 hours for LC-MS/MS analysis to determine each metabolite’s chemical stability. The stability of each sample was determined by comparing the analyte peak-area-to-peak-area ratios of CBZ, which we previously found to be stable for at least 72 hours (data not shown). Chemical reactivity experiments for MFA-AMP, MFA-CoA, and MFA-1-O-G were performed by incubating each acyl-linked metabolite (1 μM) separately in 0.1 M Kpi (pH 7.4) containing Gly, Tau, GSH, or NAC and MFA-GSH with NAC (10 mM) (n = 3) at 37°C in screw-capped glass vials in a shaking incubator (Fig. 5). Aliquots (100 μl) of the incubation mixture were taken at 0, 2, 5, 10, 30, and 60 minutes and quenched with 1 μM CBZ/ACN solution and then injected onto the column for LC-MS/MS analyses. Quantitative measurements were performed by plotting peak area ratios of MFA-GSH, MFA-Gly, MFA-Tau, or MFA-NAC to CBZ versus the concentration of each acyl-linked MFA metabolite.
Fig. 5.
Scheme of the transacylation of MFA-AMP, MFA-CoA, and MFA-1-O-G with the bionucleophiles Gly, Tau, GSH, and NAC in buffer (0.1 M Kpi; pH 7.4, 37°C).
In Vitro Studies with Rat Hepatocytes.
Freshly isolated rat (250–300 g, male Sprague-Dawley) hepatocytes were prepared according to the method of Irving et al. (1984) and greater than 85% viability was achieved routinely as determined by trypan blue exclusion testing. Incubations of hepatocytes (2 million viable cells/ml) with MFA (100 μM) were performed in Williams’ Medium E fortified with l-glutamine (4 mM) in a 50-ml round bottom flask. Incubations (n = 3) were performed with continuous rotation and gassed with 95% O2/5% CO2 at 37°C. Aliquots were taken at 0, 0.2, 0.5, 1, 2, 4, 8, 10, 20, 30, and 60 minutes and analyzed for MFA-AMP, MFA-CoA, MFA-GSH, MFA-1-O-G, MFA-Gly, MFA-Tau, and MFA-NAC by LC-MS/MS.
For the analyses of MFA-AMP, MFA-GSH, MFA-1-O-G, MFA-Gly, MFA-Tau, and MFA-NAC, aliquots (200 μl) of the incubation mixture were added directly into microcentrifuge tubes (2 ml) followed by quenching with a solution of ACN containing 3% formic acid/2 μM CBZ (200 μl). Samples were then centrifuged (14,000g, 5 minutes) and the supernatant fractions (200 μl) were transferred to HPLC autosampler vials for LC-MS/MS analysis.
For the analyses of MFA-CoA formation, aliquots (200 μl) from the same incubations were transferred directly into microcentrifuge tubes and quenched with a solution of ACN/2 μM CBZ (400 μl) followed by the addition of hexane (600 μl). The samples were vortexed (1 minute), centrifuged (14,000g, 5 minutes), and aliquots (300 μl) of the aqueous layer were transferred to an HPLC autosampler vial followed by a 1-hour evaporation of residual hexane under the fume hood. Samples were then analyzed by LC-MS/MS.
Identification and Quantification of MFA-AMP, MFA-CoA, MFA-GSH, and MFA-1-O-G.
MFA-treated rat hepatocyte extracts were analyzed by LC-MS/MS for MFA-AMP, MFA-CoA, MFA-GSH, and MFA-1-O-G as previously described (Horng and Benet, 2013). Single-reaction monitoring (SRM) in positive ionization mode with the chromatographic conditions described above were used for the quantitation with the following mass transitions: MH+ m/z 571 to m/z 224 (MFA-AMP), MH+ m/z 991 to m/z 224 (MFA-CoA), MH+ m/z 531 to m/z 224 (MFA-GSH), MH+ m/z 418 to m/z 224 (MFA-1-O-G), and MH+ m/z 237 to m/z 194 for CBZ. The elution times of each acyl-linked metabolite were as follows: 7.6 minutes (MFA-AMP), 7.3 minutes (MFA-CoA), 7.7 minutes (MFA-GSH), 7.9 minutes (MFA-1-O-G), and 9.3 minutes (CBZ). No chromatographic peaks corresponding to each acyl-linked metabolite were detected in blank incubation extracts lacking MFA. The concentration of each MFA-acyl–linked metabolite was determined by plotting peak area ratios of each metabolite to CBZ versus the concentration.
Identification and Quantification of MFA-Gly.
The identification and quantitation of MFA-Gly by LC-MS/MS was carried out by SRM using the mass transitions MH+ m/z 299 to m/z 224 and MH+ m/z 237 to m/z 194 for CBZ detection using ESI in positive mode and the chromatographic methods described above. The elution times of 8.7 minutes (Fig. 2C) and 9.3 minutes were obtained for authentic MFA-Gly and CBZ, respectively. No chromatographic peaks corresponding to MFA-Gly were detected in blank incubation extracts lacking MFA. The concentration of MFA-Gly was determined by plotting peak area ratios of MFA-Gly to CBZ versus the concentration of MFA-Gly.
Identification and Quantification of MFA-Tau.
