Abstract
Monocytic cells enhance neovascularization by releasing proangiogenic mediators and/or by transdifferentiating into endothelial-like cells. However, the mechanisms that govern this transdifferentiation process are largely unknown. Recently, monocyte chemotactic protein-1 (MCP-1)-induced protein (MCPIP) has been identified as a novel CCCH-type zinc-finger protein expressed primarily in monocytic cells. Here, we analyzed whether MCPIP might exert angiogenic effects by promoting differentiation of monocytic cells into endothelial cell (EC)-like phenotype. The expression of MCPIP increased during MCP-1-induced transdifferentiation in human bone marrow mononuclear cells (BMNCs). Knockdown of MCPIP with small interfering RNA (siRNA) abolished MCP-1-induced expression of EC markers Flk-1 and Tie-2 in human BMNCs. BMNCs transfected with MCPIP expression vector displayed EC-like morphology accompanied by downregulation of monocytic markers CD14 and CD11b, upregulation of EC markers Flk-1 and Tie-2, induction of cadherin (cdh)-12 and -19, activation of endoplasmic reticulum (ER) stress, and autophagy. Knockdown of cdh-12 or cdh-19 markedly inhibited MCPIP-induced enhancement of cell attachment and EC-marker expression. Inhibition of ER stress by tauroursodeoxycholate abolished MCPIP-induced expression of EC markers. Inhibition of autophagy by knockdown of Beclin-1 with siRNA or by an autophagy inhibitor 3′-methyladenine inhibited MCPIP-induced expression of EC markers. Expression of MCPIP in BMNCs enhanced uptake of acetylated low-density lipoprotein (acLDL), formation of EC-colony, incorporation of cells into capillary-like structure on Matrigel, and exhibited increased neovascularization in the ischemic hindlimb in mice. These results demonstrate that MCPIP may be an important regulator of inflammatory angiogenesis and provide novel mechanistic insights into the link between MCP-1 and cardiovascular diseases.
Introduction
Therapeutic angiogenesis has emerged as a promising strategy for the treatment of ischemic diseases such as coronary artery disease and limb ischemia. Transplantation of culture-expanded endothelial progenitor cells (EPCs) or bone marrow mononuclear cells (BMNCs) showed beneficial effects on left ventricular recovery in patients with myocardial infarction (Napoli et al., 2007). There is increasing evidence that the initiation of neovascularization in the setting of tissue ischemia is related to the recruitment and activation of monocytes/macrophages within the ischemic tissues (Capoccia et al., 2008; Sanberg et al., 2010). These monocytes can stimulate endothelial cell proliferation and/or migration through secretion of angiogenic growth factors and proteases (Rehman et al., 2003; Capoccia et al., 2008; Bouchentouf et al., 2010). It was also proposed that monocytes may differentiate into endothelial cells and get incorporated into the neovasculature (Anghelina et al., 2006; Sharifi et al., 2006). However, the mechanisms underlying the differentiation of monocytic cells into endothelial cell (EC)-like cells are still not well defined.
Monocytes are recruited from blood to the damaged tissue by the local production of cytokines and chemokines (Charo and Taubman, 2004; Niu and Kolattukudy, 2009). The CC chemokine CCL2 [monocyte chemotactic protein-1 (MCP-1)] is one of the most frequently observed chemokines following tissue ischemia and has been considered as one of the principal angiogenic factors associated with recruitment of monocytes (Capoccia et al., 2008; Niu et al., 2009; Schwarz et al., 2004). Administration of exogenous MCP-1 has been shown to increase monocyte/macrophage recruitment, collateral vessel formation, and blood flow to the ischemic tissue in hindlimb models of ischemia (Ito et al., 1997; Voskuil et al., 2003). When monocytes were driven into the heart by cardiomyocyte-targeted expression of MCP-1, the invading monocytes appeared to form erythrocyte-containing vessel-like tunnels (Moldovan et al., 2000). MCP-1 has been shown to mobilize and transdifferentiate bone marrow monocyte lineage cells into EC-like cells (Fujiyama et al., 2003). We recently discovered that the signaling initiated by MCP-1 binding to its receptor CCR2 in human peripheral blood monocytes triggered the induction of a novel CCCH-type zinc-finger protein, named MCP-1-induced protein (MCPIP, also known as Zc3h12a or Regnase-1) (Zhou et al., 2006; Uehata and Akira, 2013). MCPIP is a cytoplasmic protein expressed primarily in immune cells and can be induced in multiple cell types by many proinflammatory agents such as interleukin-1β (IL-1β), tumor necrosis factor α, bacterial lipopolysaccharide, and phorbol 12-myristate-13-acetate (Jura et al., 2012; Liang et al., 2008). Up-to-date reports suggest that MCPIP is a key factor controlling the inflammatory state as well as apoptosis and differentiation (Matsushita et al., 2009; Vrotsos et al., 2009; Younce and Kolattukudy, 2012; Liang et al., 2010). Furthermore, overexpression of MCPIP in human umbilical vein endothelial cells (HUVECs) showed angiogenic activity by induction of cadherin (cdh)-12, -19 (Niu et al., 2008), and involvement of endoplasmic reticulum (ER) stress and autophagy (Roy and Kolattukudy, 2012). Because MCP-1 is one of the principal angiogenic factors associated with recruitment of monocytes (Capoccia et al., 2008), we examined whether MCPIP might exert angiogenic effects by promoting differentiation of monocytic cells into EC-like phenotype. Data presented here strongly suggest that MCP-1 induces transdifferentiation of monocytic cells to endothelial cells, at least in part by the induction of MCPIP, which induces upregulation of cdh-12 and -19, resulting in ER stress and autophagy, leading to differentiation of BMNCs into EC phenotype. Transplantation of MCPIP-modified BMNCs into a murine model of hindlimb ischemia significantly enhanced capillary-like structure formation in the ischemic tissue.
Materials and Methods
Cell Cultures.
Human BMNCs purchased from StemCell Technologies (Vancouver, BC, Canada) were cultured in endothelial cell basal medium (EBM) supplemented with hydrocortisone (1 μg/ml), bovine brain extract (12 μg/ml), gentamicin (50 μg/ml), amphotericin B (50 ng/ml), epidermal growth factor (10 ng/ml), and 2% fetal bovine serum (Clonetics; Lonza Inc., Allendale, NJ) at 37°C in a humidified 5% CO2 incubator. To examine whether MCP-1-induced differentiation of BMNCs into ECs is mediated via MCPIP, BMNCs were incubated in EBM medium with the presence or absence of 100 nM MCPIP siRNA as described previously (Niu et al., 2008). Recombinant human MCP-1 (100 ng/ml) was added to the medium for 48 hours. Gene expression changes for cell markers Tie-2 and CD11b were assayed by reverse transcription–polymerase chain reaction (RT-PCR) and quantitative (q)RT-PCR as described below. Experiments were repeated at least three times to provide their reproducibility.
Cell Transfection.
BMNCs were seeded at 2 × 105 cells per well in six-well plates and cultured in the complete EBM medium for 3 days. After removal of the nonadherent cells, the attached cells were transfected with 1 μg of pEGFP/N1 vector or 1 μg of pEGFP-MCPIP plasmid for 24 hours as previously described (Zhou et al., 2006; Niu et al., 2008). The attached cells were continually cultured in the complete EBM medium for 7 days, and then harvested for gene expression analysis. For gene silence studies, the attached cells were preincubated with Opti-MEM I medium containing FuGENE 6 (Roche Diagnostics, Mannheim, Germany)/siRNA mixture (final concentration 100 nM siRNA) for 6 hours before any treatments. siRNA for MCPIP, cdh12, and cdh19 was designed by Dharmacon (Thermo Fisher Scientific, Pittsburgh, PA) as described previously (Niu et al., 2008). siRNA for Beclin-1 was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). For inhibitor intervention experiments, the attached cells were preincubated with 100 μM tauroursodeoxycholate (TUDC; Sigma-Aldrich, St. Louis, MO) or 50 μM 3′-methyladenine (3′-MA; Sigma-Aldrich) in the complete EBM medium for 6 hours before gene transfection. Experiments were repeated at least three times to prove their reproducibility.
