Abstract
Adenylyl cyclase (AC) isoforms are implicated in several physiologic processes and disease states, but advancements in the therapeutic targeting of AC isoforms have been limited by the lack of potent and isoform-selective small-molecule modulators. The discovery of AC isoform-selective small molecules is expected to facilitate the validation of AC isoforms as therapeutic targets and augment the study of AC isoform function in vivo. Identification of chemical probes for AC2 is particularly important because there are no published genetic deletion studies and few small-molecule modulators. The present report describes the development and implementation of an intact-cell, small-molecule screening approach and subsequent validation paradigm for the discovery of AC2 inhibitors. The NIH clinical collections I and II were screened for inhibitors of AC2 activity using PMA-stimulated cAMP accumulation as a functional readout. Active compounds were subsequently confirmed and validated as direct AC2 inhibitors using orthogonal and counterscreening assays. The screening effort identified SKF-83566 [8-bromo-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepin-7-ol hydrobromide] as a selective AC2 inhibitor with superior pharmacological properties for selective modulation of AC2 compared with currently available AC inhibitors. The utility of SKF-83566 as a small-molecule probe to study the function of endogenous ACs was demonstrated in C2C12 mouse skeletal muscle cells and human bronchial smooth muscle cells.
Introduction
cAMP is a crucial component of signal transduction cascades that modulate diverse physiologic processes, including metabolism (Robison et al., 1968), cell growth and differentiation (Whitfield et al., 1979), learning and memory (Kandel, 2001), cardiac contractility (Drummond and Severson, 1979), and immune responses (Serezani et al., 2008). Cellular levels of cAMP are dynamically modulated by two families of enzymes: adenylyl cyclases (ACs), which synthesize cAMP from ATP (Hanoune and Defer, 2001), and phosphodiesterases, which degrade cAMP (Bender and Beavo, 2006).
The specificity of cAMP signaling is tightly regulated, in part by the repertoire and interplay of signaling molecules present within a given cell. A major contributing factor to this specificity is that nine membrane-bound mammalian AC isoforms have been identified, each with distinct patterns of regulation by G protein subunits, protein kinases, and Ca2+ (Hanoune and Defer, 2001; Patel et al., 2001). The AC isoforms are commonly activated by the stimulatory G protein but are categorized into four subgroups based on their sequence similarities and regulatory properties (Hanoune and Defer, 2001; Patel et al., 2001). The group I ACs (AC1, AC3, and AC8) are stimulated by Ca2+/calmodulin. In contrast, group II ACs (AC2, AC4, and AC7) are insensitive to Ca2+, but are conditionally activated by G protein Gβγ subunits. Group III ACs (AC5 and AC6) are inhibited by free Ca2+, Gαi/o subunits, and phosphorylation by protein kinase A. AC9, the lone member of group IV, is distinguished by its relative insensitivity to stimulation by forskolin.
Insight from knockout and transgenic mouse studies suggests that individual AC isoforms contribute to important physiologic processes and diseases (Sadana and Dessauer, 2009). For example, knockout and transgenic overexpression models implicate both AC5 and AC6 in cardiac function (Roth et al., 1999; Tepe et al., 1999; Okumura et al., 2003; Tang et al., 2008), Further, AC5−/− mice display an increased life span and show protective effects against age-related oxidative stress, apoptosis, loss of bone quality, and cardiomyopathy (Yan et al., 2007). Several ACs have been implicated in acute and chronic pain. Specifically, morphine-induced behavioral responses such as analgesia, dependence, tolerance, and withdrawal were absent in AC5−/− mice (Kim et al., 2006). In contrast, Ca2+/calmodulin-stimulated AC knockout mice (AC1−/−/AC8−/−) retained the short-term antinociceptive effects of morphine but showed attenuated tolerance and withdrawal (Li et al., 2006). Additional studies with AC1−/− and AC1−/−/AC8−/− mice also show attenuated behavioral responses in models of deep muscle, inflammatory, and neuropathic pain (Wei et al., 2002).
The aforementioned studies suggest that selective AC modulators have therapeutic utility for the treatment of conditions involving cardiac function, aging, and pain. As such, the AC5/AC6 inhibitors 1R,4R-3-(6-aminopurin-9-yl)-cyclopentanecarboxylic acid hydroxyamide (PMC-6) and Ara-A: Adenine 9-β-D-Arabinofuranoside (AraAde) have shown efficacy in preventing cardiomyocyte apoptosis (Iwatsubo et al., 2004) and a mouse model of heart failure (Iwatsubo et al., 2012). In addition, a small-molecule AC1 inhibitor, 5-[[2-(6-Amino-9H-purin-9-yl)ethyl]amino]-1-pentanol (NB001), has been reported to have analgesic effects in animal models of neuropathic and inflammatory pain (Vadakkan et al., 2006; Wang et al., 2011). Despite these beneficial effects, PMC-6, AraAde, and NB001 (and the AC inhibitor class as a whole) require more rigorous and comprehensive characterization regarding target specificity and AC isoform selectivity (Seifert et al., 2012; Braeunig et al., 2013). Moreover, a dearth of isoform-selective small-molecule AC modulators has limited the study of AC isoforms as therapeutic targets (Pierre et al., 2009; Seifert et al., 2012). For example, AC2 is potentially involved in skeletal muscle physiology, lung diseases, neuroendocrine tumors (NETs), and colorectal cancer (Duerr et al., 2008; Drozdov et al., 2011; Yu et al., 2011; Berdeaux and Stewart, 2012); yet the pharmacological study of AC2 is difficult because most small-molecule AC inhibitors preferentially inhibit other AC isoforms (Pierre et al., 2009; Seifert et al., 2012). Boron-dipyrromethene (BODIPY)-forskolin appears to be the most potent AC2 inhibitor, but its use as a chemical probe for AC2 activity is hindered because it also partially activates AC1 and AC5 (Pinto et al., 2008; Erdorf et al., 2011). Given the shortcomings of small-molecule AC modulators and the absence of published reports involving AC2 knockout animals, the identification of selective AC2 modulators is expected to provide useful chemical probes to facilitate the study of AC2.
The present report describes the development and execution of a cell-based screening approach for the discovery of novel small-molecule inhibitors of AC2. We screened the NIH clinical collections I and II (727 compounds) for small molecules that inhibit cAMP accumulation in response to selective activation of AC2. Compounds identified as active were examined in a series of confirmation assays to validate direct AC2 inhibition and define their AC isoform-selectivity profiles. Our studies have resulted in the identification of SKF-83566 as a selective AC2 inhibitor that is expected to be a promising tool to investigate the physiologic roles of AC2.
Materials and Methods
The NIH clinical collections I and II were purchased from Evotec, Inc. (South San Francisco, CA). Oxymetholone, tranilast, amlexanox, duloxetine, and indatraline were purchased from Sequoia Research Products (Pangbourne, UK). [3H]cAMP was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). A23187, 3-isobutyl-1-methylxanthine (IBMX), loratadine, prochlorperazine, maprotiline, thioridazine, G418, Dulbecco’s modified Eagle’s medium (DMEM), and trichloroacetic acid (TCA) were purchased from Sigma-Aldrich (St. Louis, MO). Phorbol 12-myristate 13-acetate (PMA), forskolin, (6)-N-[(1R*,2R*)-2-phenylcyclopentyl]-azacyclotridec-1-en-2-amine hydrochloride (MDL-12,330A HCl) prostaglandin E2 (PGE2), [9-(tetrahydro-2-furanyl)-9H-purin-6-amine] (SQ22,536), and (±)-SKF-83566 HBr were purchased from Tocris Bioscience (Ellisville, MO). 2′5′-Dideoxyadenosine (2′5′-ddAD) was purchased from Santa Cruz Biotechnology (Dallas, TX). Lipofectamine 2000, OPTI-MEM, and antibiotic-antimycotic 100× solution were purchased from Life Technologies (Grand Island, NY). FetalClone I serum, bovine calf serum, HEPES, and Hanks’ balanced salt solution (HBSS) were purchased from Hyclone (Logan, UT). BisindoloylmaleimideI (BisI) was purchased from Calbiochem (La Jolla, CA). The HTRF cAMP and Cellul’ERK kits were purchased from Cisbio Bioassays (Bedford, MA).