The identification and quantitation of MFA-Tau by LC-MS/MS was carried out by SRM using the mass transitions MH+ m/z 349 to m/z 224 and MH+ m/z 237 to m/z 194 for CBZ detection using ESI in positive mode and the chromatographic methods described above. The elution times of 9.1 minutes (Fig. 3C) and 9.3 minutes were obtained for authentic MFA-Tau and CBZ, respectively. No chromatographic peaks corresponding to MFA-Tau were detected in blank incubation extracts lacking MFA. The concentration of MFA-Tau was determined by plotting peak area ratios of MFA-Tau to CBZ versus the concentration of MFA-Tau.
Identification and Quantification of MFA-NAC.
The identification and quantitation of MFA-NAC by LC-MS/MS was carried out by SRM using the mass transitions MH+ m/z 387 to m/z 224 and MH+ m/z 237 to m/z 194 for CBZ detection using ESI in positive mode and the chromatographic methods described above. The elution times of 9.25 minutes (Fig. 4C) and 9.3 minutes were obtained for authentic MFA-NAC and CBZ, respectively. No chromatographic peaks corresponding to MFA-NAC were detected in blank incubation extracts lacking MFA. The concentration of MFA-NAC was determined by plotting peak area ratios of MFA-NAC to CBZ versus the concentration of MFA-NAC.
Results
Identification of MFA-AMP, MFA-CoA, MFA-1-O-G, and MFA-GSH.
Analysis of rat hepatocyte extracts incubated with MFA by LC-MS/MS detection allowed for the identification of MFA-AMP, MFA-CoA, MFA-1-O-G, and MFA-GSH formed in incubations with MFA, as previously described (Horng and Benet, 2013). MFA-AMP, MFA-CoA, MFA-GSH, and MFA-1-O-G formed in rat hepatocyte extracts and authentic standards coeluted at retention times of 7.6, 7.3, 7.7, and 7.9 minutes, respectively, and the product ion spectrum of each conjugate was consistent with its chemical structure and identical to its corresponding authentic standard.
Identification of MFA-Tau.
Analysis of rat hepatocyte extracts incubated with MFA by LC-MS/MS detection allowed for the identification of MFA-Tau formed in incubations with MFA. MFA-Tau formed in rat hepatocyte extracts and authentic standard coeluted at a retention time of 9.1 minutes (Fig. 3C) and the product ion spectrum of the conjugate was consistent with its chemical structure and identical to its corresponding authentic standard. MFA-Gly and MFA-NAC were not detected in MFA rat hepatocyte incubations.