Reverse Transcription–Polymerase Chain Reaction Analysis.
Total RNA from cultured cells or tissues of mice was prepared and reverse transcribed into cDNA and subjected to semiquantitative RT-PCR with the primers as described previously (Niu et al., 2008) and the follows: human CD14, forward 5′-GGTGCCGCTGTGTAGGAAAGA-3′, reverse 5′-GGTCCTCGAGCGTCAGTTCCT-3′; human CD11b, forward 5′-TGGACACTGAAAACGCAATGAC-3′, reverse 5′-TCTTGGCGGGATTTCTCACTG-3′; human Flk-1, forward 5′-CTGGCATGGTCTTCTGTGAAGCA-3′, reverse 5′-AATACCAGTCGATGTGATGCGGT-3′; human Flt-1, forward 5′-AACCAGAAGGGCTCTGTGGA-3′, reverse 5′-ATGCCAAATGCTGATGCTTG-3′; human Tie-2, forward 5′-TGGAGCCAGAGAAGACCACA-3′, reverse 5′-TCCTGTCTTTGGCCTCAGGT-3′; human CD31, forward 5′-CAACGAGAAAATGTCAGA-3′, reverse 5′-GGAGCCTTCCGTTCTAGACT-3′; mouse CD31, forward 5′-CAAGCGGTCGTGAATGACAC-3′, reverse 5′- CACTGCCTTGACTGTCTTAAG-3′. PCR products were electrophoresed on agarose gel and RNA expression levels were normalized to β-actin as previously described (Niu et al., 2008).
Real-Time Quantitative RT-PCR.
Quantitative RT-PCR (qRT-PCR) was performed on iCycler Real-Time PCR system (Bio-Rad, Hercules, CA) using SYBR Green Premix Kit (Applied Biosystems, Foster City, CA) with the primers as described previously (Niu et al., 2008) and the follows: human Tie-2, forward 5′-TACTAATGAAGAAATGACCCTGG-3′, reverse 5′-GGAGTGTGTAATGTTGGAAATCT-3′; human Flk-1, forward 5′-TGCCTACCTCACCTGTTTC-3′, reverse 5′-GGCTCTTTCGCTTACTGTTC-3′; human vascular endothelial growth factor (VEGF), forward 5′-CCCTGGCTTTACTGCTGTAC-3′, reverse: 5′-TCTGAACAAGGCTCACAGTG-3′. The expression level of each candidate gene was normalized by subtracting the corresponding β-actin threshold cycle (Ct) values.
Western Blotting.
Lysates from cultured cells or tissues of mice were prepared as described previously (Niu et al., 2008, 2011). The lysates were then subjected to SDS-PAGE and transferred onto nitrocellulose membranes. Immunoblot analysis was completed as described previously (Niu et al., 2008, 2011). Primary rabbit polyclonal antibodies were as follows: human MCPIP, 1:500; Flk-1, 1:1000; Tie-2, 1:500; CD31, 1:500; cdh-12, 1:500 (R&D Systems, Techne Corporation, Minneapolis, MN); VEGF, 1:1000; and cdh-19, 1:1000 (Abnova, Tapei City, Taiwan). The specific bands were quantified using a densitometer equipped with an imaging system (Alpha Imager 2200).
Immunofluorescence Staining and Uptake of DiI-acLDL.
BMNCs cells were seeded at 5 × 105/ml in Laboratory-Tek chamber glass slides (Nalge Nunc International, Rochester, NY) and transfected with MCPIP expression or control vector. After culture for 7 days, cells were fixed and permeabilized in 4% paraformaldehyde in PBS with 0.1% Triton X-100 for 10 minutes at room temperature. After blocking with 3% bovine serum albumin, cells were incubated with MCPIP, CD31, and CD144 antibodies, respectively, overnight at 4°C. Cells were then incubated with Cy3- or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) for 2 hours at room temperature, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and prepared for fluorescence microscopy. Lipoproteins labeled with the fluorescent probe 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI-acLDL) have been used to visualize the uptake of lipoproteins by endothelial cells (Voyta et al., 1984; Gaffney et al., 1985). For DiI-acLDL uptake analysis, at days 7 after transfection, cells were incubated with 2.5 μg/ml DiI-acLDL (Invitrogen) at 37°C in a humidified 5% CO2 incubator for 4 hours. The cells were then washed with Dulbecco's phosphate-buffered saline (DPBS) and viewed with a Nikon fluorescence microscopy. The percentage of total positive cells was calculated in five randomly selected fields for each slide, and three slides were studied per group.
EC Colony Formation Assay.
BMNCs were seeded at 5 × 105/ml on 3.5-cm culture dishes coated with human fibronectin (Sigma-Aldrich). After 3 days of culture in the EndoCult Liquid Medium (StemCell Technologies), cells were transfected with MCPIP expression vector or control vector, and continually cultured in the EndoCult Liquid Medium for 5 days, and screened for the formation of colonies. The number of colonies was counted under an inverted microscopy from five randomly selected fields for each culture dish.
In Vitro Matrigel Analysis.
Matrigel (BD Biosciences, MA) was prepared as previously described (Niu et al., 2008). HUVECs were seeded onto the surface of the polymerized Matrigel (1.0 × 104 cells/per well). The DiI-acLDL-labeled BMNCs transfected with MCPIP expression vector or control vector were plated (1.0 × 104 cells/well) onto the Matrigel seeded with HUVECs, and incubated in EBM medium for 24 hours. Tube formation and DiI-acLDL-labeled BMCs incorporating into tube structures were observed under a phase contrast/fluorescence microscope and photographed. The DiI-acLDL-labeled BMNCs incorporating into tube-like structure were calculated and averaged by five randomly selected high power fields per well under magnification ×400.
Mouse Mode of Hindlimb Ischemia and BMNC Transplantation.
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 86-23, Revised 1996) and were approved by the Animal Care and Use Committee of the University of Central Florida. In brief, unilateral hindlimb ischemia was induced in FVB/N mice (8-week-old, 18−22 g) by excising the proximal regions of the femoral and saphenous arteries. The wound was closed and animals were brought back to the cages with free access to food and water. At 24 hours after surgery, animals (n = 5 per group) were injected intravenously via tail vein with 100 μl of EBM medium loaded with BMNCs (5 × 105/ml) transfected with MCPIP expression vector or control vector. On days 3 and 28 after cell transplantation, the adductor muscles were collected, and fixed in 10% phosphate-buffered formaldehyde or snap-frozen in liquid nitrogen. The fixed tissues were routinely processed and sliced into 5-μm sections for hematoxylin-and-eosin staining. Capillary-like structures were calculated and averaged from five randomly selected high power fields (HPF, 400-fold). Immunohistochemistry was performed to determine CD31 expression in situ with Alexa Fluor 594-labeled anti-mouse CD31 antibody. Photomicrographs were obtained under 400× magnification.
Statistical Analysis.
Data are expressed as the mean ± S.D. of a given number of observations. Results were compared between groups by Student’s t tests using SPSS 10.0 software (IBM, Armonk, NY) under Windows XP. A P value of <0.05 was considered to be significant.
Results
Knockdown of MCPIP Inhibited the MCP-1-Induced Differentiation of BMNCs into ECs.
