Abstract
Delta-opioid receptors (DOR) are present in the superficial dorsal horn and are believed to regulate the release of small afferent transmitters as evidenced by the effects of spinally delivered delta-opioid preferring peptides. Here we examined the effects of intrathecal SNC80 [(+)-4-[α(R)-α-[(2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl]-3-(methoxybenzyl)-N,N-diethylbenzamide], a selective nonpeptidic DOR agonist, in three preclinical pain models, acute thermal escape, intraplantar carrageenan-tactile allodynia, and intraplantar formalin flinches, and on the evoked release of substance P (SP) from small primary afferents. Rats with chronic intrathecal catheters received intrathecal vehicle or SNC80 (100 or 200 μg). Intrathecal SNC80 did not change acute thermal latencies or carrageenan-induced thermal hyperalgesia. However, SNC80 attenuated carrageenan-induced tactile allodynia and significantly reduced both phase 1 and phase 2 formalin-induced paw flinches, as assessed by an automatic flinch counting device. These effects were abolished by naltrindole (3 mg/kg i.p.), a selective DOR antagonist, but not CTOP (10 µg i.t.), a selective MOR antagonist. Furthermore, intrathecal SNC80 (200 μg) blocked formalin-induced substance P release otherwise evoked in the ispilateral superficial dorsal horn as measured by NK1 receptor internalization. In conclusion, intrathecal SNC80 alleviated pain hypersensitivity after peripheral inflammation in a fashion paralleling its ability to block peptide transmitter release from small peptidergic afferents, which by its pharmacology appears to represent an effect mediated by a spinal DOR.
Introduction
After initial pharmacological characterization of the delta-opioid receptor (DOR) and its pharmacological distinction from the classic mu-opioid receptor (MOR) (Hughes et al., 1975), it was shown that intrathecal enkephalins yielded a potent analgesia (Yaksh et al., 1977, 1978). Metabolically-stabilized peptide-based agonists given spinally had a potent analgesic effect in rodents, primates (Tung and Yaksh, 1982; Yaksh, 1983), and humans (Onofrio and Yaksh, 1983; Moulin et al., 1985; Krames et al., 1986). Based on differential sensitivity of the intrathecal agonists to naloxone antagonism and minimal cross-tolerance with morphine, we suggested a distinct role for DORs in spinal nociceptive modulation (Tung and Yaksh, 1982). Subsequent advances further supported the role of spinal DORs: 1) spinal actions were demonstrated by yet more selective cyclic peptides [e.g., [2-d-penicillamine,5-d-penicillamine]-enkephalin (DPDPE)] and later the nonpeptidic SNC80 [(+)-4-[α(R)-α-[(2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl]-3-(methoxybenzyl)-N,N-diethylbenzamide] with DOR/MOR potency ratios often in excess of several 100-fold, depending upon the assays (Leslie, 1987; Kramer et al., 1993; Calderon et al., 1994); 2) the distinction of DOR from MOR agonists was supported by development of antagonists such as naltrindole (Granier et al., 2012), which reverses DOR agonists at doses that have no effect upon MOR agonist actions (Drower et al., 1991; Malmberg and Yaksh, 1992; Tiseo and Yaksh, 1993; Craft et al., 1995; Yaksh et al., 1995).
Mechanistically, MOR and DOR agonists decrease opening of voltage-sensitive calcium channels, preventing mobilization of terminal neurotransmitter (Soldo and Moises, 1998; Law et al., 2000). The hypothesized presynaptic localization on sensory nociceptors suggests that these agents block neurotransmitter release from C fibers. Earlier studies with the selective peptidic DOR ligand [d-Pen2, l-Pen5]-enkephalin showed that its intrathecal delivery in vivo diminished substance P (SP) release evoked by the transient receptor potential cation channel subfamily V member 1 agonist capsaicin (Aimone and Yaksh, 1989), a finding supported by subsequent work showing that a DOR peptide blocked voltage-dependent calcium currents in rat dorsal root ganglion neurons in a naltrindole-sensitive fashion (Acosta and Lopez, 1999). More recently, however, Scherrer and colleagues (2009) in anatomic studies reported in mice that MOR are present on IB4-negative, peptidergic afferents and DOR are on IB4-positive, non-peptidergic afferents. Such an observation would argue that spinal DOR activation would not affect release from peptidergic afferents. However, both mRNA and protein of DOR were detected in peptidergic nociceptors in mice (Mennicken et al., 2003; Wang et al., 2010). Moreover, local intradermal SNC80 inhibited nerve growth factor–induced hyperalgesia in rats lacking nonpeptidergic (e.g., IB4+) nociceptors, thus arguing that DORs, at least at the peripheral terminal, may function to regulate peptidergic afferent excitability (Joseph and Levine, 2010). These conflicting findings led us to revisit the effects of spinal DOR agonists on transmitter release from peptidergic afferents. Here, we systematically examined the effects of the nonpeptide delta-opioid agonist SNC80 on nociceptive behavior and, using internalization of the NK1 receptor, we assessed in parallel the effects of IT SNC80 on SP release. We observed that IT SNC80 produced significant antinociception and prevented intraplantar formalin-evoked SP release, all in a manner reversed by naltrindole. These results support the role of DOR in regulating small peptidergic afferent terminal excitability in the rat.