Stable Cell Line Generation and Cell Culture Conditions.
Human embryonic kidney (HEK) 293 cells were cultured in DMEM supplemented with 5% bovine calf serum, 5% fetal clone I, and 1% antibiotic-antimycotic 100× solution and maintained in a humidified incubator at 37°C and 5% CO2. For generation of a clonal stable cell line, HEK293 cells were transfected with pcDNA3.1(+) encoding human AC1, AC2, or AC5 using Lipofectamine 2000 according to the manufacturer’s protocol. Stable clones were selected by growth in media containing 600 μg/ml (AC2) or 800 μg/ml (AC1 and AC5) G418. Stable expression of AC isoforms was confirmed functionally by measuring cAMP accumulation in response to selective pharmacological activation conditions. For example, AC1 was stimulated with 3 µM A23187, AC2 was stimulated with the phorbol ester PMA, and AC5 was activated by 300 nM forskolin.
The C2C12 mouse skeletal muscle cell line was purchased from the American Type Culture Collection (Manassas, VA). C2C12 myoblasts were maintained at a low confluency in DMEM media containing 10% fetal bovine serum. Myoblasts (passages 3–17) were plated in 96-well format at 5 × 104 cells/well. Differentiation into myotubes was induced once the cells reached 90% confluence by switching to medium supplemented with 2% horse serum. The growth medium was changed every 24 hours. Myotubes were allowed to mature for 5 days before the experiments were completed.
Human bronchial smooth muscle cells (hBMSCs) were purchased from LonzaBio (Basel, Switzerland) and were grown in smooth muscle basal medium supplemented with the SmGM-2 bullet kit (5% fetal bovine serum, 0.1% insulin, 0.1% human epidermal growth factor, 0.2% human fibroblast growth factor-B, and gentamicin sulfate/amphotericin B; LonzaBio). Cells were kept at 5% CO2 and 37°C, and experiments were performed on cells from passages 5–13.
Cisbio HTRF cAMP Assay.
The cellular cAMP levels were measured using either the Cisbio HTRF cAMP dynamic 2 assay kit or a dynamic 2/HiRange hybrid kit (consisting of cAMP-d2 from the dynamic 2 kit and the anti-cAMP cryptate conjugate from the HiRange kit). The cAMP assays were performed on cryopreserved cells that were rapidly thawed at 37°C and resuspended in cell suspension buffer (HBSS, 20 mM HEPES, 0.1% fatty acid–free BSA or OPTI-MEM for HEK-hAC1 cells). Cells were centrifuged at 500g, and the supernatant was aspirated. Cells were washed by resuspending in cell suspension buffer and centrifuged at 500g. The supernatant was aspirated, and cells were seeded into a 384-well plate and allowed to incubate at 37°C and 5% CO2 for 2.5 hours. Cells were then treated as indicated with ligands diluted in stimulation buffer (HBSS, 20 mM HEPES, 500 µM IBMX or OPTI-MEM, 500 µM IBMX for HEK-hAC1 cells) and incubated for 1 hour at room temperature. The stimulation was terminated by sequential addition of 10 μl/well cAMP-d2 and 10 µl/well anti-cAMP cryptate conjugate, each diluted (1:39) in lysis buffer. The experiments that used the dynamic 2 kit for cAMP detection were performed without IBMX in the stimulation buffer (to accommodate the sensitivity for cAMP detection), but with IBMX in the lysis buffer (to prevent phosphodiesterase-mediated degradation of cAMP in the lysate). After a 1-hour incubation at room temperature, the time-resolved fluorescence energy transfer (TR-FRET) was measured with a lag time of 100 μs and an integration time of 300 μs using a Synergy4 (BioTek, Winooski, VT) fluorescence plate reader (excitation filter: 330/80 nm and emission filters: 620/10 nm and 665/8 nm). The resulting cAMP concentrations were calculated in GraphPad Prism (GraphPad Software, La Jolla, CA) by applying the 620/665 nm fluorescence ratio values to a standard curve of known cAMP concentrations.
Screening Conditions.
Cryopreserved HEK-hAC2 cells were seeded into a 384-well plate at 15 μl/well using a MultiFlo (BioTek) bulk reagent dispenser. After a 2.5-hour incubation at 37°C and 5% CO2, the cell plates were allowed to equilibrate to room temperature on the bench for 15 minutes. Test compounds (80 nl) were added to the cells with a MultiPette-mounted 384-well pin tool and allowed to incubate at room temperature for 30 minutes. AC2 activity was then stimulated by the addition of 5 µl of PMA (50 nM final concentration) diluted in stimulation buffer with the MultiFlo reagent dispenser, followed by incubation at room temperature for 1 hour. The Cisbio HTRF cAMP dynamic 2 kit was used to quantify the cellular cAMP as described already. Briefly, the cAMP-d2 (containing 500 μM IBMX final volume) and anti-cAMP cryptate conjugate working reagents were sequentially added (10 μl/well each) with a MultiFlo reagent dispenser and incubated at room temperature for 1 hour. Test compounds were initially screened in singlet or duplicate, and all cAMP concentrations were converted to percent inhibition of the PMA-stimulated cAMP response.
[3H]cAMP Assay.
Cryopreserved HEK-hAC2 cells were thawed and prepared as described already. Cells were seeded at a density of 12,000 cells/well into a 384-well plate in cell suspension buffer and incubated for 2.5 hours at 37°C and 5% CO2. Test compounds were added to the cells with a MultiPette-mounted 384-well pin tool and allowed to incubate at room temperature for 30 minutes. The AC stimulation was carried out at room temperature for 1 hour, and the reaction was stopped by the addition of cold (4°C) TCA to provide a final TCA concentration of 3%. The cAMP in the lysate was then quantified using a [3H]cAMP competition assay as previously described (Przybyla and Watts, 2010). C2C12 cell cAMP experiments were performed similarly, with the exceptions that the assay was performed in 96-well format on continuously propagated cells before differentiation into myotubes, and compounds were delivered by multichannel pipette for both pretreatment and stimulation steps.
Phospho-p44/42 Mitogen-Activated Protein Kinase ERK1/2 Phosphorylation Assay.
HEK-hAC2 cells were seeded into 96-well plates at a density of 25,000 cells/well in OPTI-MEM and incubated overnight at 37°C and 5% CO2. Drug treatment was carried out in OPTI-MEM as follows. Cells were pretreated with test compound (30 µM) at 37°C for 10 minutes, and ERK1/2 phosphorylation was stimulated by the addition of PMA (50 nM) and incubation for 10 minutes at 37°C. The resulting ERK1/2 phosphorylation was measured using the Cisbio HTRF Cellul’ERK assay according to the manufacturer’s protocol (two-plate protocol). In brief, the stimulation buffer was decanted and supplemented lysis buffer was added, followed by shaking at 500 rpm at room temperature for 30 minutes. The anti-ERK-Eu3+-cryptate and anti-phospho-ERK-d2 antibodies were combined and added to a 384-well low-volume plate (white, Proxiplate 384 Plus; PerkinElmer Life and Analytical Sciences). Lysate from the stimulation or the positive or negative control lysate was then added on top of the HTRF reagents and incubated for 2 hours at room temperature. The TR-FRET was then measured on the Synergy4 (BioTek) plate reader.
Spodoptera frugiperda Cell Line Membrane AC Activity Assay.