Chemical Stability of MFA-CoA, MFA-AMP, MFA-GSH, MFA-NAC, MFA-Gly, and MFA-Tau in Buffer.
In vitro incubation of each acyl-linked metabolite of MFA in Kpi under physiologic conditions (pH 7.4, 37°C) revealed that MFA-AMP, MFA-CoA, and MFA-GSH were chemically stable for at least 24 hours, while MFA-NAC, MFA-Gly, and MFA-Tau were chemically stable with no detectable hydrolysis for at least 3 hours of incubation (data not shown). Previous studies carried out under the same conditions have shown MFA-1-O-G possesses a half-life of degradation of ∼16 hours in buffer under physiologic conditions (McGurk et al., 1996; Grillo et al., 2012).
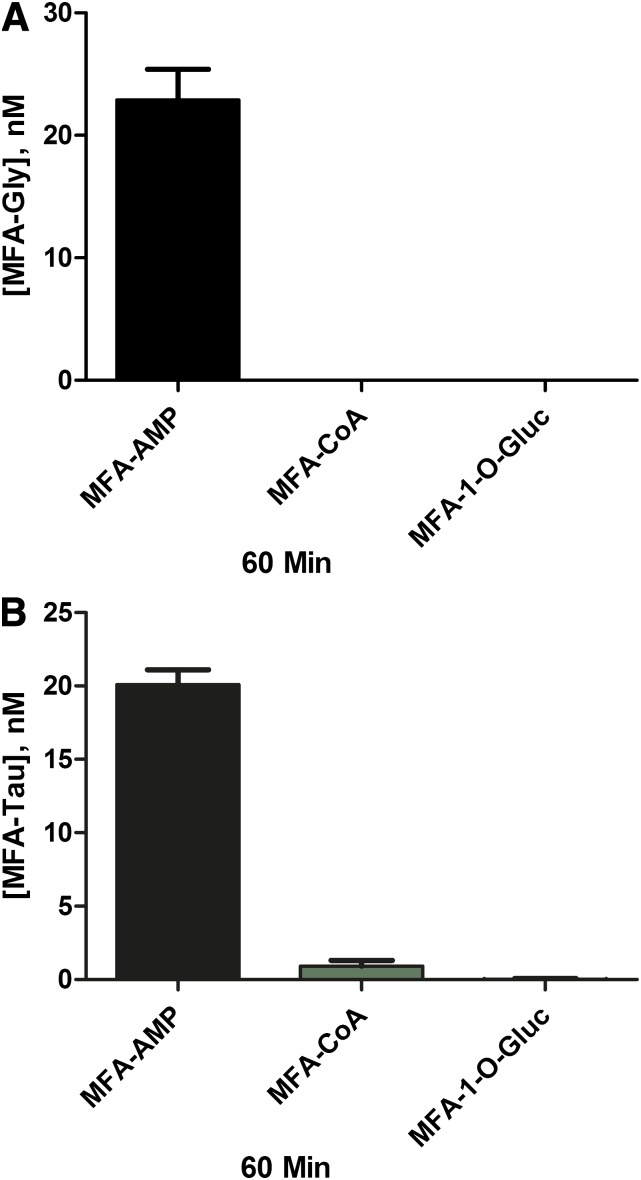
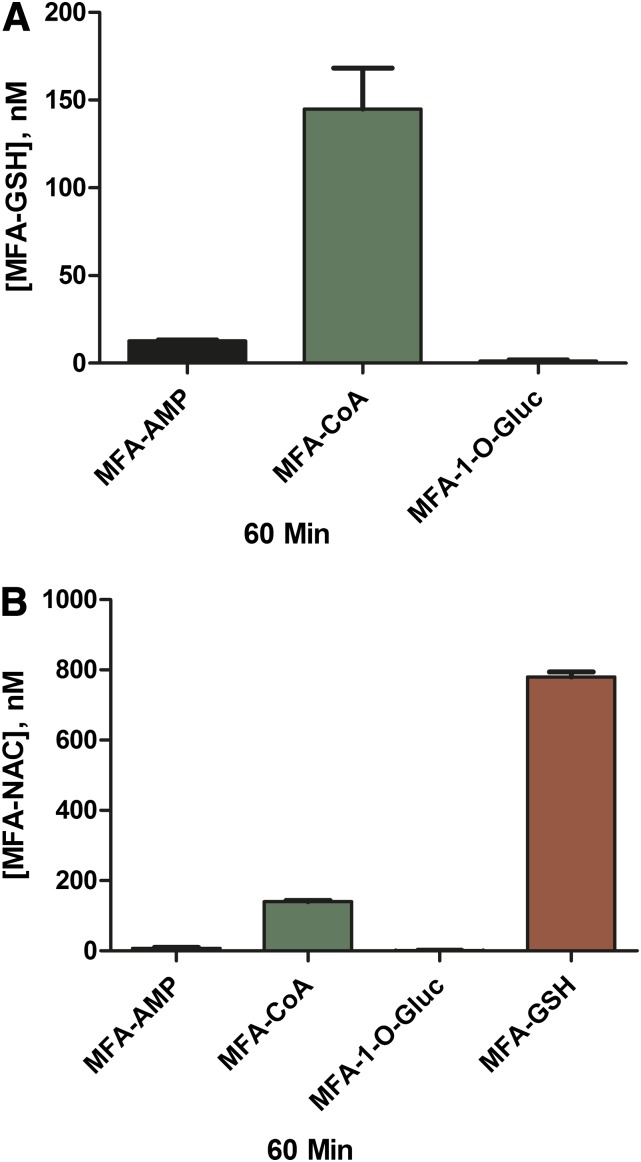
Chemical Reactivity of MFA-CoA, MFA-AMP, MFA-1-O-G, and MFA-GSH with Gly, Tau, GSH, and NAC.
Incubation of MFA-AMP (1 μM) with Gly and Tau (10 mM) in Kpi (0.1 M) under physiologic conditions (i.e., 37°C, pH 7.4) resulted in the N-amidation of both glycine and taurine, producing 23.2 ± 4.2 nM and 20.1 ± 1.8 nM of MFA-Gly and MFA-Tau conjugates, respectively, after 60 minutes of incubation (Fig. 6, A and B). Incubations of MFA-CoA (1 μM) with glycine and taurine (10 mM) resulted in minimal N-amidation, producing no MFA-Gly (limit of detection ∼0.5 nM for all MFA-conjugates) and 0.93 ± 0.65 nM of MFA-Tau at the 60-minute time-point. MFA-1-O-G (1 μM) exhibited no reactivity toward both Gly and Tau. In vitro GSH (10 mM) reactivity studies with MFA-AMP, MFA-CoA, and MFA-1-O-G (1 μM) resulted in 12.8 ± 1.0 nM, 145 ± 40 nM, and 1.3 ± 0.97 nM of MFA-GSH formation, respectively, at the 60-minute time-point (Fig. 7A). The incubation of NAC (10 mM) with MFA-AMP, MFA-CoA, and MFA-GSH (1 μM) under physiologic conditions resulted in the formation of 7.45 ± 4.8 nM, 141 ± 4.9 nM, and 780 ± 26 nM of MFA-NAC conjugates, respectively, at 60 minutes (Fig. 7B). While MFA-1-O-G continued to show no reactivity toward the nucleophile NAC.
Fig. 6.
Mean reactivity assessment ± S.D. at 60 minutes of MFA-AMP, MFA-CoA, and MFA-1-O-G toward Gly (A) and Tau (B) in buffer (0.1 M Kpi; pH 7.4, 37°C).
Fig. 7.
Mean reactivity assessment ± S.D. at 60 minutes of MFA-AMP, MFA-CoA, MFA-1-O-G, and MFA-NAC toward GSH (A) and NAC (B) in buffer (0.1 M Kpi; pH 7.4, 37°C).