Materials and Methods
Animals
Male Holtzman Sprague-Dawley rats (250–300 g; Harlan, Indianapolis, IN) were individually housed in standard cages and maintained on a 12-hour light/dark cycle (lights on at 7:00 AM). Testing occurred during the light cycle. Food and water were available ad libitum. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, Bethesda, MD) and as approved by the Institutional Animal Care and Use Committee of the University of California (San Diego, CA). A total of 100 rats was used.
Intrathecal Catheter Implantation
Rats were implanted with a single intrathecal catheter for drug delivery, as described previously (Yaksh and Rudy, 1976; Malkmus and Yaksh, 2004). In brief, rats were anesthetized by induction with 4% isoflurane in a room air/oxygen mixture (1:1), the anesthesia was maintained with 2% isoflurane delivery by mask, and the animal was placed in a head holder. The cisternal membrane was exposed through a midline incision and a polyethylene (outer diameter 0.36 mm) catheter was passed into the intrathecal space to the level of the L2–L3 spinal segments (8.5 cm). The catheter was externalized on the top of the head. Rats were given carprofen (5 mg/kg, in lactated Ringer’s solution) subcutaneously and allowed to recover under a heat lamp. Rats exhibiting motor weakness or signs of paresis were killed. Animals were allowed to recover for 5 to 7 days before the experiment.
Drug, Antibody, and Materials
SNC80, CTOP, and naltrindole were purchased from Sigma-Aldrich (St. Louis, MO). SNC80 and CTOP were dissolved in 20% dimethyl sulfoxide, 0.1 N HCl. Naltrindole was dissolved in saline. SNC80, vehicle, or CTOP was administered intrathecally in a volume of 10 μl followed by a 10 μl saline flush. Naltrindole was administered (1 ml i.p.) 20 minutes before SNC80 or vehicle. The rabbit anti-NK1 receptor polyclonal antibody was purchased from the Advanced Targeting Systems (San Diego, CA). Secondary Alexa 488-conjugated antibody was purchased from Invitrogen (Carlsbad, CA). Prolong mounting medium was from Thermo Fisher Scientific (Pittsburgh, PA).
Behavior
Thermal Escape Latency.
After an initial acclimation, the thermal escape latency was determined using a Hargreaves type thermal escape testing system (Dirig et al., 1997). In the absence of a response at 20 seconds, the stimulus was terminated, and that latency was assigned. Data were collected before and at 15, 30, 60, 90, and 120 minutes after the intrathecal injection. Each time point presents the average of the left and right paw withdrawal latency in seconds of the group.
Carrageenan-Evoked Thermal Hyperalgesia and Tactile Allodynia.
Under light anesthesia (isoflurane), rats were injected with 100 μl of λ-carrageenan (2%; Nacalai Tesque Inc., Kyoto, Japan) in the plantar surface of the left hindpaw. Thermal latency of each paw was determined as described above. Withdrawal threshold to a mechanical stimulus was assessed using von Frey filaments applied though a wire mesh grid following the “up down” protocol (Chaplan et al., 1994). Intrathecal SNC80 or vehicle was injected at 60 minutes after carrageenan. In antagonist study, rats were given naltrindole (3 mg/kg i.p.) 20 minutes before intrathecal SNC80. The size of the injected paw was measured before carrageenan and after testing at 4 hours. Rats were killed after testing.
Formalin-Induced Paw Flinching.
Animals were allowed to acclimate in individual Plexiglas chambers for 30 minutes before experimental manipulation. Rats were administered intrathecal vehicle or agonist 10 minutes before the formalin injection. Flinching was evoked by a subcutaneous injection of formalin (5%, 50 μl) into the dorsal side of left hindpaw. A soft metal band was placed around the hindpaw being injected, and flinching and shaking of the injected paw was quantified by an automatic device (Department of Anesthesiology, University of California, San Diego) (Yaksh et al., 2001). Flinches were counted in 1-minute intervals for 60 minutes. Animals were then killed.
Motor Function Evaluation.
Effects of intrathecal agents on motor function were assessed for arousal (response to a hand clap), righting reflex, and placing/stepping reflex (Malmberg and Yaksh, 1994).