Membranes from the Spodoptera frugiperda cell line, Sf9 cells, expressing AC1, AC2, or AC5 were prepared as previously described (Taussig et al., 1994). All AC activity assays were performed for 10 minutes at 30°C in a final volume of 50 µl. The final concentrations of magnesium chloride (MgCl2) and Mg-ATP in the reaction were 5 mM and 200 µM, respectively. Inhibitors were solubilized in DMSO and incubated with AC-containing membranes (15 µg) for 10 minutes on ice before the start of the reaction. The final concentration of DMSO in the reaction did not exceed 3% for either vehicle or inhibitors. Reactions were initiated upon addition of a reaction mix containing [α-32P]ATP and 30 µM forskolin. The reactions were terminated with stop solution (2.5% SDS, 50 mM ATP, and 1.75 mM cAMP), and the products were separated by sequential chromatography on Dowex-50 and aluminum oxide (Al2O3) (Dessauer, 2002). Mechanistic studies of inhibition were performed as described, but with membranes from Sf9 cells expressing AC2 (5 µg), 100 nM Gαs, and varying concentrations of Mg-ATP.
Interleukin-6 mRNA Expression in Human Bronchial Smooth Muscle Cells.
Human bronchial smooth muscle cells (HBSMCs) were pretreated with 30 µM inhibitor or vehicle for 20 minutes before 1-hour treatment with 1 µM forskolin or vehicle. Quantitative reverse-transcription polymerase chain reaction (PCR) for interleukin-6 (IL-6) was then performed using the primer pairs: GAC AGC CAC TCA CCT CTT CA (IL-6 forward) and AGT GCC TCT TTG CTG CTT TC (IL-6 reverse). The total RNA was isolated using the RNeasy kit with on-column DNase step (Qiagen Inc, Valencia, CA). RNA purity and yield were determined with a Nanodrop spectrophotometer, and 1 µg RNA was reverse-transcribed using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Indianapolis, IN) and oligo (dT)18 primer. PCR was carried out on Roche Lightcycler 480 and amplification was detected by SYBR green (KAPA Biosystems, Woburn, MA). Single PCR products were confirmed by melting-curve analysis, and the fold regulation was calculated by the ΔΔCt method with normalization to the RPL13A housekeeping gene.
Results
Assay Development and Screening of the NIH Clinical Collections I and II.
The lack of robust inhibitors of AC2 (Pavan et al., 2009; Pierre et al., 2009; Seifert et al., 2012) and the absence of published reports on AC2−/− mice suggest that the discovery of AC2 inhibitors will provide important research tools. Thus, one initial goal of the present study was to develop an approach to identify and validate novel inhibitors of AC2 activity in intact cells. To achieve this goal, we developed and optimized assay parameters for the measurement of intracellular cAMP in 384-well format in a semiautomated fashion, a tactic that may ultimately allow for large-scale high-throughput screening (HTS) to identify novel AC2 inhibitors.
HEK293 cells are known to express endogenously multiple AC isoforms (Hellevuo et al., 1993; Ludwig and Seuwen, 2002). Therefore, to study AC2 modulation specifically, it is important to identify pharmacological stimulation conditions that selectively activate recombinant AC2 when expressed in HEK293 cells. We and others have previously reported that the protein kinase C (PKC)-activating phorbol ester PMA selectively stimulates cAMP accumulation in HEK293 cells stably expressing recombinant rat AC2 (Yoshimura and Cooper, 1993; Cumbay and Watts, 2001). For this study, HEK293 cells stably expressing human AC2 (hAC2) were constructed and screened for cAMP accumulation in response to PMA. It is notable that the basal level of cAMP in the HEK-hAC2 cells was higher than the HEK-wt cells (data not shown) and is likely due to constitutive activity of AC2, a property that has been previously observed (Pieroni et al., 1995; Pinto et al., 2008). As expected, PMA treatment had no effect on cAMP in HEK-wt cells but provided an ∼8-fold enhancement of cAMP in HEK-hAC2 cells (data not shown and Fig. 1A). These results suggest that recombinant hAC2 can be selectively activated by PMA in an HEK293 cell background.
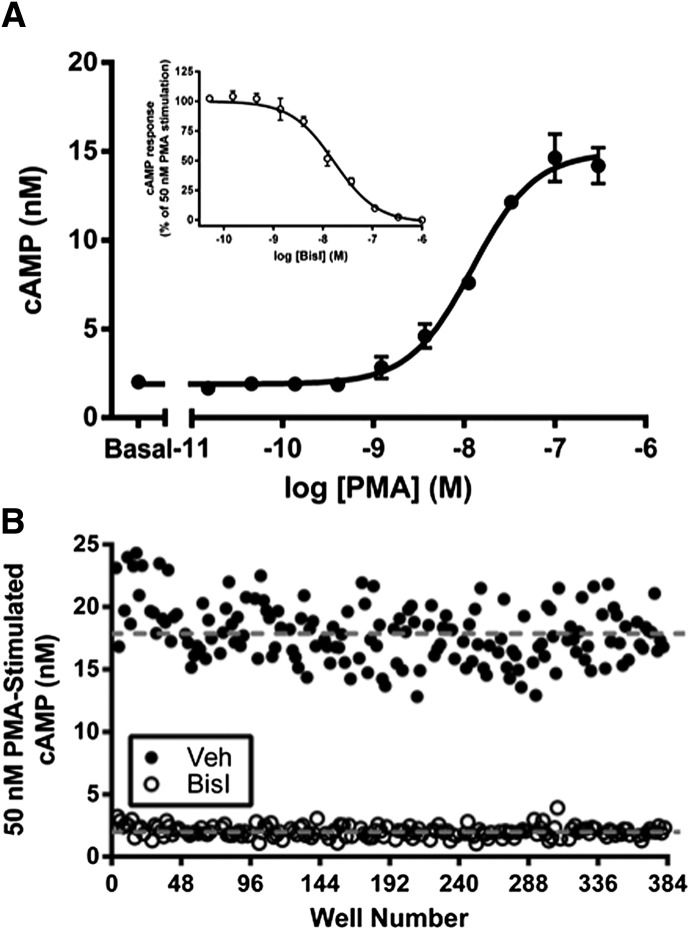
Fig. 1.
Optimization of conditions for an intact-cell assay that is capable of high-throughput screening for small-molecule inhibitors of AC2. (A) Concentration-response curve analysis of PMA for stimulation of an AC2-mediated cAMP response in HEK-hAC2 cells. Data are the mean ± S.E.M. of three independent experiments. Inset: Inhibition of 50 nM PMA-stimulated AC2 activity with the PKC inhibitor BisI. Data are the mean ± S.E.M. of three independent experiments. (B) Evaluation of assay robustness by Z′ analysis. Data are representative of three independent experiments.
After verification of PMA treatment as a strategy for selective activation of AC2, potential screening parameters were further explored by performing a more in-depth evaluation of the effects of PMA in HEK-hAC2 cells. PMA treatment provided a concentration-dependent increase in cAMP with an EC50 value of 16 ± 5.0 nM (n = 3) (Fig. 1A). We chose to use 50 nM PMA (∼EC85 concentration) to stimulate AC2 for the study of AC2 inhibitors. As a control for the inhibition of AC2 activity, the PKC inhibitor BisI was used to inhibit the phorbol ester–mediated activation of AC2. Treatment with BisI provided full inhibition of PMA-stimulated AC2 activity with an IC50 of 16 ± 1.9 nM (n = 3), suggesting that 1 μM BisI is sufficient to inhibit completely AC2 activity stimulated by 50 nM PMA (Fig. 1A, inset).