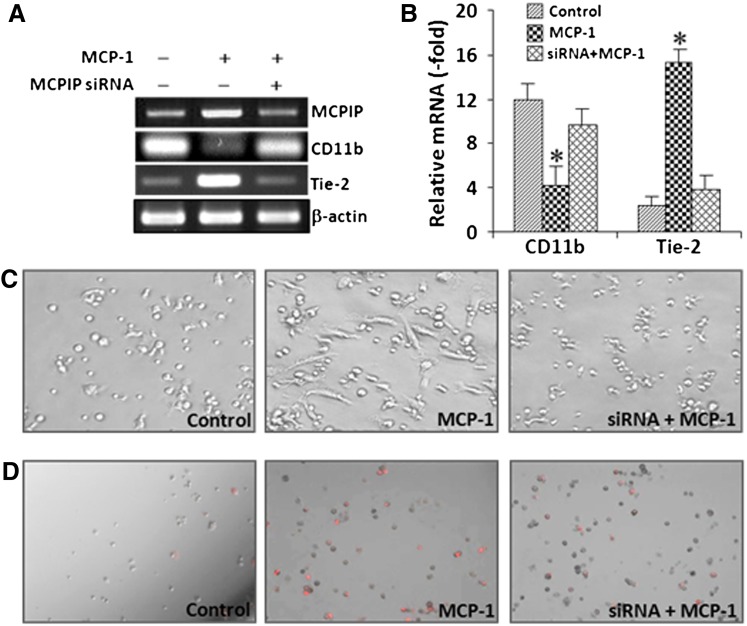
Bone marrow monocyte lineage cells have been shown to promote re-endothelialization in a MCP-1-dependent manner (Fujiyama et al., 2003), and MCPIP is expressed in human peripheral blood monocytes by treatment of MCP-1 (Zhou et al., 2006). We tested whether MCPIP plays a role in MCP-1-mediated differentiation of BMNCs into ECs. Differentiation of BMNCs was assessed by analysis of the expression of CD11b, a cell-surface marker of differentiated monocytic cells (Alessio M, et al., 1996; Kamiya et al., 2011), and Tie-2, a surface marker of differentiated ECs (Sharifi et al., 2006; Kim et al., 2009). MCP-1 treatment of BMNCs resulted in upregulation of Tie-2 and downregulation of CD11b (Fig. 1, A and B), suggesting transdifferentiation of BMMCs into ECs. Pretreatment with siRNA against MCPIP significantly inhibited MCP-1-induced upregulation of Tie-2 and downregulation of CD11b (Fig. 1, A and B). Moreover, MCP-1-induced formation of cobblestone-like morphology and uptake of DiI-acLDL, a widely used marker for assay of endothelial function (Voyta et al., 1984; Gaffney et al., 1985), in BMNCs was markedly inhibited by siRNA against MCPIP (Fig. 1, C and D), suggesting that MCPIP participates in MCP-1-induced transdifferentiation of BMNCs into ECs.
Fig. 1.
MCPIP participates in MCP-1-induced transdifferentiation of BMNCs into ECs. BMNCs were plated in EBM medium on six-well plate coated with fibronectin and treated with MCP-1 in the absence or presence of siRNA against MCPIP for 48 hours. (A) Expression of monocytic cell marker CD11b and EC marker Tie-2 was evaluated by RT-PCR. β-actin served as internal control. (B) Relative mRNA expression of cell surface markers is presented in bar graphics. *P < 0.01 versus untreated control or in the presence of MCPIP siRNA. (C) Representative photomicrographs after 3 days in culture show numbers of cells displaying cobblestone-like morphology in MCP-1-treated BMNCs and siRNA against MCPIP inhibited MCP-1-induced morphology changes in BMNCs. (D) Representative photomicrographs after 3 days in culture show numbers of cells uptaking DiI-acLDL in MCP-1-treated BMCs and siRNA against MCPIP inhibited DiI-acLDL uptake by MCP-1-treated BMNCs. Cells uptaking DiI-acLDL were observed under a phase-contrast/fluorescent microscopy. The red fluorescence indicates intracellular DiI-acLDL.
Forced Expression of MCPIP Promotes Endothelium-Like Morphology in BMNCs.
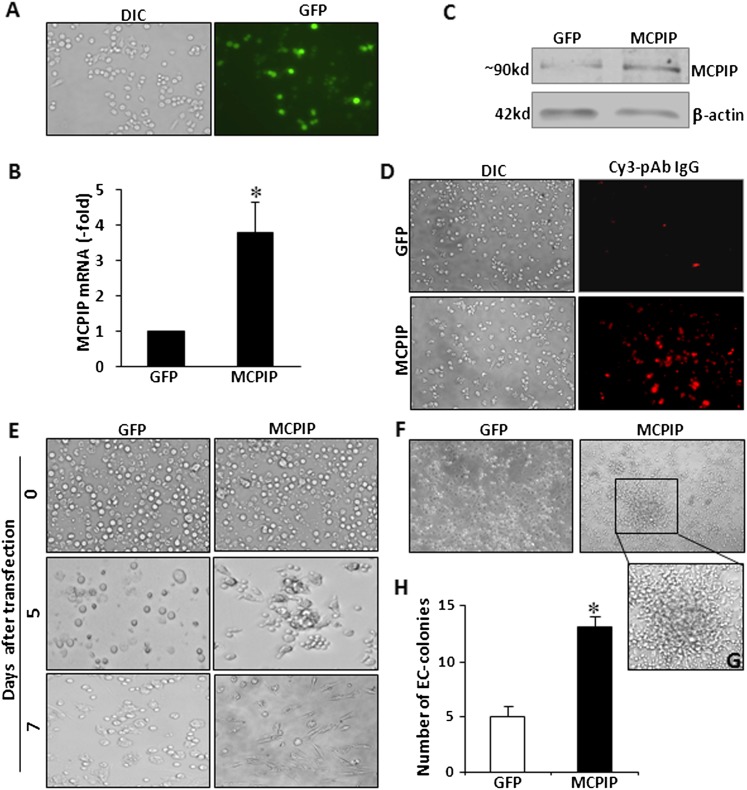
To examine the possible role of MCPIP in angiogenic differentiation of monocytic cells, we determined the effects of expression of MCPIP on transdifferentiation of BMNCs into ECs. BMNCs were transfected with MCPIP–green fluorescent protein (GFP) expression vector or GFP control vector. Expression of MCPIP was confirmed by GFP-positive cells (Fig. 2A), qRT-PCR (Fig. 2B), immunoblots, and immunocytochemistry analysis (Fig. 2, C and D). Morphologic changes of the BMNCs were monitored under light microscopy. Upon 5 days of culture, some cells attached to the culture plate in round shape, and the numbers of attached cells in the MCPIP-transfected BMNCs were much higher than in the GFP-transfected BMNCs (Fig. 2E). We also observed that the attached cells in the MCPIP-transfected BMNCs displayed an array-like arrangement rather than a random dispersal of round shape cells observed in GFP control–transfected cells (Fig. 2E). After 7 days of culture, most cells in the MCPIP-transfected BMNCs exhibited cobblestone-like morphology, whereas only a few of cells in the GFP-transfected BMNC showed a spindle shape and the remaining cells retained their rounded shape (Fig. 2E). Furthermore, when cells were cultured in the EndoCult Liquid Medium for 5 days, we observed an increase in numbers of EC-colony in BMNCs transfected with MCPIP when compared with BMNCs transfected with GFP control (Fig. 2, F and H). These morphologic changes indicated that expression of MCPIP in BMNCs promoted differentiation of BMNCs into endothelial-like cells.
Fig. 2.
Expression of MCPIP induces endothelial cell–like morphology and EC-colony formation in BMNCs. After 3 days of culture, BMNCs were transfected with MCPIP-GFP expression vector or GFP control vector. Representative photomicrographs of differential interference contrast (DIC) image and GFP fluorescent (green) image at 48 hours after transfection (A). Expression of MCPIP in BMCs was assessed by qRT-PCR (B) and immunoblots (C). The immunofluorescence images (D) show cells expressing MCPIP (red). (E) Cell morphology was observed by light microscopy 0, 5, and 7 days after transfection. Cells transfected with MCPIP expression vector displayed an array-like arrangement rather than random dispersal. (F) Cells were cultured in EC-colony medium. Endothelial cells colony forming units comprised clusters of round cells centrally and sprouts of spindle-shaped cells at the periphery and these began to appear at day 5 in BMNCs transfected with MCPIP expression vector. (G) One selected EC-colony from the (F) is magnified. Original magnification ×100. (H) Results of three independent experiments are presented as a mean of numbers of colonies formed per 1 × 106 cells plated. *P < 0.01 versus cells transfected with GFP control.