Measurement of Dorsal Horn Substance P Release
The release of substance P from spinal sensory afferent was quantified by examining the percentage of NK1 receptor-bearing neurons in the ipsilateral and contralalateral superficial dorsal horns that displayed internalization of the NK1 receptor. In this experiment, the rats received formalin (5%, 50 µl) in the dorsum of one hindpaw. Ten minutes after the injection, the animals were perfused with 4% paraformaldehyde. In the exogenous SP-induced NK1R internalization experiment, rats were injected with intrathecal SP (30 nmol) or saline and perfused 30 minutes later. The lumbar block of the spinal cord was removed and postfixed in 4% paraformaldehyde overnight. After cryoprotection in 30% sucrose, coronal sections (30 μm) were cut on a cryostat (CM1800; Leica), mounted on slides, and prepared for fluorescent immunohistochemistry. In brief, sections were incubated with rabbit anti-NK1R polyclonal antibody (1:3000 in phosphate-buffered saline containing 3% normal goat serum and 0.3% Triton X-100) overnight at room temperature. After rinsing in phosphate-buffered saline, sections were incubated for 120 minutes at room temperature in a goat anti-rabbit secondary antibody conjugated with Alexa Fluor 488 (1:1000). All sections were finally rinsed and placed under a coverslip with ProLong mounting medium. The incidence of NK1R internalization was counted under a fluorescence microscope (Olympus Optical, Tokyo, Japan) equipped with a 60× oil-immersion objective lens according to the standard of previous reports (Mantyh et al., 1995; Abbadie et al., 1997; Nazarian et al., 2008). Neuronal profiles that had 10 or more NK1R-labeled endosomes were considered positive for NK1R internalization. The total number of NK1R immunoreactive neurons in lamina І/II, with and without NK1R internalization, was counted and taken to calculate the fraction of cells showing internalization. Counting was carried out without knowledge of treatments. Mean counts from three to five sections per segment were used as representative counts for a given animal. Images were taken using Magna FIRE SP and processed by Adobe Photoshop CS4.
Treatment Paradigm
There were three sets of experiments:
Effects of intrathecal SNC80 or its vehicle on acute thermal threshold, carrageenan-evoked tactile allodynia, and formalin-evoked flinching alone or in the presence of systemically delivered naltrindole.
Effects of intrathecal SNC80 or its vehicle upon the NK1R internalization after intraplantar formalin when given alone or with a naltrindole pretreatment.
Effects of intrathecal SNC80 on NK1R internalization induced by exogenous substance P (e.g., in the absence of afferent stimulation).
Statistical Analysis
Changes in formalin-induced paw-flinching behavior in phases I and II was analyzed using one-way ANOVA followed by Tukey’s post hoc analysis. Carrageenan data were analyzed by two-way ANOVA. The analysis for NK1R internalization data consisted of t test or one-way ANOVA. In all analysis, probability to detect the difference was set at the 5% level (P < 0.05).
Results
Tactile Allodynia Induced by Carrageenan.
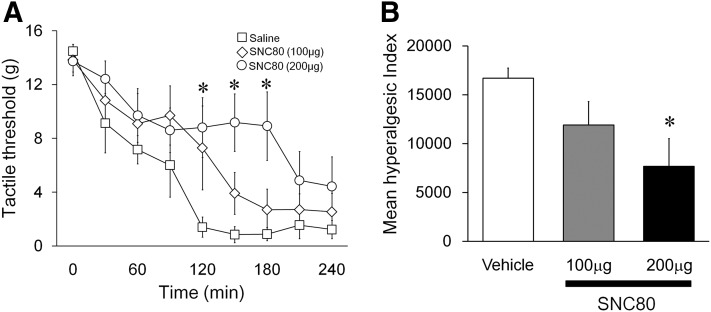
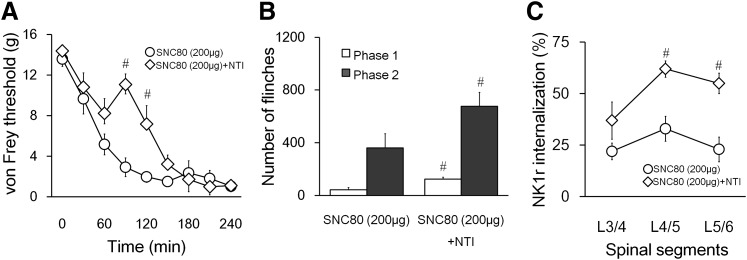
A continuous decrease in the tactile threshold of the ipsilateral paw was observed after intraplantar carrageenan injection. Intrathecal SNC80 at 60 minutes after carrageenan reversed the allodynia in a dose-dependent manner (Fig. 1A, P < 0.05). In animals treated with 200 μg of SNC80, the paw withdrawal thresholds were significantly different from the vehicle control at 120, 150, and 180 minutes (Fig. 1A, P < 0.05). A significant difference between 200 μg and the control group was also noted in the hyperalgesic index values that represent the degree of the allodynia (Fig. 1B, P < 0.05).
Fig. 1.
Effects of intrathecal SNC80 on λ-carrageenan-induced tactile allodynia. (A) Tactile threshold in grams plotted as a function of time after λ-carrageenan. Vehicle or SNC80 (100 or 200 μg) was administrated intrathecally at time 60 minutes. At 120, 150, and 180 minutes, intrathecal 200 μg of SNC80 significantly increased the withdrawal threshold of von Frey filaments. (B) Histogram presents the effects of vehicle and SNC80 (100 and 200 μg) on the hyperalgesia index (area under the curve). Intrathecal 200 μg, but not 100 μg, of SNC80 significantly depressed the hyperalgesic index. Data are presented as mean ± S.E.M. n = 5–7, *P < 0.05.
SNC80 (200 μg) did not produce significant analgesic effects in the acute thermal Hargreaves’ test (Supplemental Fig. 1, A and B) nor did it reverse thermal hyperalgesia induced by carrageenan (Supplemental Fig. 2).