Ultimately, our approach for the identification of AC inhibitors relies on the development of a cell-based assay that is capable of high-throughput screening of small-molecule libraries. Therefore, it was important to evaluate the robustness of the HEK-hAC2 cell cAMP assay when converted to a semiautomated format that is amenable to high-throughput screening (see Materials and Methods). Specifically, assay robustness was examined by performing a Z′ analysis for the assay parameters developed for screening (Zhang et al., 1999). The Z′ value was calculated using 50 nM PMA as the maximum stimulation control and 1 μM BisI as the minimum stimulation control. Our AC2 screening assay provided a Z′ = 0.44 ± 0.02 (n = 3), suggesting that the assay is appropriate for small-molecule library screening (Fig. 1B) (National Institutes of Health Chemical Genomics Center Assay Guidance Manual, www.ncbi.nlm.nih.gov/books/NBK53196/).
The NIH clinical collections I and II consist of 727 total test compounds that have a history of use in human clinical trials (www.nihclinicalcollection.com). The collections contain drug-like molecules with documented biologic activity and safety profile information. The modest size of the collections, paired with the reasons stated, make the NIH clinical collections a good starting collection for early screening efforts in the search for AC2 inhibitors. The NIH clinical collections were screened for the ability of test compounds (25 μM) to inhibit PMA-stimulated AC2 activity in HEK-hAC2 cells. Of the 727 compounds screened, 10 compounds identified as active for the inhibition of PMA-stimulated cAMP accumulation in HEK-hAC2 cells (each displaying >30% inhibition at 25 µM) were chosen for confirmation and validation of activity (Table 1; see Supplemental Table 1 for screening results for all compounds that provided at least 10% inhibition).
TABLE 1.
Screening of NIH Clinical Collections I and II for inhibition of AC2 activity
The NIH Clinical Collections I and II (25 µM) were screened for inhibition of PMA-stimulated (50 nM) cAMP accumulation in HEK-hAC2 cells using the Cisbio HTRF cAMP dynamic 2 detection method. The data represent the average percent inhibition of the PMA-stimulated cAMP response from duplicate plates (see Materials and Methods).
| Compound Name | Inhibition |
|---|---|
| % | |
| SKF-83566 | 85 |
| Tranilast | 69 |
| Loratadine | 64 |
| Thioridazine | 58 |
| Duloxetine | 51 |
| Amlexanox | 41 |
| Indatraline | 39 |
| Oxymetholone | 37 |
| Prochlorperazine | 35 |
| Maprotiline | 33 |
Confirmation and Validation of Activity.
Confirmation studies were carried out using freshly prepared powders that were purchased from commercial sources. The initial confirmation of active small molecules used a single concentration (30 µM) of the test compounds for the inhibition of AC2 activity. HEK-hAC2 cells were incubated with 50 nM PMA to activate AC2 selectively in the presence of test compound, and the resulting cAMP accumulation was measured with the Cisbio HTRF cAMP dynamic 2 kit (identical to the assay format used for small-molecule library screen). All test compounds provided inhibition of AC2 activity at 30 μM, confirming the activity observed in the initial screen (Table 2).
TABLE 2.
Confirmation of the inhibitory activity of test compounds identified in the screen of NIH Clinical Collections I and II
Active compounds (30 µM) were tested for inhibition of PMA-stimulated cAMP in HEK-hAC2 cells using either the Cisbio HTRF cAMP dynamic 2 technology or a [3H]cAMP competition method for detection of cAMP. Data are reported as the mean ± S.E.M. of the percent inhibition of the PMA response from three independent experiments.
| Compound Name | Inhibition |
|
|---|---|---|
| TR-FRET | [3H]cAMP | |
| % | ||
| SKF-83566 | 95 ± 1.6 | 94 ± 2.8 |
| Tranilast | 76 ± 2.9 | 79 ± 5.3 |
| Loratadine | 61 ± 0.5 | 71 ± 8.1 |
| Thioridazine | 36 ± 5.7 | ND |
| Duloxetine | 39 ± 3.8 | ND |
| Amlexanox | 45 ± 5.7 | ND |
| Indatraline | 60 ± 2.0 | 53 ± 5.5 |
| Oxymetholone | 58 ± 1.9 | 58 ± 2.9 |
| Prochlorperazine | 41 ± 8.3 | ND |
| Maprotiline | 26 ± 1.8 | ND |
ND, not determined.
The screening assay and initial confirmation of active compounds rely on TR-FRET for the detection of cAMP (see Materials and Methods). It is possible that the active compounds have inherent fluorescence that can be measured in the same wavelengths as those used for cAMP detection, thereby skewing the measured fluorescence and affecting the resulting estimation of the cAMP concentration (Degorce et al., 2009). Thus, we assessed the ability of the best compounds (i.e., those that provided at least 50% inhibition in the confirmation assay) to inhibit AC2 activity in a non–fluorescence-based assay. The active compounds SKF-83566, oxymetholone, tranilast, indatraline, and loratadine were tested for their ability to inhibit PMA-stimulated cAMP accumulation in a [3H]cAMP competitive binding assay. All of the compounds tested retained the ability to inhibit PMA-stimulated cAMP accumulation in HEK-hAC2 cells, indicating bona fide reduction of cAMP rather than interference with the fluorescence detection methods (Table 2). Furthermore, the extent of inhibition of PMA-stimulated AC2 activity by the test compounds in the [3H]cAMP assay was nearly identical to that observed in the TR-FRET-based cAMP detection method.
Our screening strategy used PMA to stimulate AC2 selectively via phosphorylation mediated by PKC (Jacobowitz and Iyengar, 1994). Thus, it is possible that the compounds identified as active may exert their cAMP-attenuating effects through inhibition of PKC rather than directly inhibiting AC2. PMA-mediated PKC activation is known to stimulate ERK1/2 phosphorylation in HEK293 cells (DellaRocca et al., 1997), allowing for a simple counterscreen to eliminate false positives (Fig. 2A). Specifically, we measured ERK1/2 phosphorylation in response to PKC activation in HEK-hAC2 cells in the absence and presence of the active compounds (Fig. 2B). As expected, PMA treatment resulted in a significant enhancement of ERK1/2 phosphorylation (5.7 ± 0.2-fold over basal) that was inhibited completely by the PKC inhibitor BisI. In contrast, SKF-83566, oxymetholone, tranilast, and loratadine did not significantly alter the PMA-mediated ERK1/2 phosphorylation in HEK-hAC2 cells, suggesting that these compounds do not inhibit PKC. Indatraline, however, inhibited the PMA-mediated ERK1/2 phosphorylation by ∼80%.
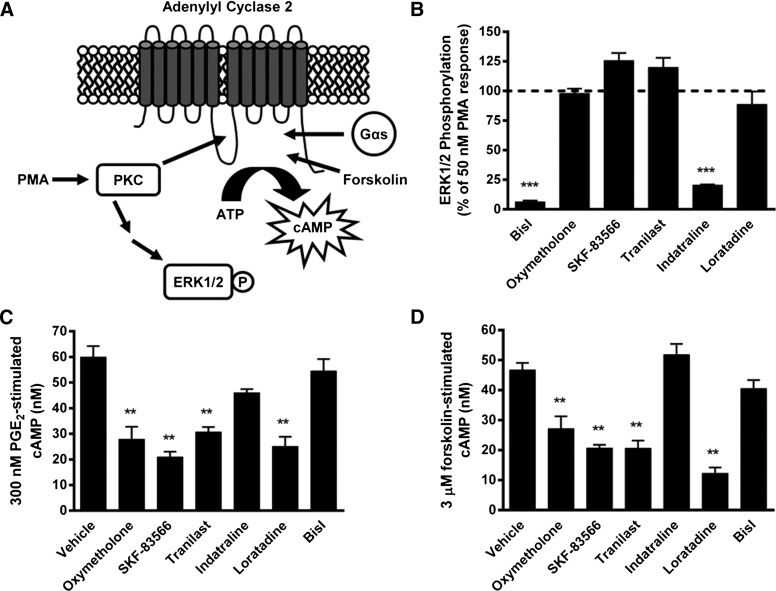
Fig. 2.