Forced Expression of MCPIP Induces Transdifferentiation of BMNCs into Functional ECs.
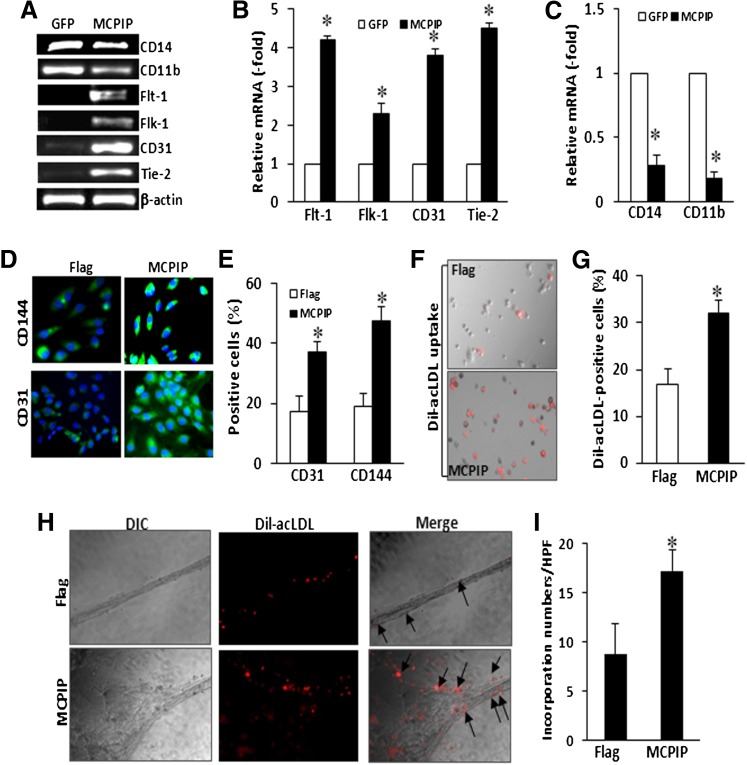
We further examined the expression changes of cell surface markers in BMNCs at day 7 after transfection with MCPIP expression vector. RT-PCR analysis showed that expression of MCPIP induced upregulation of EC-specific markers, flt-1, flk-1, Tie-2, and CD31 and downregulation of monocytic-specific markers, CD14 and CD11b, compared with that of the GFP-transfected BMNCs (Fig. 3, A–C). We also immunocytochemically examined the expression of CD31 and CD144 (VE-cadherin), two specific EC-surface markers, in BMCs transfected with FLAG-tagged MCPIP or FLAG-tagged empty vector. At day 7 after transfection, a much larger percentage of BMNCs showed an intense cytoplasmic fluorescence for CD31 and CD144 in MCPIP-transfected BMNCs, when compared with BMNCs transfected with control vector (Fig. 3, D and E).
Fig. 3.
Expression of MCPIP induces upregulation of EC phenotypic markers and uptake of acLDL in BMNCs. BMNCs were transfected with Flag-tagged MCPIP expression vector or Flag-tagged empty vector and cultured in EC cultured medium. At day 7 after transfection, expression of monocytic and endothelial cell phenotypic markers was evaluated by RT-PCR (A). Relative mRNA expression of these markers is represented in bar graphics (B and C). *P < 0.05 versus cells transfected with control vector. (D) Representative photomicrographs of indirect immunofluorescence using fluorescein isothiocyanate–labeled secondary antibody (green) for EC surface markers CD31 and CD144. Nuclei were counterstained with DAPI in blue. Numbers of cells expressing CD31 and CD144 are presented in a bar graph (E). *P < 0.05 versus cells transfected with control vector. (F) Representative photomicrographs of cells uptaking DiI-acLDL under a phase-contrast/fluorescent microscopy. The red fluorescence indicates intracellular DiI-acLDL. Numbers of cells uptaking DiI-acLDL are presented in a bar graph (G). *P < 0.05 versus cells transfected with control vector. HUVECs and DiI-acLDL-labeled BMNCs were cocultured in EBM medium on 24-well plate coated with Matrigel. (H) Representative photomicrographs of DIC images and DiI-acLDL-loaded images at 24 hours after coculture. The red fluorescence indicates intracellular acLDL. Arrows indicate DiI-acLDL-labeled cells incorporating into tube-like structure (original magnification, ×400). (I) Cells incorporating into tube-like structure were calculated and averaged by five randomly selected high power fields. *P < 0.01 versus cells transfected with control vector.
Uptake of acLDL is a feature of endothelial cells and has been used for assay of endothelial cell function (Voyta et al., 1984; Gaffney et al., 1985). We performed the DiI-acLDL uptake assay on BMNCs 7 days after transfection with MCPIP expression vector. A much larger proportion of the BMNCs transfected with MCPIP were positive for DiI-acLDL when compared with FLAG-tagged empty vector (Fig. 3, F and G), suggesting that BMNCs transfected with MCPIP behaved as endothelial cells.
Next, we examined whether expression of MCPIP could enhance the ability of the BMNCs to incorporate into tube-like structure formed with HUVECs in vitro. After transfection with FLAG-tagged MCPIP expression vector or control vector for 7 days, BMNCs were labeled with DiI-acLDL and were cocultured with HUVECs in EBM medium on Matrigel for 24 hours. We observed incorporation of DiI-acLDL-labeled cells into vascular networks (Fig. 3H, arrows), and cells transfected with MCPIP showed enhanced incorporation in comparison with the empty-vector transfected cells (Fig. 3I), suggesting that expression of MCPIP in BMNCs promoted differentiation of BMNCs into functional endothelial cells.
Signaling Pathways of MCPIP-Mediated Endothelium-Like Differentiation of BMNCs.
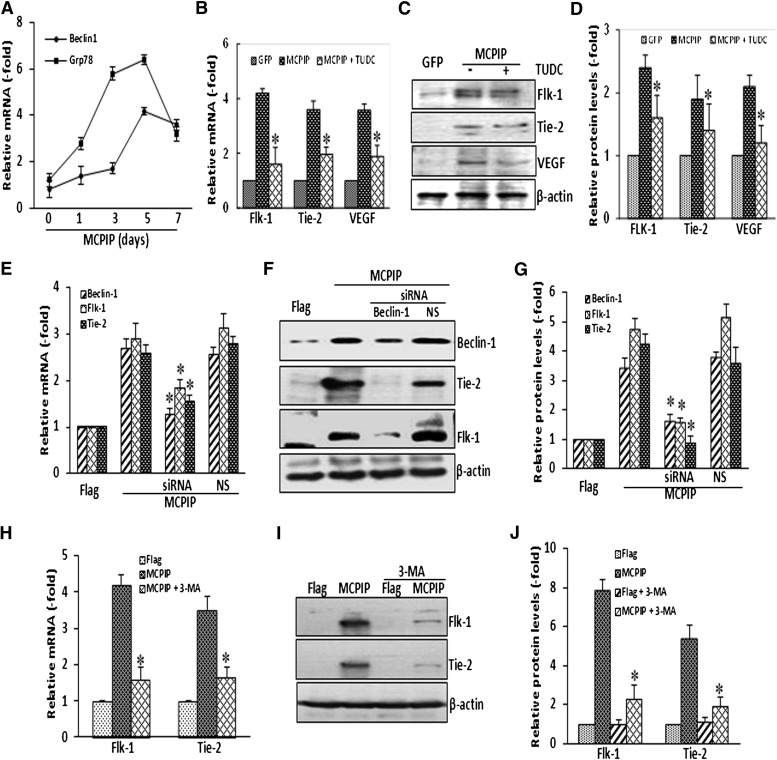
ER stress and autophagy have been implicated in MCPIP-mediated differentiation processes (Wang et al., 2011; Roy and Kolattukudy, 2012; Younce and Kolattukudy, 2012). To test whether such processes are involved in the transdifferentiation of BMNCs to ECs, we examined the expression of Grp78, an ER stress marker, and Beclin-1, an autophagy marker, in MCPIP-transfected BMNCs. These ER stress and autophagy markers were induced in the BMNCs transfected with MCPIP expression vector (Fig. 4A). Furthermore, when cells were treated with TUDC, a specific inhibitor of ER stress (Ozcan et al., 2006), MCPIP-induced upregulation of Tie-2, Flk-1, and VEGF was significantly inhibited at both mRNA and protein levels (Fig. 4, B–D). Similarly, inhibition of autophagy by knockdown of Beclin-1 with siRNA inhibited MCPIP-mediated induction of Tie-2 and Flk-1 in BMCs (Fig. 4, E–G). Treatment of BMNCs with 3-MA, a widely used inhibitor of autophagy (Seglen and Gordon, 1982; Kovacs, et al., 1998), also led to a significant inhibition of MCPIP-induced expression of Tie-2 and Flk-1 in BMNCs (Fig. 4, H–J). These results suggest that MCPIP-mediated transdifferentiation of BMNCs into EC-like phenotype involves ER stress and autophagy.