Formalin-Induced Paw Flinches
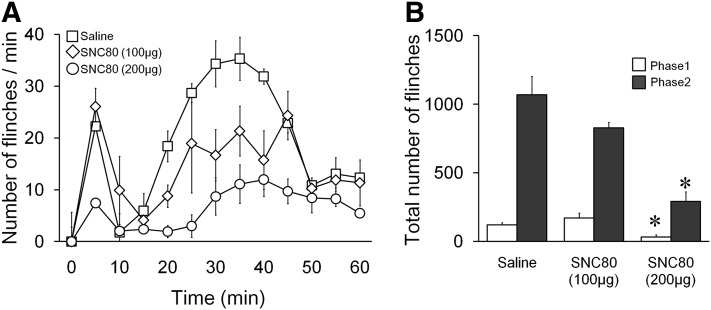
The effects of intrathecal SNC80 (100 or 200 µg) or vehicle on formalin-induced paw flinches were shown in Fig. 2, A and B. SNC80 (200 µg but not 100 µg) significantly reduced the number of formalin-induced paw flinches in phase 2 but not phase 1 compared with the vehicle control.
Fig. 2.
Effects of intrathecal SNC80 on formalin-induced paw-flinching behavior. (A) Time course over 60 minutes of flinches after formalin (5%, 50 μl) paw injection in rats with intrathecal injection of SNC80 (100, 200 μg) or vehicle. (B) Cumulative flinches in each phase, phase 1: 1–9 minutes; phase 2: 10–60 minutes. Intrathecal 200 μg, but not 100 μg, of SNC80 reduced both phase 1 and phase 2 of formalin-induced paw flinching. Data are presented as mean ± S.E.M. n = 4–6, *P < 0.05.
Formalin-Induced NK1 Receptor Internalization
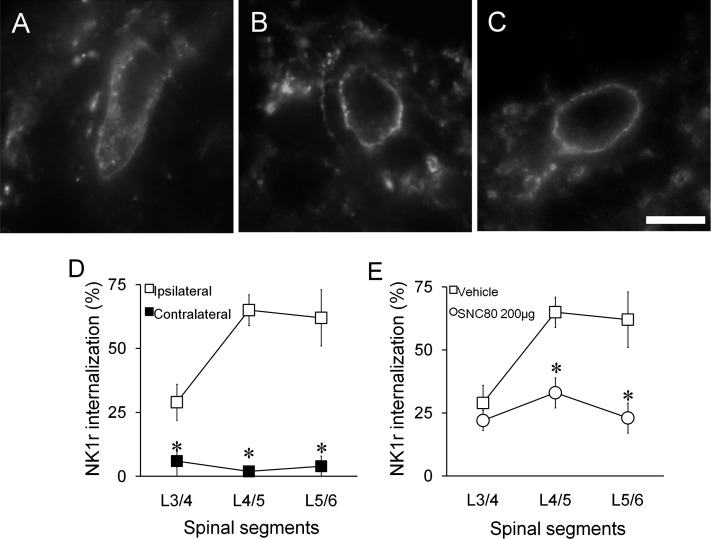
NK1R immunoreactivity was typically observed outlining the cell membrane in many superficial dorsal horn neurons. Unilateral intraplantar injection of formalin (5%, 50 μl) produced a robust NK1R internalization in ipsilateral superficial dorsal horn (Fig. 3, A and D) but not in contralateral dorsal horn (Fig. 3, B and D). Intrathecal SNC80 (200 µg) treatment resulted in a prominent reduction of formalin-evoked NK1R internalization in the ipsilateral L5 and L6 spinal cord (Fig. 3, C and E, P < 0.05).
Fig. 3.
Effects of intrathecal SNC80 on formalin-induced neurokinin 1 receptor (NK1R) internalization. (A–C) Representative fluorescent microscope images of NK1R in the lumbar superficial dorsal horn. (A) A lamina I neuron with NK1R internalization in the ipsilateral spinal cord from a rat pretreated with intrathecal vehicle 10 minutes after paw formalin. Note the lack of homogeneous cell membrane labeling and the presence of NK1R (+) endosomes in the cytoplasm. (B) A lamina I neuron in the contralateral spinal cord with no NK1R internalization. (C) A lamina I neuron in the ipsilateral spinal cord from a rat pretreated with intrathecal SNC80. Scale bar, 10 µm. (D) Percentage of NK1R (+) neurons showing internalization in the ipsilateral and contralateral lumbar spinal cord. Rats were treated with intrathecal vehicle followed by intraplantar formalin. (E) Percentage of NK1R (+) neurons showing internalization in the ipsilateral lumbar spinal cord. Rats received intrathecal vehicle or SNC80 (200 μg) followed by intraplantar formalin. Data are presented as mean ± S.E.M. n = 3, *P < 0.05.