Counterscreening for validation of active compounds as AC2 inhibitors. (A) Schematic for PKC-dependent and PKC-independent activation of AC2. (B) The effects of test compounds (30 µM) on PMA-stimulated ERK1/2 phosphorylation were measured in HEK-hAC2 cells. Data are mean ± S.E.M. of three independent experiments. ***P < 0.001 (one sample t test compared with 100). (C) The effects of test compounds (30 µM) on 300 nM PGE2-stimulated cAMP accumulation and (D) 3 µM forskolin-stimulated cAMP accumulation was measured in HEK-hAC2 cells. Data are mean ± S.E.M. of three independent experiments. **P < 0.01, compared with vehicle condition, one-way analysis of variance followed by Dunnett’s post hoc test.
The lack of inhibition of PKC-dependent ERK1/2 phosphorylation by SKF-83566, oxymetholone, tranilast, and loratadine is consistent with a direct inhibition of AC2 activity by these drugs. To test this supposition further, we examined the ability of SKF-83566, oxymetholone, tranilast, and loratadine to inhibit AC2 activity stimulated via other mechanisms. AC2 is also stimulated in a PKC-independent manner by Gαs in response to activation of Gs-coupled receptors and directly via the small molecule AC activator forskolin. PGE2 is known to bind and activate the Gs-coupled EP2/4 prostanoid receptors that are endogenously expressed in HEK293 cells (Willoughby et al., 2007; Bogard et al., 2012). As expected, PGE2 treatment resulted in a concentration-dependent increase in cAMP accumulation in HEK-hAC2 cells (EC50: 160 ± 78 nM, n = 3). To examine the effects of inhibitors on Gαs-stimulated AC2 activity, the identified active compounds were then tested for the inhibition of 300 nM PGE2-stimulated cAMP in HEK-hAC2 cells (Fig. 2C). Oxymetholone, SKF-83566, tranilast, and loratadine significantly inhibited PGE2-stimulated cAMP in HEK-hAC2 cells. Next, they were similarly tested for their ability to inhibit the cAMP generated in response to direct AC stimulation by forskolin (Fig. 2D). Oxymetholone, SKF-83566, tranilast, and loratadine significantly inhibited forskolin-stimulated cAMP accumulation in HEK-hAC2 cells. These data are in agreement with the effects of the test compounds on PKC-mediated ERK1/2 phosphorylation. Specifically, indatraline and BisI inhibited PKC-mediated ERK1/2 phosphorylation but not Gαs- or forskolin-stimulated cAMP in HEK-hAC2 cells. Taken together, these observations suggest that inhibition of PMA-stimulated cAMP by indatraline in HEK-hAC2 cells is due to inhibition of PKC. More importantly, our data indicate that SKF-83566, oxymetholone, tranilast, and loratadine inhibit multiple modes of AC2 stimulation (i.e., PKC-, Gαs-, and forskolin-mediated) but do not inhibit PKC activity.
AC Isoform-Selectivity Profiles.
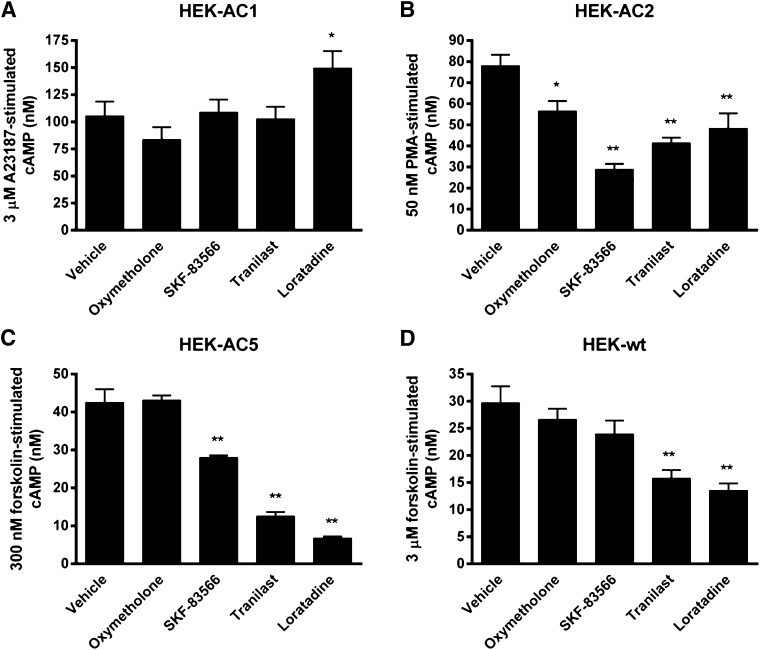
The selectivity profiles of the active compounds for inhibition of AC isoforms were explored using intact cell cAMP assays. The ability of compounds to modulate cAMP levels in HEK-hAC2 cells was compared with that of HEK-hAC1 and HEK-hAC5 cells, as well as HEK-wt cells (Fig. 3). AC1 and AC5 were chosen as representative ACs from group I and group III ACs, respectively. AC1 activity was selectively activated by the calcium ionophore A23187 (3 µM) and AC5 was stimulated by 300 nM forskolin in HEK293 cells stably expressing each isoform (data not shown). Test compounds (30 µM) were evaluated for the ability to modulate selective activation of AC1 and AC5 activity in HEK293 cells. None of the test compounds inhibited AC1 activity; however, loratadine significantly potentiated A23187-stimulated cAMP by ∼150%. Studies with HEK-hAC5 cells revealed that loratadine and tranilast strongly inhibited forskolin-stimulated cAMP in HEK-hAC5 cells, whereas SKF-83566 had more modest activity (∼35% inhibition). Tranilast and loratadine also significantly inhibited forskolin-stimulated AC activity in HEK-wt cells, whereas SKF-83566 had no significant effect. Oxymetholone modestly but significantly inhibited PMA-stimulated cAMP in HEK-hAC2 cells, but it had no effect on the AC responses in HEK-hAC1, -hAC5, or -wt cells. These results suggest that the active compounds show distinct patterns of cAMP modulation in HEK293 cells stably expressing recombinant AC1, AC2, or AC5.
Fig. 3.
AC isoform-selectivity profile of test compounds in intact-cell studies. AC isoform selectivity was assessed by testing the ability of test compounds (30 µM) to modulate (A) 3 µM A23187-stimulated cAMP in HEK-hAC1 cells; (B) 50 nM PMA-stimulated cAMP in HEK-hAC2 cells; (C) 300 nM forskolin-stimulated cAMP in HEK-hAC5 cells; and (D) 3 µM forskolin-stimulated cAMP in HEK-wt cells. *P < 0.05; **P < 0.01 compared with vehicle condition, one-way analysis of variance followed by Dunnett’s post hoc test.