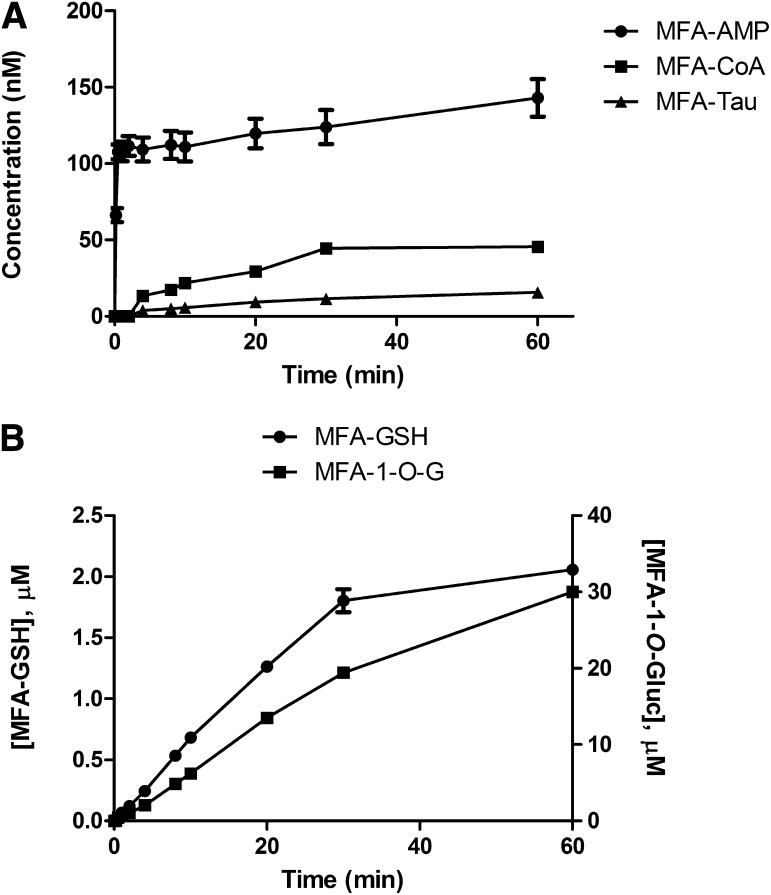
Time-Course of Formation of MFA-AMP, MFA-Tau, and MFA-Gly in Rat Hepatocyte Incubations.
The incubation of MFA (100 μM) in rat hepatocytes under physiologic conditions (37°C, pH 7.4) resulted in the detection of MFA-AMP, MFA-CoA, MFA-GSH, MFA-1-O-G, and MFA-Tau. The initial rise of MFA-AMP formation was very rapid attaining a concentration of 107.7 nM at ∼30 seconds (Fig. 8A). MFA-CoA was not detectable until the 4-minute time-point, reaching a concentration of 45.6 nM at 60 minutes. MFA-Tau levels were undetectable until 4 minutes, reaching a concentration of 15.7 nM at 60 minutes. The formation of MFA-GSH was linear, reaching a concentration of 2.1 μM at 60 minutes, while the formation of MFA-1-O-G increased to a concentration of 30.0 μM at 60 minutes (Fig. 8B). MFA-Gly and MFA-NAC conjugates were undetectable during the 60 minutes incubation period.
Fig. 8.
Mean time-dependent formation ± S.D. of MFA-AMP, MFA-CoA, and MFA-Tau (A), MFA-1-O-G (right axis units) and MFA-GSH (left axis units) (B) in rat hepatocyte incubations.
Discussion
Carboxylic acid-containing drugs are metabolized into reactive electrophilic acyl-linked metabolites that can form irreversible adducts with proteins, potentially causing an allergic reaction in hypersensitive individuals (Stogniew and Fenselau, 1982). MFA is a nonsterodial anti-inflammatory drug prescribed for its analgesic and anti-inflammatory activity via inhibition of cyclooxygenase-dependent prostanoid formation (Hawkey, 1999). Commonly used to treat pain, MFA has been implicated in several cases of hepatic and renal disturbances and hypersensitivity reactions (Handisurya et al., 2011). These toxicities are proposed to occur via bioactivation of MFA into reactive acyl-linked metabolites covalently binding onto macromolecules. MFA undergoes conjugation via the free carboxyl group into the 1-O-acyl glucuronide, MFA-1-O-G (Glazko, 1966; Sato et al., 1993), which has been shown to irreversibly bind to albumin (McGurk et al., 1996). MFA also undergoes further conjugation into MFA-AMP, MFA-CoA, and MFA-GSH (Grillo et al., 2012; Horng and Benet, 2013) in rat hepatocytes, all of which possess an increased chemical electrophilicity and are reactive toward protein nucleophiles.
Electrophilicity assessment of metabolites in drug development involve screens utilizing nucleophilic trapping agents. Glutathione is a commonly used biomarker of reactivity for bioactivation studies. Presumably, the greater the in vitro nucleophilic adduct formation, the greater the probability it will covalently bind onto proteins and elicit a toxic reaction. However, not all protein covalent bindings result in the onset of toxicity, and thus the challenge is to identify those protein targets that are critical for the onset of a drug-induced toxicity. In addition to thioesters (Van Breemen and Fenselau, 1985), acyl-linked metabolites have also been shown to react with proteins via oxygen ester (Wells et al., 1987) and amide-linkage (van Breemen and Fenselau, 1986; Mitamura et al., 2011). In the present study, we investigate the selective nonenzymatic acylation of amino and thiol functional groups of four biologic nucleophiles: Gly, Tau, GSH, and NAC.