SNC80 on NK1 Receptor Internalization Induced by Exogenous Substance P
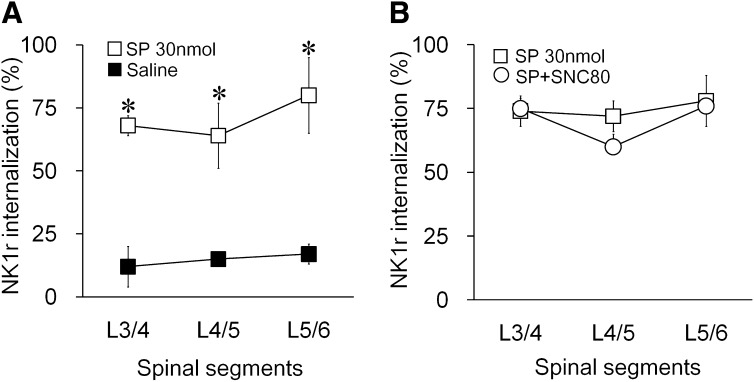
To investigate whether SNC80 directly blocks NK1r internalization in postsynaptic spinal neurons, we induced NK1R internalization by exogenous substance P (30 nmol) by intrathecal administration. When examined 30 minutes after injection, substance P (30 nmol) produced widespread NK1R internalization at L4–L6 levels of spinal cord in lamina І compared with intrathecal saline (Fig. 4A). Intrathecal SNC80 at 200 μg, a dose that completely blocked formalin-induced NK1R internalization, did not alter the exogenous substance P-induced NK1R internalization when given 10 minutes before the substance P (Fig. 4B). This result confirmed that SNC80 did not inhibit NK1R internalization directly. Rather, SNC80 blocked formalin-induced NK1R internalization by inhibiting SP release.
Fig. 4.
The effects of SNC80 on the neurokinin 1 receptor (NK1R) internalization induced by exogenous substance P. (A) Percentage of NK1R (+) neurons showing internalization in lamina I of the lumbar spinal cord 30 minutes after intrathecal SP. Rats were treated with intrathecal substance P or saline. (B) Percentage of NK1R (+) neurons showing internalization in lamina I of the lumbar spinal cord. Rats were treated with intrathecal vehicle or SNC80 before receiving intrathecal substance P. Data are presented as mean ± S.E.M. n = 3, *P < 0.05
Naltrindole and CTOP on SNC80 Effects
Intraperitoneal administration of naltrindole (3 mg/kg), a selective DOR antagonist, significantly reversed the effect of SNC80 (200 µg) on carrageenan-induced tactile allodynia (Fig. 5A). Naltrindole also significantly blocked the effect of SNC80 on formalin-induced paw flinches (Fig. 5B). Furthermore, the effect of SNC80 on formalin-induced NK1R internalization was reversed by intraperitoneal naltrindole (Fig. 5C). On the other hand, CTOP (10 μg), an MOR-selective antagonist, did not block the effects of SNC80 in the formalin flinching test (Supplemental Fig. 3). These data collectively suggest that the effects of intrathecal SNC80 are mainly mediated through DOR.
Fig. 5.
The effects of naltrindole (3 mg/kg) on SNC80 (200 μg)-mediated antinociception and block of substance P release after formalin. (A) Tactile threshold in grams plotted as a function of time after intraplantar λ-carrageenan. Naltrindole (i.p.) or saline (i.p.) was administered at 40 minutes after carrageenan and SNC80 (i.t.) was injected 20 minutes after naltrindole. (B) Cumulative flinches after intraplantar formalin in phase 1 (1–9 minutes) and phase 2 (10–60 minutes). Rats were treated with naltrindole (i.p.) or saline followed by intrathecal SNC80 (20 minutes after naltridole) and intraplantar formalin (10 minutes after SNC80). (C) Percentage of NK1R (+) neurons showing internalization in lamina I of the lumbar spinal cord 10 minutes after paw formalin. Rats were treated the same way as in B. Data are presented as mean ± S.E.M. n = 5–7 (A and B), n = 3 (C), *P < 0.05.
Discussion
Considerable evidence demonstrates the analgesic effects of intrathecally delivered DOR agonists. Here our major findings indicate that spinally administered SNC80, a nonpeptidic small molecule, resulted in the robust inhibition of the ipsilateral release of lumbar dorsal horn substance P otherwise evoked by the unilateral intraplantar injection of formalin into the hindpaw and was active at doses that reduced formalin-evoked flinching; both effects were reversed by naltrindole. SNC80 is one of the first highly selective, nonpeptidic, and systemically active delta agonists. It is 500-fold more selective for DOR than MOR based on in vivo and in vitro assays (Calderon et al., 1994; Bilsky et al., 1995). Naltrindole is a selective DOR competitive antagonist (Granier et al., 2012). Given that the predominant source of SP involved in the formalin-evoked internalization is the peptidergic transient receptor potential cation channel subfamily V member 1 (+) primary afferent (Kondo et al., 2005), these observations emphasize that in the rat, DOR receptors exert a presynaptic effect upon on peptidergic terminals and are functionally coupled to regulate their excitability.
Effects of Intrathecal SNC80 on Presynaptic Substance P Release.