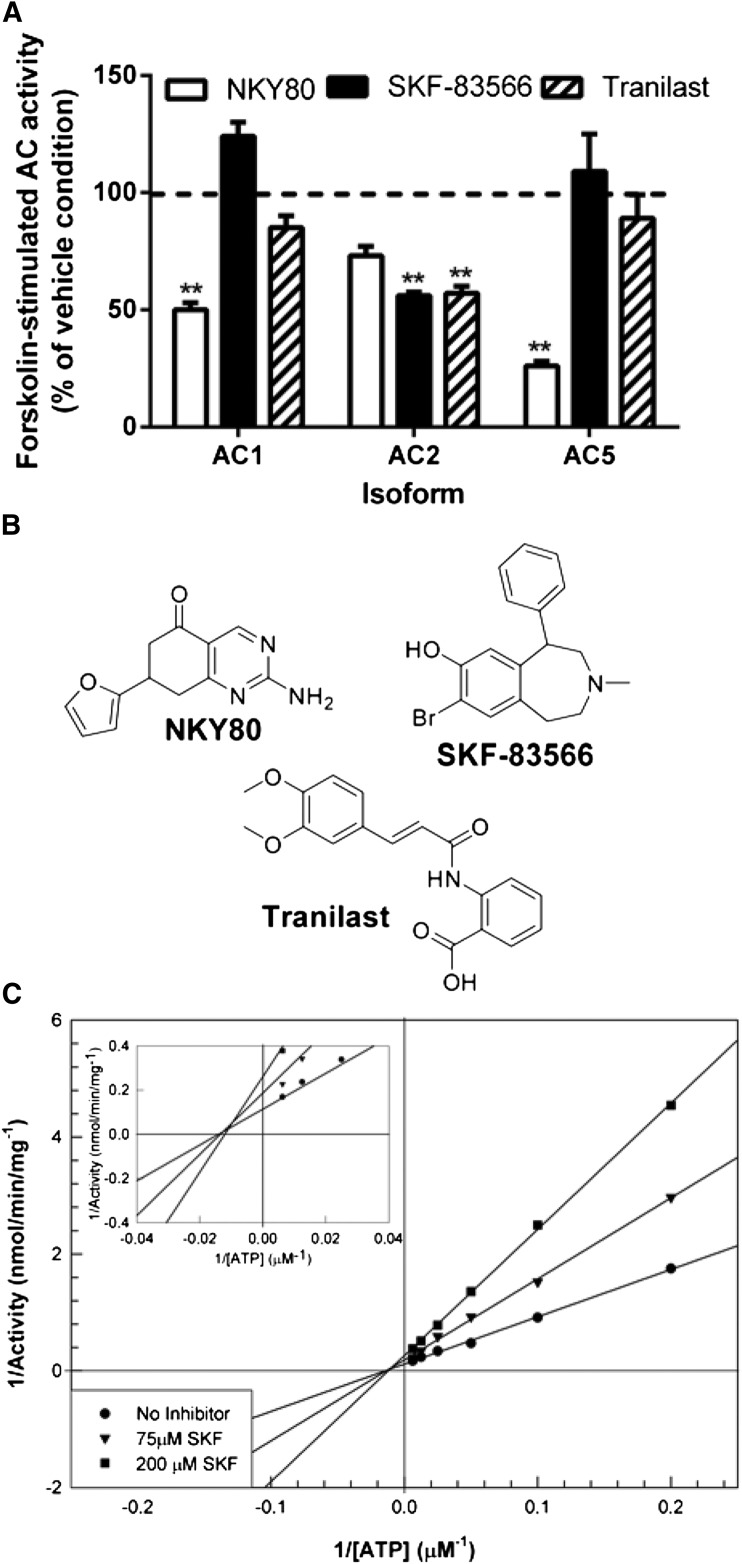
The direct modulation of AC isoforms was explored using a cell-free, reconstituted system to assess directly the effects of test compounds on AC activity. Specifically, the effects of the test compounds were studied in AC activity assays that were performed using membranes from Sf9 insect cells expressing recombinant AC1, AC2, or AC5. As expected, SKF-83566 significantly inhibited forskolin-stimulated AC2 activity (>40%), suggesting a direct mode of inhibition (Fig. 4A). SKF-83566 was inactive against AC1 or AC5. A similar pattern of activity was observed with tranilast (Fig. 4A). In contrast to SKF-83566 and tranilast, a commercially available AC inhibitor, 2-Amino-7-(furanyl)-7, 8-dihydro-5(6H)-quinazolinone (NKY80), showed marked inhibition of AC1 and AC5 but only modest inhibition of AC2 activity (Fig. 4A; see Fig. 4B for chemical structures). These studies identified SKF-83566 as the most favorable compound to carry into further studies, as it provided the most robust AC2 inhibition while having no effect on forskolin-stimulated cAMP levels in HEK-wt cells and retention of AC2 inhibition in vitro. To determine the mechanism for SKF-83566 inhibition of AC2 activity, Gαs-stimulated AC2 membranes were tested under varying concentrations of ATP in the absence or presence of 75 µM or 200 µM SKF-83566. The predicted Vmax values changed with increasing concentrations of SKF-83566, but the predicted Km remained relatively stable (Fig. 4C), suggesting that SKF-83566 is a noncompetitive AC2 inhibitor.
Fig. 4.
Inhibition of AC activity in vitro. (A) The effects of 100 µM NKY80, SKF-83566, and tranilast on forskolin-stimulated (30 µM) AC activity were measured in membranes of Sf9 cells expressing AC1, AC2, or AC5. **P < 0.01 (t test compared with vehicle condition). (B) Chemical structures of NKY80, SKF-83566, and tranilast. (C) Double-reciprocal plot of AC activity from Sf9 membranes expressing AC2 (5 µg) in the presence of 100 nM Gαs and in the absence or presence of SKF-83566 (75 µM or 200 µM) and the indicated concentrations of ATP. The data suggest that SKF-83566 noncompetitively inhibited AC2 activity with respect to ATP.
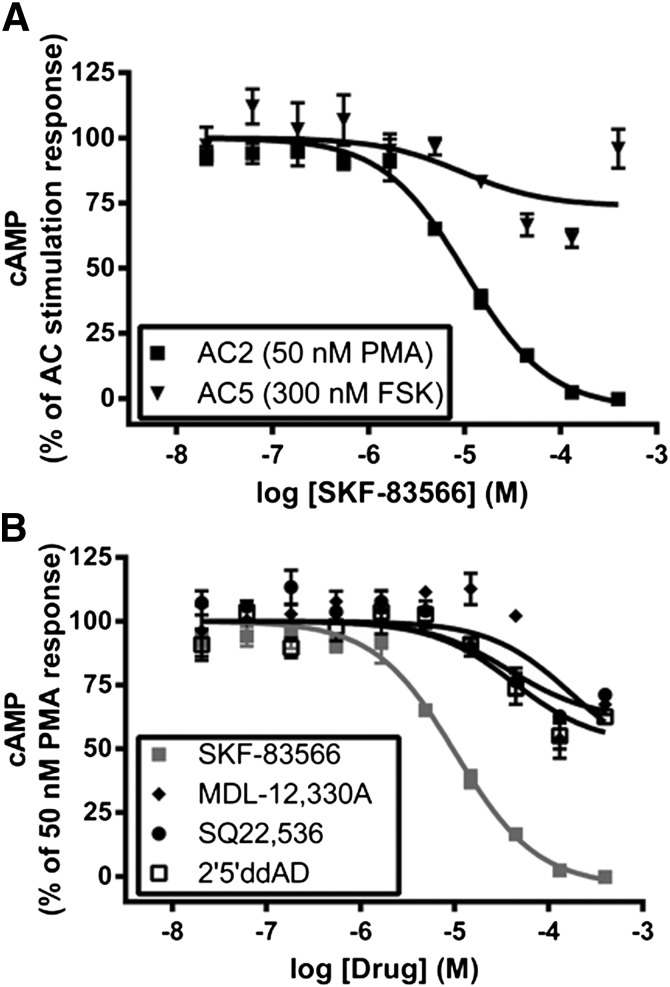
The AC isoform selectivity of SKF-83566 was further characterized by performing a concentration-response analysis for inhibition of cAMP in HEK-hAC2 and HEK-hAC5 cells. SKF-83566 fully inhibited PMA-stimulated cAMP in HEK-hAC2 cells with an IC50 value of 10 ± 1.4 µM and maximum inhibition of 104 ± 2% (Fig. 5A). Additionally, the potency and efficacy values of SKF-83566 for inhibition of 3 µM forskolin stimulation (IC50: 19 ± 3.3 µM and maximum inhibition: 113 ± 2%, n = 3) and 300 nM PGE2 stimulation (IC50: 21 ± 4.5 µM and maximum inhibition: 117 ± 2%, n = 3) in HEK-hAC2 cells were similar to those observed for inhibition of the PMA response in HEK-hAC2 cells. The robust inhibition below basal levels presumably reflects inhibition of the constitutive AC2 activity. As anticipated from the single-point studies, SKF-83566 only partially inhibited forskolin-stimulated cAMP in HEK-hAC5 cells (<40%) at a concentration of 130 µM. We consistently observed less inhibition of AC5 at 400 µM, suggesting a biphasic response (Fig. 5A). Nonetheless, these results, taken together with the results presented in Fig. 4A, indicate marked selectivity of SKF-83566 for inhibition of AC2 over AC1 and AC5.