Fig. 4.
Participation of ER stress and autophagy in MCPIP-mediated upregulation of EC markers. Expression of ER stress marker GRP78 and autophagy marker Beclin-1was examined by qRT-PCR in BMNCs transfected with MCPIP expression vector at the time points indicated. Relative mRNA levels were expressed as fold change compared with cells transfected with control vector (A). Inhibition of ER stress by TUDC suppresses MCPIP-induced upregulation of EC markers Flk-1, Tie-2, and VEGF in the BMCs assayed by qRT-PCR (B) and immunoblots (C). The bar graphs represented the densitometric analysis normalized by β-actin protein levels and expressed as fold change compared with cells transfected with control vector (D). *P < 0.05 versus cells transfected with MCPIP expression vector. Inhibition of autophagy by siRNA against Beclin-1 suppresses MCPIP-induced upregulation of EC markers Flk-1 and Tie-2 in BMNCs assayed by qRT-PCR (E) and immunoblots (F). The bar graphs represented the densitometric analysis normalized by β-actin protein levels and expressed as fold change compared with cells transfected with control vector (G). *P < 0.05 versus cells transfected with Flagged-tagged MCPIP vector only and/or with nonspecific siRNA (NS). Inhibition of autophagy by 3-MA suppresses MCPIP-induced upregulation of EC markers Flk-1 and Tie-2 in BMNCs assayed by qRT-PCR (H) and immunoblots (I). The bar graphs represented the densitometric analysis normalized by β-actin protein levels and expressed as fold change compared with cells transfected with control vector (J). *P < 0.05 versus cells transfected with MCPIP expression vector.
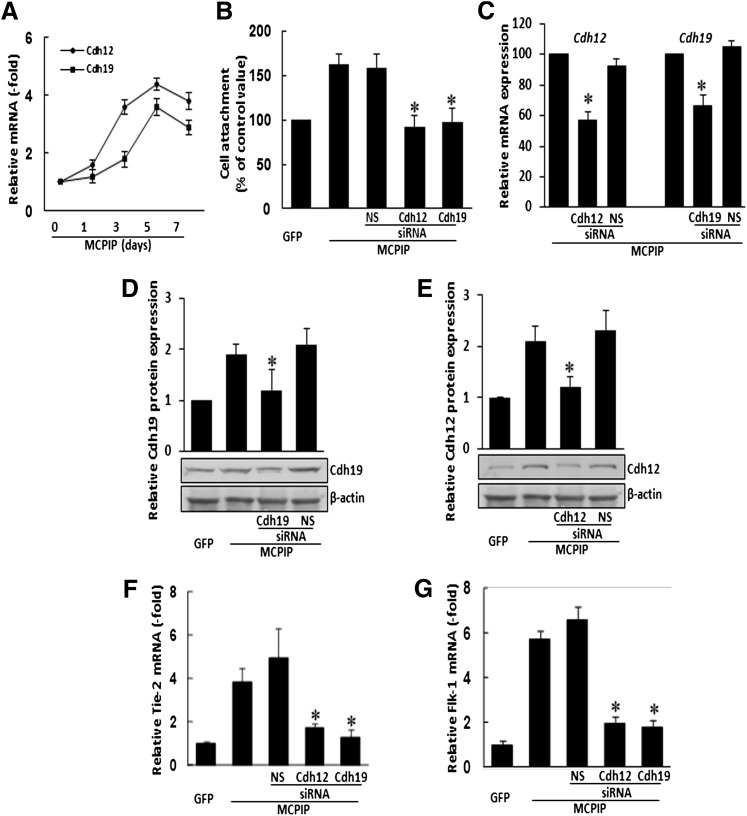
Cell attachment is a feature seen in the early stages of differentiation and incorporation of EPCs into the neovasculature. N-cadherin is a major factor mediating cell attachment (Blaschuk and Rowlands, 2000; Luo and Radice, 2005). We tested whether cdh12 and cdh19, two target genes of MCPIP (Niu et al., 2008), might be involved in MCPIP-mediated attachment by BMNCs. Real-time PCR analysis showed that expression of cdh12 and cdh19 were induced in BMNCs after transfection with MCPIP-GFP expression vector compared with cells transfected with the GFP control (Fig. 5A). Furthermore, knockdown of cdh 12 or cdh19 with siRNA specific for either cdh12 or cdh19 significantly reduced MCPIP-induced enhancement of BMNCs attachment (Fig. 5B). Specificity of knockdown for cdh12 or cdh19 was indicated by the real-time PCR and immunoblot analysis showing that MCPIP-induced expression of cdh12 or cdh19 at both transcript and protein levels were markedly suppressed by treatment with siRNA for either cdh12 or cdh19 but not nonspecific siRNA (Fig. 5, C–E). Importantly, we observed that siRNA for cdh12 or cdh19 markedly suppressed MCPIP-induced upregulation of Tie-2 and Flk-1 (Fig. 5, F and G), suggesting that induction of cdh12 and cdh19 is involved in MCPIP-mediated endothelial differentiation in BMNCs.
Fig. 5.
MCPIP-mediated upregulation of EC markers is associated with induction of cdh12 and cdh19 in BMNCs. BMNCs were cultured in EBM medium and transfected with MCPIP expression vector or control vector. (A) qRT-PCR was performed to determine the expression of cdh12 and cdh19 in BMNCs at the time points indicated. Relative mRNA levels were expressed as fold change compared with cells transfected with control vector. (B) Treatment of cells with siRNA against cdh12 and cdh19 inhibited MCPIP-mediated enhancement of cell attachment. (C) qRT- PCR analysis of cdh12 and cdh19 mRNA in BMNCs transfected with the expression vector for MCPIP-GFP with or without cdh12- and cdh19-specific or nonspecific siRNA showed that only siRNA specific for the particular cdh gene showed knockdown. *P < 0.05 versus MCPIP vector- or nonspecific siRNA (NS)–transfected BMNCs. (D and E) Histograms depicting the average cdh12 and cdh19 expression levels in the examined groups shown in the Western blots. (F and G) Treatment of cells with siRNA against cdh12 and cdh19 inhibited MCPIP-mediated upregulation of endothelial cell markers Tie-2 and Flk-1 in BMNCs. *P < 0.05 versus cells transfected with MCPIP only or nonspecific siRNA (NS).