In vitro GSH reactivity assessment of MFA-AMP, MFA-CoA, and MFA-1-O-G revealed MFA-CoA to be 11.5-fold more reactive than MFA-AMP toward the thiol functional group of GSH (Fig. 7A), consistent with our previous studies (Horng and Benet, 2013), while MFA-CoA was 19.5-fold more reactive toward the thiol groups of NAC than its corresponding MFA-AMP (Fig. 7B). Alternatively, incubations with Gly and Tau revealed that MFA-AMP is more reactive toward N-acyl-amidation than its corresponding MFA-CoA, producing a significant amount of MFA-Gly, while MFA-CoA did not react with Gly (Fig. 6A). The amidation of Tau by MFA-AMP was also 17.5-fold greater than by MFA-CoA (Fig. 6B). MFA-1-O-G exhibited little to no reactivity toward all four bionucleophiles. Reactivity data were linear during the incubation and therefore the 60-minute time-point was used to calculate the slope of conjugate formation (Table 1). Reactivity studies utilizing cholic acid have also demonstrated a greater reactivity of CA-AMP compared with CA-CoA toward the N-acyl-amidation of glycine and taurine, while CA-CoA exhibited a greater reactivity toward the acylation of the cysteine-sulfhydryl group of GSH and NAC (Mitamura et al., 2011). Studies in buffer have also shown that acyl-adenylates can spontaneously react with the amino groups of substance P (Goto et al., 2001), lysosomes (Goto et al., 2005), and histones (Mano et al., 2004), further suggesting that acyl-adenylates have a high reactive affinity toward amino functional groups, and that acyl-AMPs and acyl-CoAs are selective in their nonenzymatic acylation of amino-versus-thiol functional groups. This selectivity may be attributed to differences in the degree of electrophilicity of MFA-AMP versus MFA-CoA and the nucleophilicity of the amino versus the thiol functional groups, suggesting different reactivity mechanisms toward protein nucleophilic sites.
TABLE 1.
Mean rates ± S.D.s of formation of MFA conjugates incubated with Gly, Tau, GSH, and NAC (10 mM) in physiologic buffer (pH 7.4, 37°C) (n = 3)
| Conjugate | MFA-Gly | MFA-Tau | MFA-GSH | MFA-NAC |
|---|---|---|---|---|
| 1 μM | nM/min | |||
| MFA-AMP | 0.39 ± 0.07 | 0.35 ± 0.01 | 0.21 ± 0.02 | 0.12 ± 0.08 |
| MFA-CoA | N.D. | 0.02 ± 0.01 | 2.42 ± 0.67 | 2.34 ± 0.08 |
| MFA-GSH | N.A. | N.A. | N.A. | 13.0 ± 0.44 |
| MFA-1-O-G | N.D. | N.D. | N.D. | N.D. |
N.A., not applicable; N.D., not detected.
Acyl-GSH conjugates share a common structural moiety, thioester, to that of the acyl-CoA. Therefore, it is conceivable that MFA-GSH is just as, if not more, reactive than its corresponding acyl-CoA derivative. NAC reactivity assessments show that MFA-GSH is indeed highly reactive, exhibiting a 5.5- and 108-fold greater reactivity toward NAC than MFA-CoA and MFA-AMP, respectively (Fig. 7B). Grillo and Benet (2002) have demonstrated that the reactive potential of S-acyl-glutathione conjugates with NAC correlates to the degree of α-carbon substitution of the acyl-linkage. Increasing α-carbon substitution results in decreasing reactivity, which is in agreement with the relative degradation rates associated with acyl glucuronides and assumed to be identical for that of their respective S-acyl-CoA thioesters. Therefore, if MFA toxicity is the result of covalent binding by reactive intermediates, then it is conceivable that the unusually high formation of MFA-GSH in rat hepatocytes (Grillo et al., 2012; Horng and Benet, 2013), compared with diclofenac (Grillo et al., 2003), zomepirac (Olsen et al., 2005), (R)-ibuprofen (Grillo and Hua, 2008), and (R)-flunoxaprofen (Grillo et al., 2010), may be responsible for the tissue injury associated with MFA. This assumption is based on the high covalent binding values in animals dosed with drugs known to cause hepatotoxicity in humans, such as isoniazid (Nelson et al., 1978) and acetaminophen (Matthews et al., 1997).