Fig. 5.
Concentration-response analysis of SKF-83566 for inhibition of cAMP. (A) Dose-response curves of SKF-83566 for inhibition of PMA-stimulated (50 nM) cAMP in HEK-hAC2 cells and forskolin-stimulated (300 nM) cAMP in HEK-hAC5 cells. (B) Dose-response curves of SKF-83566, MDL-12,330A, SQ 22,536, and 2′5′-ddAD for inhibition of PMA-stimulated (50 nM) cAMP in HEK-hAC2 cells. The SKF-83566 data in (A) and (B) are from the same experiments; these studies were performed simultaneously. Data are expressed as a percentage of the stimulation response and are reported as the mean ± S.E.M. of three independent experiments.
The ability of SKF-83566 to inhibit PMA-stimulated AC2 activity was directly compared with several known AC inhibitors (i.e., SQ 22,536, MDL-12,330A, and 2′5′-ddAD; Fig. 5B). Efficacy comparisons revealed marked differences between SKF-83566 (>100% inhibition) and the known inhibitors (i.e., SQ22,536, 29 ± 3%; MDL-12,330A, 33 ± 4%; and 2′5′-ddAD, 38 ± 3%), each at a concentration of 400 µM. These results demonstrate that SKF-83566 displays superior potency and efficacy for inhibition of AC2 activity when directly compared with SQ 22,536, MDL12,330A, and 2′5′-ddAD in HEK-hAC2 cells.
SKF-83566 as a Tool to Probe AC2 Function.
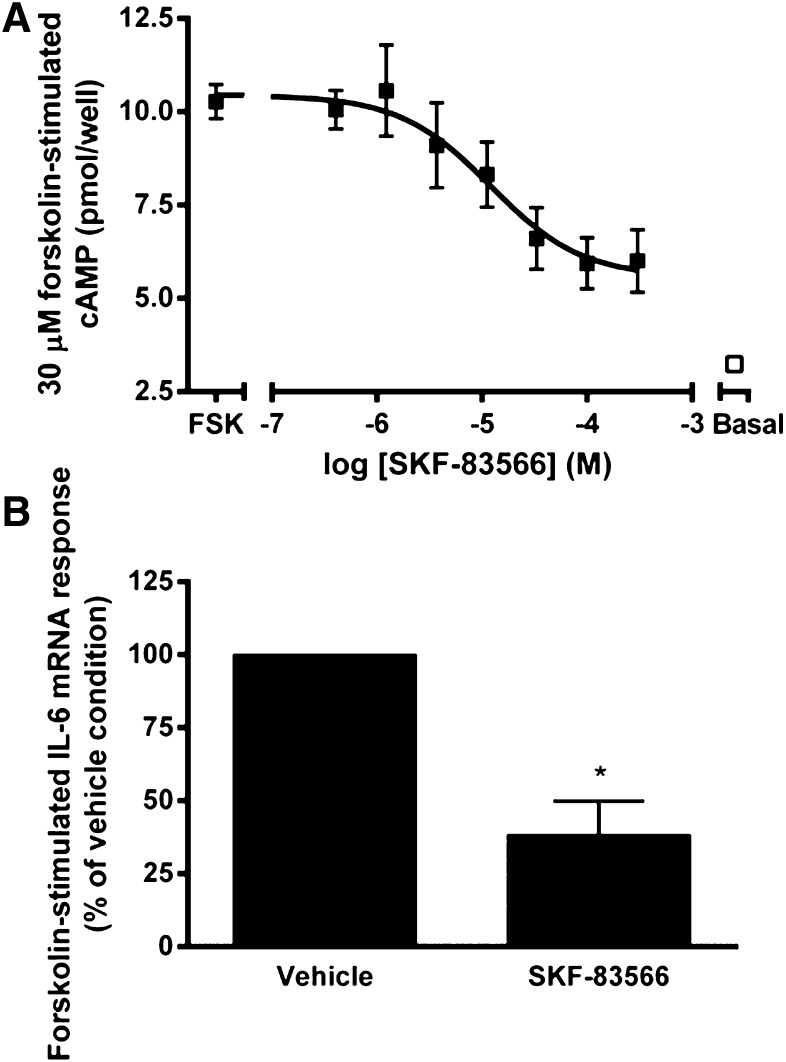
The most potent and selective AC2 inhibitor (SKF-83566) was examined in cell models where AC2 is natively expressed (along with other AC isoforms), allowing confirmation of its activity in a more physiologic context and evaluation of its use as a tool to probe AC2 function. AC2 is reported to be abundantly expressed in skeletal muscle tissues (Torgan and Kraus, 1996; Suzuki et al., 1998; Ludwig and Seuwen, 2002). Therefore, the ability of SKF-83566 to inhibit forskolin-stimulated cAMP accumulation was studied in differentiated mouse C2C12 skeletal muscle myotubes (Fig. 6A). As expected, SKF-83566 inhibited forskolin-stimulated cAMP with an IC50 value of 15 ± 6.5 µM and maximum inhibition of 69 ± 8.8%, consistent with SKF-83566 inhibiting endogenous AC2 activity (Fig. 6A).
Fig. 6.
SKF-83566 as a chemical probe for native AC2 activity. (A) The effect of SKF-83566 on forskolin-stimulated (30 µM) cAMP was measured in mouse C2C12 skeletal muscle cells that were differentiated into myotubes. Data are mean ± S.E.M. of three independent experiments. (B) The effect of SKF-83566 on forskolin-stimulated (1 µM) IL-6 mRNA expression was measured in hBSMCs. Data are expressed as a percentage of the vehicle condition and are the mean ± S.E.M. of four independent experiments. *P < 0.05; **P < 0.01 (one sample t test compared with 100).
Human bronchial smooth muscle cells express AC2, AC4, and AC6 (Bogard et al., 2011), and recent studies suggest that forskolin-stimulated IL-6 expression in hBSMCs is selectively mediated by AC2 (A.S. Bogard and R.S. Ostrom, unpublished observations). Therefore, the effect of SKF-83566 on forskolin-stimulated IL-6 mRNA expression was measured in hBMSCs using quantitative reverse transcription-PCR (Fig. 6B). SKF-83566 treatment reduced the forskolin-stimulated IL-6 mRNA to 38 ± 11% of the vehicle-treated cells. These results indicate that SKF-83566 inhibited the AC2-mediated upregulation of IL-6 mRNA expression in hBSMCs, suggesting that SKF-83566 may be a useful tool to assess the function of AC2.
Discussion
Several studies have implicated AC isoforms in physiologic functions and disease states, leading to the hypothesis that ACs are potentially novel therapeutic targets (Pierre et al., 2009; Sadana and Dessauer, 2009). However, further research is required to validate AC isoforms as therapeutic targets, and advancements have been limited because of the current paucity of small-molecule modulators that are potent and AC isoform selective. The need for additional small-molecule tools to assess the in vivo activity of AC isoforms is further reinforced in the case of AC2, where there is both a lack of selective small-molecule modulators and an absence of published transgenic or knockout mouse studies for AC2. Therefore, we developed an HTS-compatible intact-cell small-molecule screening approach and subsequent validation paradigm for the discovery of AC2 inhibitors.