MCPIP-Modified BMNCs Contribute to Tissue Vascularization in Ischemic Limbs.
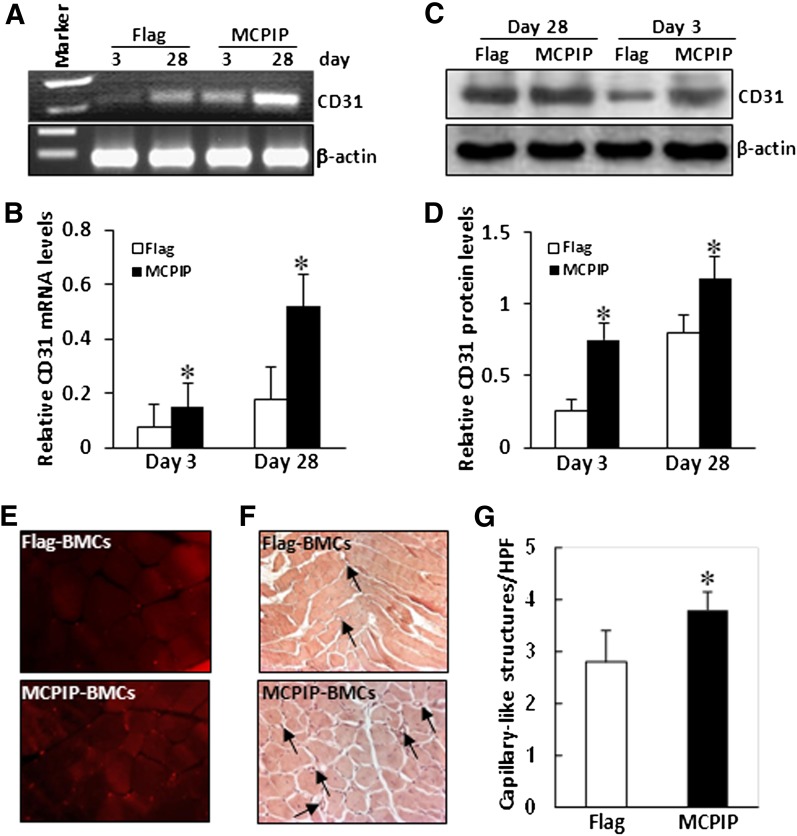
Finally, we evaluated whether transplantation of MCPIP-transfected BMNCs systemically would enhance ischemic neovascularization in mice limbs. Expression of CD31 in the ischemic tissues at days 3 and 28 after transplantation were examined by RT-PCR and immunoblot analysis. A significant increase in CD31 expression in ischemic tissues was found in mice transplanted with MCPIP-transfected BMNCs compared with mice that were transplanted with empty vector–transfected BMCs (Fig. 6, A–D). In histologic analysis, we observed CD31-positive cells in the ischemic limb at days 28 after cell transplantation, and the number of CD31-positive cells in the ischemic zone was significantly higher in mice that received MCPIP-transfected BMNCs compared with mice transplanted with empty vector–transfected BMNCs (Fig. 6E). An increase in the number of capillary-like structures in ischemic zone of mice transplanted with MCPIP-modified BMNCs was also observed when compared with mice transplanted with empty vector-modified BMNCs (Fig. 6, F and G). These results indicate that MCPIP-transfected BMNCs could cause enhanced neovascularization in the ischemic limbs.
Fig. 6.
Transplantation of MCPIP-modified BMNCs enhances new vessel formation in the ischemic limb. BMNCs were transfected with MCPIP expression vector or control vector as described in Materials and Methods. Equal numbers of cells were transplanted intravenously into the mice with limb ischemia via tail vein. At days 3 and 28 after transplantation, RT-PCR (A) and immunoblots (C) were performed to analyze expression of endothelial marker CD31 in the ischemic limb. The bar graphs (B and D) represented the quantification of CD31 expression by densitometric analysis normalized by β-actin. (E) Representative photomicrographs show CD31-positive cells in the ischemic limb at days 28 after cell transplantation. (F) Representative hematoxylin-and-eosin–stained sections obtained 28 days after transplantation indicate formation of capillary-like structures (arrows) in the ischemic limb. (G) Capillary-like structures were calculated and averaged by five randomly selected high power fields. *P < 0.05 versus mice that received BMNCs transfected with control vector (n = 5).
Discussion
Neovascularization is an essential process of tissue repair, and monocytes/macrophages, the major component of the infiltrated leukocytes, have long been recognized as critical regulators of angiogenesis in tissue repair as they are recruited to sites of injury and exhibit angiogenic phenotype under the postischemic microenvironment (Capoccia et al., 2008; Sanberg et al., 2010). However, the mechanisms that govern monocyte angiogenic phenotype are largely unknown. We have recently found that MCP-1-induced protein (MCPIP), a novel zinc-finger protein expressed on myeloid cells, plays a critical role in controlling innate immune activation. In the present study, we report that MCPIP could promote endothelial cell-like morphology formation, upregulate EC-specific surface markers CD31, Tie-2, and Flk-1 expression and downregulate monocytic cell-specific markers CD14 and CD11b in BMNCs. We also demonstrated that forced expression of MCPIP in BMNCs could enhance EC-colony formation, uptake of acLDL and incorporation into tube-like structures in vitro. We present evidence to suggest that this transdifferentiation process is likely mediated by MCPIP-induced cell attachment, ER stress, and autophagy. We further demonstrated that transplantation of MCPIP-modified BMNCs into a murine model of hindlimb ischemia could significantly enhance tissue vascularization in the ischemic hindlimb. These findings indicate a novel angiogenic differentiation capability for MCPIP, which promotes endothelial cell differentiation from BMNCs and offers new approach to achieve the formation of EPCs for therapeutic angiogenesis.
BMNCs are known to transdifferentiate into endothelial cells. Several factors, in particular MCP-1, have been considered as major angiogenic factors associated with recruitment of monocytes (Moldovan et al., 2000; Fujiyama et al., 2003; Schwarz et al., 2004; Capoccia et al., 2008). The invading monocytes within the ischemic myocardium have been shown to form erythrocyte containing vessel-like tunnels (Moldovan et al., 2000). Recently, these results have been supported by in vivo findings showing BMNCs adhere on injured endothelium and accelerate re-endothelialization in an MCP-1-dependent manner (Fujiyama et al., 2003). MCPIP was originally discovered as a protein induced by MCP-1 in human peripheral blood monocytes and is expressed in mononuclear cells in the highly vascularized zones of ischemic myocardium (Zhou et al., 2006). We postulated that MCPIP may be involved in the differentiation of BMNCs into endothelial cells. Data presented here indicated that knockdown of MCPIP inhibits MCP-1-induced upregulation of EC maker Tie-2 and downregulation of monocytic cell marker CD11b. Forced expression of MCPIP also stimulated EC-like morphology formation in BMNCs cultured in EC culture medium and significantly increased the expression of Tie-2, Flk-1, and CD31, which were accompanied by decreased expression of CD14 and CD11b. Consistent alterations were also observed in the morphology and function of BMNCs transfected with MCPIP expression construct, with increased cobblestone-like formation and uptake of DiI-acLDL. When cocultured with HUVECs, expression of MCPIP also markedly increased the number of BMNCs incorporating or adjacent to the tube-like networks of HUVECs, suggesting a potent EC differentiation-promoting activity of MCPIP on BMNCs. MCPIP-induced changes observed in cell culture of the monocytic cells appears to be relevant to ischemic neovascularization in intact animal. Transplantation of MCPIP-modified BMNCs resulted in detectable enhancement of neovascularization in ischemic hindlimb. The findings are consistent with the reports of use of myeloid angiogenic cells to enhance ischemic neovascularization in models of myocardial infarction and hindlimb ischemia (Kawamoto et al., 2001; Urbich et al., 2003). It is likely that these MCPIP-modified BMNCs from the circulation reached the ischemic area and served as a source for EPCs required for neovascularization. It is also possible that MCPIP-modified BMNCs may act as supporting cells during vascular repair by the release of proangiogenic factors. Indeed, monocytes/macrophages have been shown to compensate for neovascularization by drilling metalloelastase-positive tunnels in ischemic myocardium (Moldovan et al., 2000). Adult bone marrow contains only a small subpopulation of EPCs that can proliferate and differentiate into mature endothelial cells. We demonstrated that forced expression of MCPIP in BMNCs enhances the ability of BMNCs to function as EPCs for neovascularization in ischemic tissue. This finding not only provides an efficient approach to achieve the functional EPCs from BMNCs but also has potential clinical application for therapeutic angiogenesis in ischemic diseases.