Acyl-CoA thioester synthesis is a two-step reaction. This reaction occurs when the AMP moiety of ATP is transferred to the acyl group of the carboxylic acid via acyl-CoA synthetase, forming an acyl-adenylate intermediate. The enzyme-bound activated intermediate is then displaced by coenzyme A to yield the associated acyl-CoA product and free AMP (Vlahcevic et al., 1999). Acyl-CoA and acyl-AMP metabolites both spontaneously and enzymatically, via glutathione-S-transferase, form GSH-conjugates (Li et al., 2002; Grillo et al., 2012; Horng and Benet, 2013). In addition to GSH, glycine (Keller, 1842) and, taurine (James et al., 1971; Hutson and Casida, 1978) are two of the most commonly cited metabolic amino acid conjugation reactions. Amino acid conjugation occurs through the transfer of the acyl group from the acyl-CoA to an amino acid via N-acetyltransferase. However, previous and our current studies have shown that acyl-adenylates are capable of spontaneously reacting to both Gly and Tau (Mitamura et al., 2011), suggesting that acyl-adenylate intermediates have a greater inherent chemical affinity toward amino groups than both the acyl-CoAs and acyl-1-O-G. Rat hepatocyte MFA incubation resulted in rapid MFA-AMP formation (Cmax 107 nM at ∼30 seconds), while MFA-CoA was undetectable until 4 minutes, achieving a concentration of 45.6 nM at 60 minutes (Fig. 8A). This sequence is in agreement with the acyl-CoA biosynthetic pathway. MFA-1-O-G and MFA-GSH were also shown to increase linearly, achieving concentrations of 30.0 μM and 2.1 μM at 60 minutes, respectively (Fig. 8B). Our experiments also revealed the presence of MFA-Tau, undetectable until 4 minutes, reaching a concentration of 15.7 nM at 60 minutes. It was not determined if the formed MFA-Tau primarily occurs nonenzymatically from MFA-AMP or enzymatically via the MFA-CoA thioester. MFA-Gly was not detected in these incubations, possibly due to an insufficiency in analytical sensitivity (limit of detection for all MFA-conjugates was ∼0.5 nM). Previous zomepirac (ZP) rat hepatocyte incubations revealed the presence of ZP-CoA, ZP-1-O-G, ZP-Tau, and ZP-Gly (Olsen et al., 2005). The high concentration of ZP-1-O-G compared with ZP-Gly and ZP-Tau may be reflective of dose, a determinant of whether a drug undergoes glucuronidation or amino acid conjugation (Hutt and Caldwell, 1990). At lower doses, carboxylic acids tend to undergo amino acid conjugation, while at higher doses, glucuronidation dominates. Amino acid conjugation is a high-affinity, low-capacity system, while glucuronidation is a high-capacity pathway with a broader substrate selectivity. Dose also influences the type of amino acid conjugate formed (Hirom et al., 1977), with dosage increases of phenylacetic acid resulting in decreases in glycine/taurine conjugate ratios. Rat liver experiments with tolmetin (Tol) also identified the acyl-CoA–derived conjugates, Tol-Gly and Tol-Tau conjugates in rat urine (Olsen et al., 2003), whose concentrations were unaffected by clofibric acid, confirming a high-affinity/low-capacity metabolic pathway (Hutt and Caldwell, 1990). Oral administration of RS-ibuprofen to humans also led to the detection of ibuprofen-Tau but not the glycine conjugate (Shirley et al., 1994), signifying a minor biotransformation pathway. Gly and Tau conjugates are believed to be formed enzymatically (N-acyltransferases) via the acyl-CoA; however, we have demonstrated that Gly and Tau conjugates also form spontaneously via acyl-AMP conjugates. MFA-NAC conjugates were not detected in rat hepatocyte incubations, due to the lack of detectable N-acylcysteinylglycine and glutamyl transpeptidase (γ-GT) activity in rat livers (Hinchman and Ballatori, 1990). However, in humans, NAC formation would be expected to occur.
In conclusion, MFA-AMP selectively reacts nonenzymatically with the amino functional groups of glycine and lysine, while MFA-CoA selectively reacts nonenzymatically with the thiol functional groups of GSH and NAC. MFA-GSH is also reactive toward NAC, which may be of toxicological significance considering that MFA produces a high amount of GSH adducts in hepatocytes. MFA-1-O-G was not chemically reactive toward both amino and thiol functional groups. This preferential reactivity between MFA-AMP and MFA-CoA provides MFA the ability to covalently bind onto a broad range of nucleophilic sites on proteins, which increases its probability of covalently modifying critical protein targets and inducing a toxic reaction. Therefore, acyl-linked metabolites may be important in the formation of drug-protein adducts and the onset of an idiosyncratic toxicity, suggesting that the bioactivation of carboxylic acid-containing drugs into acyl-linked metabolites should be further evaluated to allow for structural modification of drug candidates, thereby reducing bioactivation and improving drug safety.
Acknowledgments
The authors thank Dr. Mark Grillo (Department of Pharmacokinetics and Drug Metabolism, Amgen, Inc., South San Francisco, CA) for many helpful discussions during the course of this work, and Chris Her (UCSF Liver Center Cell Biology Core) for assistance in the rat hepatocyte preparations.
Abbreviations
- ACN
acetonitrile
- CA
cholic acid
- CBZ
carbamazepine
- CID
collision-induced dissociation
- CoA
coenzyme A
- DMSO
dimethylsulfoxide
- ECF
ethyl chloroformate
- ESI
electrospray ionization
- GSH
l-glutathione
- HPLC
high-performance liquid chromatography
- Kpi
potassium phosphate buffer
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MFA
mefenamic acid
- MFA-1-O-G
mefenamic acid-1-O-acyl-glucuronide
- MFA-AMP
mefenamic acid-acyl-adenylate
- MFA-CoA
mefenamic acid-S-acyl-coenzyme A
- MFA-Gly
mefenamic acid-glycine
- MFA-GSH
mefenamic acid-S-acyl-glutathione
- MFA-NAC
mefenamic acid-N-acetylcysteine
- MFA-Tau
mefenamic acid-taurine
- NAC
N-acetylcysteine
- SRM
single-reaction monitoring
- THF
tetrahydrofuran
- Tol
tolmetin
- ZP
zomepirac
Authorship Contributions
Participated in research design: Horng, Benet.
Conducted experiments: Horng.
Performed data analysis: Horng, Benet.