Neurokinin 1 receptor (NK1R) is a target for substance P released from nociceptive peptidergic C fibers. Occupancy of NK1R leads to an internalization of the receptor, which serves as a quantitative indicator of primary afferent SP release (Mantyh et al., 1995; Mantyh, 2002; Marvizon et al., 2003). A number of in vivo studies have demonstrated agents that regulate SP release from sensory C fibers can indeed reduce internalization. Several examples include mu opiates such as [D-Ala2, N-MePhe4, Gly-ol]-enkephalin and morphine (Gu et al., 2005; Kondo et al., 2005) with DOR preferring peptide DPDPE (Kondo et al., 2005), deltorphin II (Beaudry et al., 2011), and N-type calcium channel blockers (Takasusuki and Yaksh, 2011). Several points emphasize that intrathecal SNC80 has a potent effect on C-fiber terminal release through DOR. 1) Intrathecal SNC80, at a dose altering formalin-evoked flinching, significantly inhibited NK1R internalization after intraplantar formalin. 2) The effects of SNC80 on NK1R internalization were reversed by naltrindole. 3) Intrathecal SNC80 had no effect on the internalization produced by exogenous SP, emphasizing that the change in formalin-evoked internalization after SNC80 was not due to a direct effect on the internalization process. These results showing an effect of intrathecal SNC 80 on SP release differ from those expected from the report by Scherrer et al. (2009). They reported that DOR is not expressed in peptidergic afferents in mice, which was in sharp contrast to a study by Riedl et al. (2009). However, the current results with rats are consistent with the observations reported by Joseph and Levine (2010), also in rats. Importantly these results are also in agreement with work showing that DOR agonists can have a presynaptic effect upon glutamatergic excitatory postsynaptic currents in rats (Ikoma et al., 2007) and upon excitatory postsynaptic current evoked by TRPA1 agonists in rats (Wrigley et al., 2010) in superficial dorsal horn neurons. Our previous work showed that intrathecal DPDPE in vivo blocked SP release evoked by intrathecal capsaicin in rats (Aimone and Yaksh, 1989). In addition, K+-evoked release of calcitonin gene-related peptide was inhibited by deltorphin II in spinal cord synaptosomes (Riedl et al., 2009) and slice preparations (Overland et al., 2009). These studies collectively suggest that peptide and nonpeptide DOR agonists in rats suppress primary afferent terminal excitability.
Pre- Versus Postsynaptic Site of Action.
Our work does not establish a presynaptic action inhibition as the sole mechanism underlying analgesia mediated by DOR. DORs are expressed in intrinsic spinal neurons (Wang and Wessendorf, 2001; Mennicken et al., 2003) in the dorsal horn, an area with high level expression of G protein–coupled inwardly rectifying potassium channels (Marker et al., 2004). DOR agonists are known to increase the conductance of inwardly rectifying potassium channels (North et al., 1987; Ikeda et al., 1995), which leads to hyperpolarization and inhibition of the neurons. Thus, analgesia mediated by intrathecal DOR agonists was attenuated in G protein–coupled inwardly rectifying potassium channel knockout mice (Marker et al., 2005).
Effects of Intrathecal SNC80 on Facilitated Pain States.
The involvement of spinal DOR in the regulation of pain behavior initiated by inflammatory insults is well established (Hong and Abbott, 1995; Hammond et al., 1998; Fraser et al., 2000; Gaveriaux-Ruff et al., 2008; Towett et al., 2009). Here we show that spinal SNC80 is effective in alleviating pain in two such models, carrageenan-induced allodynia and formalin-induced flinches. Intraplantar carrageenan induces profound local inflammation and persistent tactile and thermal hyperalgesia, in which both peripheral and central sensitization play roles. In the dorsal root ganglion, DOR is expressed in both large-diameter A fibers and small-diameter nonpeptidergic C fibers (Scherrer et al., 2009; Wang et al., 2010), two populations implicated in noxious mechanical nociception. In the present work, we observed a reversal of carrageenan-induced allodynia after intrathecal injection of SNC80, an effect blocked by naltrindole. This result suggests DOR activation attenuates central sensitization to a mechanical stimulus after peripheral injury. Paw formalin injection elicits biphasic flinching in experimental animals. Although phase I flinching behavior is considered to represent a direct nociceptive process, phase II has an important central sensitization component (Yaksh et al., 2001). Recently, it was shown that deltorphin-II, a DOR agonist, inhibited TRPA1-enhanced glutamatergic excitatory postsynaptic currents (Wrigley et al., 2010). Interestingly, TRPA1 has been shown to mediate formalin-induced pain (McNamara et al., 2007). Consistent with those findings, we observed a significant inhibition of phase II flinches with SNC80. It has been suggested that these actions of spinal DOR agonists are predominantly presynaptic (Cheng et al., 1995; Hammond et al., 1998; Abbadie et al., 2002).
The Lack of Effects by SNC80 on Thermal Nociception.
Unexpectedly, we did not detect SNC80-mediated analgesia on acute thermal pain or carrageenan-induced thermal hyperalgesia as assessed by paw withdrawal latency at a dose as high as 200 μg (440 nmol). This is in contrast with several previous reports studying spinal SNC80 (Bilsky et al., 1995; Negus et al., 1998). The result was puzzling given that SNC80 was indeed able to reduce SP release from peptidergic primary afferents, which are proposed to play a critical role in mediating thermal nociception. We believe that this discrepancy indicates neurotransmitter release from additional primary afferents other than DOR-expressing peptidergic nociceptors is needed for the expression of thermal pain in the particular tests, which warrants further studies.