Enhancement of transdifferentiation of BMNCs into ECs by MCPIP was, at least in part, mediated by the induction of its target genes cdh12 and cdh19, both of which belong to N-cadherin family, which is a transmembrane glycoprotein that mediates cell-matrix adhesion (Blaschuk and Rowlands, 2000; Luo and Radice, 2005). Both cdh12 and cdh19 have been shown to be transcriptionally activated by MCPIP (Niu et al., 2008). The relevance of MCPIP-mediated induction of cdh12 and cdh19 to EC differentiation process was demonstrated by the results of siRNA knockdown experiments showing knockdown of cdh12 and cdh19 inhibited MCPIP-induced upregulation of Tie-2, Flk-1, and CD31 in BMNCs. Recently, it was reported that siRNA against N-cadherin inhibited MCP-1-mediated capillary tube formation of human microvascular endothelial cells (Lu et al., 2009). Thus, expression of MCPIP and the consequent induction of N-cadherins might contribute to the process of differentiation and neovascularization in the ischemic microenvironment, where MCP-1 chemotactically brings circulating monocytic cells.
Cell differentiation involves the significant expansion of the ER, resulting in ER stress to support the elevated production of a new set of proteins (Wiest et al., 1990; Franco et al., 2010; Oh et al., 2012). Differentiation also involves disappearance of one set of proteins by a self-digestion process such as autophagy, which has been demonstrated to be involved in differentiation in several contexts (Aymard et al., 2011; Salemi et al., 2012). The results presented here demonstrate that enhanced expression of MCPIP induced ER stress and autophagy during the process of MCPIP-mediated EC differentiation. The important role of ER stress and autophagy in monocyte differentiation into endothelial cells was further shown by the findings that treatment of BMNCs with TUDC, a specific inhibitor of ER stress, inhibited MCPIP-induced upregulation EC markers Tie-2 and Flk-1. Similarly, inhibition of autophagy by 3-MA or knockdown of Beclin-1 resulted in the downregulation of MCPIP-induced EC markers Tie-2 and Flk-1. This observation suggests that both ER stress and autophagy are involved in endothelial cell transdifferentiation process. In agreement with these findings, transgenic expression of MCP-1 triggers ER stress in the heart (Azfer et al., 2006) and the invading monocytes in the heart appeared to form erythrocyte-containing vascular-like tunnels (Moldovan et al., 2000), suggesting that ER stress may be an important angiogenesis promoter in MCP-1-induced vascular-like tunnel formation in vivo. ER stress was recently reported to promote angiogenesis by activation of VEGF transcription (Pereira et al., 2010). Autophagy has also been reported to be an important angiogenesis enhancer, as inhibition of autophagy by 3-MA or knockdown of ATG5 inhibits tube-like structure formation in bovine aortic endothelial cells (Du et al., 2012). Thus, it is likely that during EC differentiation from BMNCs by MCPIP, the ER might undergo structural and functional reorganization to perform the new cell functions, resulting in ER stress. Autophagy is also activated to perform the necessary reprograming of protein synthesis needed for cell morphologic and structural changes.
Monocytic cells have pleiotropic functions. The recruitment of circulating blood monocytes into damaged tissue and the ensuing monocyte/macrophage angiogenic differentiation are critical to postischemic neovascularization and tissue repair. A mechanistic understanding of monocyte-specific activities in angiogenesis is just beginning to emerge. In the current study we focused on well-established and frequently used in vitro assays, demonstrating that MCPIP has the ability to upregulate EC markers and angiogenic properties in human bone marrow monocytic cells, and that this phenotypic switch is associated with MCPIP-induced upregulation of cadherins, ER stress, and autophagy. This finding provides a mechanistic explanation for the proangiogenic effect of MCP-1. However, it should be noted that MCPIP-mediated upregulation of EC marker proteins in monocytic cells does not seem to be associated with a real differentiation of these cells for integration into vascular network because these cells were detected adjacent to the tube-like networks of HUVECs and displayed spherical shape rather than stretched-out long shape. This evidence is in agreement with the findings that deletion of Tie-2-positive BM-derived cells blocked tumor angiogenesis, although these cells are not integrated into the tumor vessels but are detected adjacent to the vessel (De Palma et al., 2003). This finding suggests that therapeutic angiogenesis based on delivering monocytes and other BM-derived cells should not be restricted to generation of EPCs incorporating into capillaries. MCPIP-modified BMNCs might function as supporting cells to enhance neovascularization by releasing proangiogenic factors in a paracrine manner. This hypothesis is supported by our recent findings that myeloid cell–specific expression of MCPIP represented alternatively activated M2 macrophages (J. Niu and P.E. Kolattukudy, unpublished data), which are characterized as being proangiogenic and anti-inflammatory cells involved in tissue repair and vascular remodeling (Varin and Gordon, 2009; Medina et al., 2011). Indeed, certain environmental conditions, such as tissue ischemia and necrosis, could trigger the infiltration of monocytic cells to the site of injury, resulting in cellular inflammation, tissue repair, and vascular remodeling. Importantly, MCPIP is expressed mainly in monocytic cells and can be induced by various stimuli such as oxidative stress and inflammatory cytokines. Thus, induction of MCPIP in such cells could trigger a phenotypic switch to an angiogenic phenotype in inflamed tissues. These findings will help us to better understand the mechanisms of inflammatory angiogenesis and warrant further evaluation of MCPIP as a suitable target to modulate angiogenesis for therapeutic purpose.
Acknowledgments
The authors thank Edilu Becerra for the care of the animals used in this study.
Abbreviations
- acLDL
acetylated low-density lipoprotein
- BMNC
bone marrow mononuclear cell
- DiI-acLDL
lipoproteins labeled with the 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate
- EBM
endothelial cell basal medium
- EC
endothelial cell
- EPC
endothelial progenitor cell
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- HUVEC
human umbilical vein endothelial cells
- MCP-1
monocyte chemotactic protein-1
- MCPIP
MCP-1-induced protein
- qRT-PCR
quantitative RT-PCR
- RT-PCR
reverse transcription–polymerase chain reaction
- siRNA
small interfering RNA
- TUDC
tauroursodeoxycholate
- VEGF
vascular endothelial growth factor
Authorship Contributions
Participated in research design: Niu, Kolattukudy.
Conducted experiments: Niu, Wang, Zhelyabovska.
Performed data analysis: Niu, Kolattukudy.
Contributed new reagents: Saad.
Wrote or contributed to the writing of the manuscript: Niu, Kolattukudy.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL69458].