Wrote or contributed to the writing of the manuscript: Horng, Benet.
Footnotes
This work was supported in part by the National Institutes of Health [Grant GM36633]; and by the University of California, San Francisco, Liver Center Cell Biology Core through the National Institutes of Health [Grant P30 DK026743]. The authors declare no competing financial interests.
References
- Benet LZ, Spahn-Langguth H, Iwakawa S, Volland C, Mizuma T, Mayer S, Mutschler E, Lin ET. (1993) Predictability of the covalent binding of acidic drugs in man. Life Sci 53:PL141–PL146 [DOI] [PubMed] [Google Scholar]
- Drury PL, Asirdas LG, Bulger GV. (1981) Mefenamic acid nephropathy: further evidence. Br Med J (Clin Res Ed) 282:865–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazko AJ. (1966) Experimental observations on flufenamic, mefenamic and meclofenamic acids. 3. Metabolic disposition. Ann Phys Med Suppl:23–36 [PubMed] [Google Scholar]
- Goto J, Nagata M, Mano N, Kobayashi N, Ikegawa S, Kiyonami R. (2001) Bile acid acyl adenylate: a possible intermediate to produce a protein-bound bile acid. Rapid Commun Mass Spectrom 15:104–109 [DOI] [PubMed] [Google Scholar]
- Goto T, Shibata A, Sasaki D, Suzuki N, Hishinuma T, Kakiyama G, Iida T, Mano N, Goto J. (2005) Identification of a novel conjugate in human urine: bile acid acyl galactosides. Steroids 70:185–192 [DOI] [PubMed] [Google Scholar]
- Grillo MP, Benet LZ. (2002) Studies on the reactivity of clofibryl-S-acyl-CoA thioester with glutathione in vitro. Drug Metab Dispos 30:55–62 [DOI] [PubMed] [Google Scholar]
- Grillo MP, Hua F. (2008) Enantioselective formation of ibuprofen-S-acyl-glutathione in vitro in incubations of ibuprofen with rat hepatocytes. Chem Res Toxicol 21:1749–1759 [DOI] [PubMed] [Google Scholar]
- Grillo MP, Hua F, Knutson CG, Ware JA, Li C. (2003) Mechanistic studies on the bioactivation of diclofenac: identification of diclofenac-S-acyl-glutathione in vitro in incubations with rat and human hepatocytes. Chem Res Toxicol 16:1410–1417 [DOI] [PubMed] [Google Scholar]
- Grillo MP, Tadano Lohr M, Wait JC. (2012) Metabolic activation of mefenamic acid leading to mefenamyl-S-acyl-glutathione adduct formation in vitro and in vivo in rat. Drug Metab Dispos 40:1515–1526 [DOI] [PubMed] [Google Scholar]
- Grillo MP, Wait JC, Tadano Lohr M, Khera S, Benet LZ. (2010) Stereoselective flunoxaprofen-S-acyl-glutathione thioester formation mediated by acyl-CoA formation in rat hepatocytes. Drug Metab Dispos 38:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handisurya A, Moritz KB, Riedl E, Reinisch C, Stingl G, Wöhrl S. (2011) Fixed drug eruption caused by mefenamic acid: a case series and diagnostic algorithms. J Dtsch Dermatol Ges 9:374–378 [DOI] [PubMed] [Google Scholar]
- Hawkey CJ. (1999) COX-2 inhibitors. Lancet 353:307–314 [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Ballatori N. (1990) Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol 40:1131–1135 [DOI] [PubMed] [Google Scholar]
- Hirom PC, Idle JR, Millburn P, Williams RT. (1977) Glutamine conjugation of phenylacetic acid in the ferret [proceedings]. Biochem Soc Trans 5:1033–1035 [DOI] [PubMed] [Google Scholar]
- Horng H, Benet LZ. (2013) Characterization of the acyl-adenylate linked metabolite of mefenamic Acid. Chem Res Toxicol 26:465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson DH, Casida JE. (1978) Taurine conjugation in metabolism of 3-phenoxybenzoic acid and the pyrethroid insecticide cypermethrin in mouse. Xenobiotica 8:565–571 [DOI] [PubMed] [Google Scholar]
- Hutt AJ, Caldwell J. (1990) Amino acid conjugation, in Conjugation Reactions in Drug Metabolism (Mulder G. ed) Taylor & Francis, New York [Google Scholar]
- Ikegawa SH, Ishikawa H, Oiwa H, Nagata M, Goto J, Kozaki T, Gotowda M, Asakawa N. (1999) Characterization of cholyl-adenylate in rat liver microsomes by liquid chromatography/electrospray ionization-mass spectrometry. Anal Biochem 266:125–132 [DOI] [PubMed] [Google Scholar]
- Irving MG, Roll FJ, Huang S, Bissell DM. (1984) Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology 87:1233–1247 [PubMed] [Google Scholar]