In conclusion, intrathecal SNC80 demonstrated antinociceptive effects in several models of nociception and at similar doses blocked SP release from primary afferents in the superficial spinal dorsal horn, all though a naltrindole-sensitive mechanism.
Supplementary Material
Acknowledgments
The authors thank Shelly Malkmus and Christine Radewicz for their technical assistance.
Abbreviations
- ANOVA
analysis of variance
- CTOP
d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2
- DOR
delta-opioid receptor
- DPDPE
[d-Pen2,d-Pen5]-enkephalin
- IB4
isolectin B4
- MOR
mu-opioid receptor
- NK1R
neurokinin 1 receptor
- SNC80
(+)-4-[α(R)-α-[(2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl]-3-(methoxybenzyl)-N,N-diethylbenzamide
- SP
substance P
- TRPA1
transient receptor potential cation channel subfamily A member 1
Authorship Contributions
Participated in research design: Yaksh, Xu.
Conducted experiments: Kouchek, Takasusuki, Terashima.
Performed data analysis: Kouchek, Takasusuki, Terashima, Xu.
Wrote or contributed to the writing of the manuscript: Kouchek, Takasusuki, Terashima, Xu, Yaksh.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant NIH-DA02110].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI. (2002) Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res 930:150–162 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. (1997) Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci 17:8049–8060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta CG, López HS. (1999) delta opioid receptor modulation of several voltage-dependent Ca(2+) currents in rat sensory neurons. J Neurosci 19:8337–8348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone LD, Yaksh TL. (1989) Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides 10:1127–1131 [DOI] [PubMed] [Google Scholar]
- Beaudry H, Dubois D, Gendron L. (2011) Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci 31:13068–13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. (1995) SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther 273:359–366 [PubMed] [Google Scholar]
- Calderon SN, Rothman RB, Porreca F, Flippen-Anderson JL, McNutt RW, Xu H, Smith LE, Bilsky EJ, Davis P, Rice KC. (1994) Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(alpha R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3- methoxybenzyl]-N,N-diethylbenzamide (SNC 80): a highly selective, nonpeptide delta opioid receptor agonist. J Med Chem 37:2125–2128 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63 [DOI] [PubMed] [Google Scholar]
- Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, Inturrisi CE, Pickel VM. (1995) Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci 15:5976–5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Henley SR, Haaseth RC, Hruby VJ, Porreca F. (1995) Opioid antinociception in a rat model of visceral pain: systemic versus local drug administration. J Pharmacol Exp Ther 275:1535–1542 [PubMed] [Google Scholar]
- Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. (1997) Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods 76:183–191 [DOI] [PubMed] [Google Scholar]
- Drower EJ, Stapelfeld A, Rafferty MF, de Costa BR, Rice KC, Hammond DL. (1991) Selective antagonism by naltrindole of the antinociceptive effects of the delta opioid agonist cyclic[D-penicillamine2-D-penicillamine5]enkephalin in the rat. J Pharmacol Exp Ther 259:725–731 [PubMed] [Google Scholar]
- Fraser GL, Pradhan AA, Clarke PB, Wahlestedt C. (2000) Supraspinal antinociceptive response to [D-Pen(2,5)]-enkephalin (DPDPE) is pharmacologically distinct from that to other delta-agonists in the rat. J Pharmacol Exp Ther 295:1135–1141 [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. (2008) Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci 27:2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. (2012) Structure of the δ-opioid receptor bound to naltrindole. Nature 485:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Kondo I, Hua XY, Yaksh TL. (2005) Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: parallels with tolerance and dependence. J Pharmacol Exp Ther 314:1362–1369 [DOI] [PubMed] [Google Scholar]
- Hammond DL, Wang H, Nakashima N, Basbaum AI. (1998) Differential effects of intrathecally administered delta and mu opioid receptor agonists on formalin-evoked nociception and on the expression of Fos-like immunoreactivity in the spinal cord of the rat. J Pharmacol Exp Ther 284:378–387 [PubMed] [Google Scholar]
- Hong Y, Abbott FV. (1995) Peripheral opioid modulation of pain and inflammation in the formalin test. Eur J Pharmacol 277:21–28 [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. (1975) Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 258:577–580 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kobayashi T, Ichikawa T, Usui H, Kumanishi T. (1995) Functional couplings of the delta- and the kappa-opioid receptors with the G-protein-activated K+ channel. Biochem Biophys Res Commun 208:302–308 [DOI] [PubMed] [Google Scholar]
- Ikoma M, Kohno T, Baba H. (2007) Differential presynaptic effects of opioid agonists on Adelta- and C-afferent glutamatergic transmission to the spinal dorsal horn. Anesthesiology 107:807–812 [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. (2010) Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience 171:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. (2005) Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci 25:3651–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer TH, Davis P, Hruby VJ, Burks TF, Porreca F. (1993) In vitro potency, affinity and agonist efficacy of highly selective delta opioid receptor ligands. J Pharmacol Exp Ther 266:577–584 [PubMed] [Google Scholar]
- Krames ES, Wilkie DJ, Gershow J. (1986) Intrathecal D-Ala2-D-Leu5-enkephalin (DADL) restores analgesia in a patient analgetically tolerant to intrathecal morphine sulfate. Pain 24:205–209 [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. (2000) Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40:389–430 [DOI] [PubMed] [Google Scholar]