References
- Alessio M, De Monte L, Scirea A, Gruarin P, Tandon NN, Sitia R. (1996) Synthesis, processing, and intracellular transport of CD36 during monocytic differentiation. J Biol Chem 271:1770–1775 [DOI] [PubMed] [Google Scholar]
- Anghelina M, Krishnan P, Moldovan L, Moldovan NI. (2006) Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol 168:529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymard E, Barruche V, Naves T, Bordes S, Closs B, Verdier M, Ratinaud MH. (2011) Autophagy in human keratinocytes: an early step of the differentiation? Exp Dermatol 20:263–268 [DOI] [PubMed] [Google Scholar]
- Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. (2006) Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291:H1411–H1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk OW, Rowlands TM. (2000) Cadherins as modulators of angiogenesis and the structural integrity of blood vessels. Cancer Metastasis Rev 19:1–5 [DOI] [PubMed] [Google Scholar]
- Bouchentouf M, Paradis P, Forner KA, Cuerquis J, Boivin MN, Zheng J, Boulassel MR, Routy JP, Schiffrin EL, Galipeau J. (2010) Monocyte derivatives promote angiogenesis and myocyte survival in a model of myocardial infarction. Cell Transplant 19:369–386 [DOI] [PubMed] [Google Scholar]
- Capoccia BJ, Gregory AD, Link DC. (2008) Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol 84:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Taubman MB. (2004) Chemokines in the pathogenesis of vascular disease. Circ Res 95:858–866 [DOI] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Roca C, Naldini L. (2003) Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med 9:789–795 [DOI] [PubMed] [Google Scholar]
- Du J, Teng RJ, Guan T, Eis A, Kaul S, Konduri GG, Shi Y. (2012) Role of autophagy in angiogenesis in aortic endothelial cells. Am J Physiol Cell Physiol 302:C383–C391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Almanza G, Burns JC, Wheeler M, Zanetti M. (2010) Endoplasmic reticulum stress drives a regulatory phenotype in human T-cell clones. Cell Immunol 266:1–6 [DOI] [PubMed] [Google Scholar]
- Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, et al. (2003) Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res 93:980–989 [DOI] [PubMed] [Google Scholar]
- Gaffney J, West D, Arnold F, Sattar A, Kumar S. (1985) Differences in the uptake of modified low density lipoproteins by tissue cultured endothelial cells. J Cell Sci 79:317–325 [DOI] [PubMed] [Google Scholar]
- Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. (1997) Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 80:829–837 [DOI] [PubMed] [Google Scholar]
- Jura J, Skalniak L, Koj A. (2012) Mocyte chemotactic protein-1-indiced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions. Biochim Biophys Acta 1823:1905–1913 [DOI] [PubMed] [Google Scholar]
- Kamiya T, Makino J, Hara H, Inagaki N, Adachi T. (2011) Extracellular-superoxide dismutase expression during monocytic differentiation of U937 cells. J Cell Biochem 112:244–255 [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, et al. (2001) Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103:634–637 [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee CS, Hur J, Cho HJ, Jun SI, Kim TY, Lee SW, Suh JW, Park KW, Lee HY, et al. (2009) Priming with angiopoietin-1 augments the vasculogenic potential of the peripheral blood stem cells mobilized with granulocyte colony-stimulating factor through a novel Tie2/Ets-1 pathway. Circulation 120:2240–2250 [DOI] [PubMed] [Google Scholar]
- Kovács AL, Gordon PB, Grotterød EM, Seglen PO. (1998) Inhibition of hepatocytic autophagy by adenosine, adenosine analogs and AMP. Biol Chem 379:1341–1347 [DOI] [PubMed] [Google Scholar]
- Liang J, Wang J, Azfer A, Song W, Tromp G, Kolattukudy PE, Fu M. (2008) A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J Biol Chem 283:6337–6346 [DOI] [PubMed] [Google Scholar]
- Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE, Fu M. (2010) MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J Exp Med 207:2959–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen Q, Corey E, Xie W, Fan J, Mizokami A, Zhang J. (2009) Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin Exp Metastasis 26:161–169 [DOI] [PubMed] [Google Scholar]
- Luo Y, Radice GL. (2005) N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol 169:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. (2009) Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 458:1185–1190 [DOI] [PubMed] [Google Scholar]
- Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW. (2011) Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med 17:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. (2000) Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res 87:378–384 [DOI] [PubMed] [Google Scholar]
- Napoli C, Maione C, Schiano C, Fiorito C, Ignarro LJ. (2007) Bone marrow cell-mediated cardiovascular repair: potential of combined therapies. Trends Mol Med 13:278–286 [DOI] [PubMed] [Google Scholar]
- Niu J, Kolattukudy PE. (2009) Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 117:95–109 [DOI] [PubMed] [Google Scholar]
- Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. (2008) Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem 283:14542–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Wang K, Graham S, Azfer A, Kolattukudy PE. (2011) MCP-1-induced protein attenuates endotoxin-induced myocardial dysfunction by suppressing cardiac NF-κB activation via inhibition of IκB kinase activation. J Mol Cell Cardiol 51:177–186 [DOI] [PubMed] [Google Scholar]
- Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, Cella M, Bernal-Mizrachi C. (2012) Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem 287:11629–11641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira ER, Liao N, Neale GA, Hendershot LM. (2010) Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PLoS ONE 5:e12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, March KL. (2003) Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107:1164–1169 [DOI] [PubMed] [Google Scholar]
- Roy A, Kolattukudy PE. (2012) Monocyte chemotactic protein-induced protein (MCPIP) promotes inflammatory angiogenesis via sequential induction of oxidative stress, endoplasmic reticulum stress and autophagy. Cell Signal 24:2123–2131 [DOI] [PubMed] [Google Scholar]
- Salemi S, Yousefi S, Constantinescu MA, Fey MF, Simon HU. (2012) Autophagy is required for self-renewal and differentiation of adult human stem cells. Cell Res 22:432–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg PR, Park DH, Kuzmin-Nichols N, Cruz E, Hossne NA, Jr, Buffolo E, Willing AE. (2010) Monocyte transplantation for neural and cardiovascular ischemia repair. J Cell Mol Med 14:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz ER, Meven DA, Sulemanjee NZ, Kersting PH, Tussing T, Skobel EC, Hanrath P, Uretsky BF. (2004) Monocyte chemoattractant protein 1-induced monocyte infiltration produces angiogenesis but not arteriogenesis in chronically infarcted myocardium. J Cardiovasc Pharmacol Ther 9:279–289 [DOI] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. (1982) 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA 79:1889–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi BG, Zeng Z, Wang L, Song L, Chen H, Qin M, Sierra-Honigmann MR, Wachsmann-Hogiu S, Shah PK. (2006) Pleiotrophin induces transdifferentiation of monocytes into functional endothelial cells. Arterioscler Thromb Vasc Biol 26:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata T, Akira S. (2013) mRNA degradation by the endoribonuclease regnase-1/ZC3H12a/MCPIP-1. Biochim Biophys Acta 1829:708–713 [DOI] [PubMed] [Google Scholar]
- Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. (2003) Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation 108:2511–2516 [DOI] [PubMed] [Google Scholar]
- Varin A, Gordon S. (2009) Alternative activation of macrophages: immune function and cellular biology. Immunobiology 214:630–641 [DOI] [PubMed] [Google Scholar]
- Voskuil M, van Royen N, Hoefer IE, Seidler R, Guth BD, Bode C, Schaper W, Piek JJ, Buschmann IR. (2003) Modulation of collateral artery growth in a porcine hindlimb ligation model using MCP-1. Am J Physiol Heart Circ Physiol 284:H1422–H1428 [DOI] [PubMed] [Google Scholar]
- Voyta JC, Via DP, Butterfield CE, Zetter BR. (1984) Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 99:2034–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrotsos EG, Kolattukudy PE, Sugaya K. (2009) MCP-1 involvement in glial differentiation of neuroprogenitor cells through APP signaling. Brain Res Bull 79:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Niu J, Kim H, Kolattukudy PE. (2011) Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J Mol Cell Biol 3:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest DL, Burkhardt JK, Hester S, Hortsch M, Meyer DI, Argon Y. (1990) Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J Cell Biol 110:1501–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younce C, Kolattukudy PE. (2012) MCP-1 induced protein promotes adipogenesis via oxidative stress, endoplasmic reticulum stress and autophagy. Cell Physiol Biochem 30:307–320 [DOI] [PubMed] [Google Scholar]
- Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. (2006) Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res 98:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]