- James M, Smith RL, Williams RT. (1971) Conjugates of phenylacetic acid with taurine and other amino acids in various species. Biochem J 124:15P–16P [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. (1842) On the conversion of benzoic acid into hippuric acid. Prov Med J Retrosp Med Sci 4:256–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley M, Vessey DA. (1994) Determination of the mechanism of reaction for bile acid: CoA ligase. Biochem J 304:945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Benet LZ, Grillo MP. (2002) Studies on the chemical reactivity of 2-phenylpropionic acid 1-O-acyl glucuronide and S-acyl-CoA thioester metabolites. Chem Res Toxicol 15:1309–1317 [DOI] [PubMed] [Google Scholar]
- Mano N, Goto T, Uchida M, Nishimura K, Ando M, Kobayashi N, Goto J. (2004) Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J Lipid Res 45:295–300 [DOI] [PubMed] [Google Scholar]
- Mano N, Uchida M, Okuyama H, Sasaki I, Ikegawa S, Goto J. (2001) Simultaneous detection of cholyl adenylate and coenzyme A thioester utilizing liquid chromatography/electrospray ionization mass spectrometry. Anal Sci 17:1037–1042 [DOI] [PubMed] [Google Scholar]
- Matthews AM, Hinson JA, Roberts DW, Pumford NR. (1997) Comparison of covalent binding of acetaminophen and the regioisomer 3′-hydroxyacetanilide to mouse liver protein. Toxicol Lett 90:77–82 [DOI] [PubMed] [Google Scholar]
- McGurk KA, Remmel RP, Hosagrahara VP, Tosh D, Burchell B. (1996) Reactivity of mefenamic acid 1-o-acyl glucuronide with proteins in vitro and ex vivo. Drug Metab Dispos 24:842–849 [PubMed] [Google Scholar]
- Mitamura K, Aoyama E, Sakai T, Iida T, Hofmann AF, Ikegawa S. (2011) Characterization of non-enzymatic acylation of amino or thiol groups of bionucleophiles by the acyl-adenylate or acyl-CoA thioester of cholic acid. Anal Bioanal Chem 400:2253–2259 [DOI] [PubMed] [Google Scholar]
- Nelson SD, Mitchell JR, Snodgrass WR, Timbrell JA. (1978) Hepatotoxicity and metabolism of iproniazid and isopropylhydrazine. J Pharmacol Exp Ther 206:574–585 [PubMed] [Google Scholar]
- Olsen J, Bjørnsdottir I, Honorè Hansen S. (2003) Identification of coenzyme A-related tolmetin metabolites in rats: relationship with reactive drug metabolites. Xenobiotica 33:561–570 [DOI] [PubMed] [Google Scholar]
- Olsen J, Li C, Bjørnsdottir I, Sidenius U, Hansen SH, Benet LZ. (2005) In vitro and in vivo studies on acyl-coenzyme A-dependent bioactivation of zomepirac in rats. Chem Res Toxicol 18:1729–1736 [DOI] [PubMed] [Google Scholar]
- Robertson CE, Ford MJ, Van Someren V, Dlugolecka M, Prescott LF. (1980) Mefenamic acid nephropathy. Lancet 2:232–233 [DOI] [PubMed] [Google Scholar]
- Sato J, Yamane Y, Ito K, Bando H. (1993) Structures of mefenamic acid metabolites from human urine. Biol Pharm Bull 16:811–812 [DOI] [PubMed] [Google Scholar]
- Shirley MA, Guan X, Kaiser DG, Halstead GW, Baillie TA. (1994) Taurine conjugation of ibuprofen in humans and in rat liver in vitro. Relationship to metabolic chiral inversion. J Pharmacol Exp Ther 269:1166–1175 [PubMed] [Google Scholar]
- Somchit N, Sanat F, Gan EH, Shahrin IA, Zuraini A. (2004) Liver injury induced by the non-steroidal anti-inflammatory drug mefenamic acid. Singapore Med J 45:530–532 [PubMed] [Google Scholar]
- Stadtman TC, Elliott P. (1957) Studies on the enzymic reduction of amino acids. II. Purification and properties of D-proline reductase and a proline racemase from Clostridium sticklandii. J Biol Chem 228:983–997 [PubMed] [Google Scholar]
- Stogniew M, Fenselau C. (1982) Electrophilic reactions of acyl-linked glucuronides. Formation of clofibrate mercapturate in humans. Drug Metab Dispos 10:609–613 [PubMed] [Google Scholar]
- Taha A, Lenton RJ, Murdoch PS, Peden NR. (1985) Non-oliguric renal failure during treatment with mefenamic acid in elderly patients: a continuing problem. Br Med J (Clin Res Ed) 291:661–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J. (2007) Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol 47:513–539 [DOI] [PubMed] [Google Scholar]
- van Breemen RB, Fenselau C. (1985) Acylation of albumin by 1-O-acyl glucuronides. Drug Metab Dispos 13:318–320 [PubMed] [Google Scholar]
- van Breemen RB, Fenselau CC. (1986) Reaction of 1-O-acyl glucuronides with 4-(p-nitrobenzyl)pyridine. Drug Metab Dispos 14:197–201 [PubMed] [Google Scholar]
- Vlahcevic ZR, Pandak WM, Stravitz RT. (1999) Regulation of bile acid biosynthesis. Gastroenterol Clin North Am 28:1–25, v [DOI] [PubMed] [Google Scholar]
- Wells DS, Janssen FW, Ruelius HW. (1987) Interactions between oxaprozin glucuronide and human serum albumin. Xenobiotica 17:1437–1449 [DOI] [PubMed] [Google Scholar]
- Woods KL, Michael J. (1981) Mefenamic acid nephropathy. Br Med J (Clin Res Ed) 282:1471. [DOI] [PMC free article] [PubMed] [Google Scholar]