- Leslie FM. (1987) Methods used for the study of opioid receptors. Pharmacol Rev 39:197–249 [PubMed] [Google Scholar]
- Malkmus SA, Yaksh TL. (2004) Intrathecal catheterization and drug delivery in the rat. Methods Mol Med 99:109–121 [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. (1994) Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci 14:4882–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. (1992) Isobolographic and dose-response analyses of the interaction between intrathecal mu and delta agonists: effects of naltrindole and its benzofuran analog (NTB). J Pharmacol Exp Ther 263:264–275 [PubMed] [Google Scholar]
- Mantyh PW. (2002) Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry 63 (Suppl 11):6–10 [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. (1995) Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci USA 92:2622–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Luján R, Loh HH, Wickman K. (2005) Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J Neurosci 25:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Stoffel M, Wickman K. (2004) Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci 24:2806–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizón JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. (2003) Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience 118:535–545 [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. (2003) Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol 465:349–360 [DOI] [PubMed] [Google Scholar]
- Moulin DE, Max MB, Kaiko RF, Inturrisi CE, Maggard J, Yaksh TL, Foley KM. (1985) The analgesic efficacy of intrathecal D-Ala2-D-Leu5-enkephalin in cancer patients with chronic pain. Pain 23:213–221 [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 104:13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Gu G, Gracias NG, Wilkinson K, Hua XY, Vasko MR, Yaksh TL. (2008) Spinal N-methyl-D-aspartate receptors and nociception-evoked release of primary afferent substance P. Neuroscience 152:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. (1998) Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther 286:362–375 [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ. (1987) Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA 84:5487–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrio BM, Yaksh TL. (1983) Intrathecal delta-receptor ligand produces analgesia in man. Lancet 1:1386–1387 [DOI] [PubMed] [Google Scholar]
- Overland AC, Kitto KF, Chabot-Doré AJ, Rothwell PE, Fairbanks CA, Stone LS, Wilcox GL. (2009) Protein kinase C mediates the synergistic interaction between agonists acting at alpha2-adrenergic and delta-opioid receptors in spinal cord. J Neurosci 29:13264–13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MS, Schnell SA, Overland AC, Chabot-Doré AJ, Taylor AM, Ribeiro-da-Silva A, Elde RP, Wilcox GL, Stone LS. (2009) Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol 513:385–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo BL, Moises HC. (1998) mu-opioid receptor activation inhibits N- and P-type Ca2+ channel currents in magnocellular neurones of the rat supraoptic nucleus. J Physiol 513:787–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasusuki T, Yaksh TL. (2011) Regulation of spinal substance p release by intrathecal calcium channel blockade. Anesthesiology 115:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiseo PJ, Yaksh TL. (1993) Dose-dependent antagonism of spinal opioid receptor agonists by naloxone and naltrindole: additional evidence for delta-opioid receptor subtypes in the rat. Eur J Pharmacol 236:89–96 [DOI] [PubMed] [Google Scholar]
- Towett PK, Kanui TI, Maloiy GM, Juma F, Olongida Ole Miaron J. (2009) Activation of micro, delta or kappa opioid receptors by DAMGO, DPDPE, U-50488 or U-69593 respectively causes antinociception in the formalin test in the naked mole-rat (Heterocephalus glaber). Pharmacol Biochem Behav 91:566–572 [DOI] [PubMed] [Google Scholar]
- Tung AS, Yaksh TL. (1982) In vivo evidence for multiple opiate receptors mediating analgesia in the rat spinal cord. Brain Res 247:75–83 [DOI] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW. (2001) Equal proportions of small and large DRG neurons express opioid receptor mRNAs. J Comp Neurol 429:590–600 [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, et al. (2010) Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci USA 107:13117–13122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley PJ, Jeong HJ, Vaughan CW. (2010) Dissociation of μ- and δ-opioid inhibition of glutamatergic synaptic transmission in superficial dorsal horn. Mol Pain 6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. (1983) In vivo studies on spinal opiate receptor systems mediating antinociception. I. Mu and delta receptor profiles in the primate. J Pharmacol Exp Ther 226:303–316 [PubMed] [Google Scholar]
- Yaksh TL, Frederickson RC, Huang SP, Rudy TA. (1978) In vivo comparison of the receptor populations acted upon in the spinal cord by morphine and pentapeptides in the production of analgesia. Brain Res 148:516–520 [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Huang SP, Rudy TA. (1977) The direct and specific opiate-like effect of met5-enkephalin and analogues on the spinal cord. Neuroscience 2:593–596 [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Malmberg AB, Ro S, Schiller P, Goodman M. (1995) Characterization of the spinal antinociceptive activity of constrained peptidomimetic opioids. J Pharmacol Exp Ther 275:63–72 [PubMed] [Google Scholar]
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. (2001) An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol 90:2386–2402 [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.