The present study used the HTRF cAMP detection technology from Cisbio to develop a robust and scalable HTS assay. The development of an intact-cell assay for small-molecule AC modulators was designed to reduce cell-permeability issues that have plagued the utility of several small-molecule AC modulators (Seifert et al., 2012). However, the intact-cell screening format presents several challenges that are associated with apparent reductions in the cAMP signal that are independent of direct AC inhibition, including fluorescence detection artifacts, cell death or toxicity, and indirect modes of cAMP reduction that require subsequent validation experiments. The effectiveness of the validation experiments to identify PKC inhibitors was evident in the case of indatraline, as it was found to inhibit PMA-stimulated ERK1/2 phosphorylation but unable to modulate PKC-independent stimulation of cAMP in HEK-hAC2 cells. The complementary cAMP studies in wild-type and stably transfected HEK293 cells validated that the compounds selectively inhibit the exogenously expressed AC isoform. For example, SKF-83566 appeared to have activity for inhibition of cAMP in HEK-hAC2 and HEK-hAC5 cells but no activity for the inhibition of cAMP in the HEK293 cell background. Conversely, the inhibition observed by tranilast and loratadine was more difficult to interpret because each of these compounds strongly inhibited the forskolin-stimulated cAMP accumulation in the HEK293 cell background, in addition to their apparent activity at the exogenously expressed ACs. Therefore, tranilast and loratadine appeared to have effects on multiple AC isoforms, including endogenous ACs expressed in HEK293 cells. For example, our in vitro studies suggested direct modulation of AC2 by tranilast but indirect modulation of AC1 and AC5. The identification of SKF-83566 as a selective and direct AC2 inhibitor demonstrated the utility of the screening approach and the complementary validation experiments. The success of this initial study, combined with additional optimization for increased assay robustness, offers promise for future screening efforts of larger and more diverse chemical libraries.
The identification of SKF-83566 as a selective AC2 inhibitor represents another key contribution of the present report. SKF-83566 was originally reported as an antagonist at D1 dopamine and serotonin receptors (Ohlstein and Berkowitz, 1985). Nevertheless, the differences in potency between the receptor antagonism (Ohlstein and Berkowitz, 1985) and AC2 inhibition by SKF-83566 (i.e., 0.5–30 nM for dopamine or serotonin receptor antagonism versus ∼10 µM for AC2 inhibition), together with our control validation assays, suggested that we were observing AC2 inhibition in HEK-hAC2 cells. It is noteworthy that a recent study suggests that D1/D5 dopamine receptors are closely linked to AC by ligand similarity (Lin et al., 2013), perhaps suggesting that receptor antagonism and AC2 inhibition may have overlapping chemical requirements. Future studies with SKF-83566 should be focused on enhancing its pharmacological properties, including its specificity for AC and selectivity for AC2. For example, we used a racemic mixture of SKF-83566; therefore, pharmacological studies of the resolved enantiomers are expected to provide enhanced potency for inhibition of AC2. Additionally, classic structure-activity relationship studies, together with molecular modeling of the interaction of the catalytic subunits with SKF-83566, would also be useful to identify chemical moieties and functional groups that are important for AC2 inhibition and to understand the molecular determinants for the enhancement of specificity and isoform selectivity for AC2.
Despite the potential drawbacks associated with its receptor antagonism, SKF-83566 remains an important addition to the repertoire of AC modulators. For example, SKF-83566 displayed unmatched potency and efficacy for inhibition of AC2 when directly compared with several commonly used AC inhibitors (i.e., NKY80, MDL-12,330A, SQ 22,536, and 2′5′-ddAD). SKF-83566 also selectively inhibited AC2 (vs. AC1 and AC5), perhaps offering advantages over the diterpene analog, BODIPY-forskolin, that nonselectively modulates AC isoforms with a bidirectional modulation profile (i.e., inhibition of AC2 and partial activation of AC1 and AC5) (Pinto et al., 2008; Erdorf et al., 2011). The bidirectional modulation makes it difficult to use BODIPY-forskolin as a chemical probe in systems where multiple AC isoforms are expressed. In contrast, our studies with C2C12 mouse skeletal muscle cells and hBSMCs demonstrated the applicability of SKF-83566 as a tool to assess native AC function in a physiologic context, suggesting the possibility for its use as an in vivo probe. However, caution should be exercised when using SKF-83566 to examine AC function, as cAMP levels may also be influenced by potential AC2-independent effects of SKF-83566 (e.g., receptor antagonism or inhibition of other AC isoforms).
The identification of additional selective AC2 modulators is expected to contribute to the understanding of the physiologic roles of AC2. There is currently little direct evidence that suggests AC2 as a therapeutic target, but this may be due to the limited availability of research tools and strategies for studying AC2. Despite these limitations, AC2 has been associated with several diseases, offering a wealth of opportunities for the use of SKF-83566 as a chemical probe for AC2 function. For example, a potential role for AC2-mediated signaling in skeletal muscle physiology is suggested by the abundant expression of AC2 in adult skeletal muscle (Torgan and Kraus, 1996; Suzuki et al., 1998; Ludwig and Seuwen, 2002), and increased cAMP signaling is implicated in several aspects of muscle physiology, including hypertrophy, muscle repair, regeneration, and functional adaptation (Berdeaux and Stewart, 2012). However, the contribution of individual AC isoforms is not well understood in these physiologic processes (Berdeaux and Stewart, 2012), suggesting that SKF-83566 can be used as a chemical tool to study the contribution of AC2-mediated cAMP signaling to muscle physiology and skeletal muscle pathologies, including Duchenne muscular dystrophy and muscle atrophy associated with cancer, aging, and AIDS.
Recent studies also suggest a role for AC2 in the airway, as AC2 mediates IL-6 expression in hBSMCs (A.S. Bogard and R.S. Ostrom, unpublished observations). Consistent with this observation, we observed that SKF-83566 was able to inhibit a forskolin-stimulated IL-6 response in hBSMCs. Increased IL-6 expression has been detected in asthma patients (Neveu et al., 2010), and IL-6 is thought to play an active role in the pathogenesis of lung diseases such as asthma and chronic obstructive pulmonary disease (Neveu et al., 2010; Rincon and Irvin, 2012). Taken together, these findings indicate that it is possible that AC2 contributes to lung disease pathology by mediating elevated IL-6 in hBSMCs. Thus, SKF-83566 can be used to determine whether AC2 mediates the increased IL-6 levels in lung diseases and whether this event contributes to pathogenesis.
AC2 also appears to be involved in neuroendocrine tumors (NETs), as ADCY2 expression is upregulated in a “malignant cluster” of pancreatic NETs (Duerr et al., 2008) and is identified as a component of an upregulated cAMP/PKA/c-AMP response element-binding protein pathway in small intestinal NETs (Drozdov et al., 2011). Further in vitro expression and pharmacological analysis suggested that AC2 may be a functional mediator for upregulation of cAMP response element-binding protein–regulated transcripts that are associated with proliferation in small intestinal NETs (Drozdov et al., 2011). It is also noteworthy that ADCY2 expression is inversely correlated with survival in patients with colorectal cancer (Yu et al., 2011). The studies described herein suggest that AC2 has a potential role in the progression of NETs and colorectal cancer, but it is unclear whether the enhanced AC2 expression is pathologic, protective, or merely a marker of these disease states. SKF-83566 could be used as a chemical probe to test the functional role of AC2 catalytic activity in these pathophysiological states and, in the case of a causal relationship, suggest therapeutic utility for targeting AC2.
In conclusion, the present report describes the development and implementation of an HTS-capable intact-cell screening assay and subsequent validation strategy to identify small-molecule inhibitors of AC2. This initial screening effort identified SKF-83566 as a selective AC2 inhibitor with superior pharmacological properties for selective modulation of AC2 when directly compared with the currently available AC inhibitors.
Supplementary Material
Acknowledgments
The authors thank Ian Soderling for technical assistance with modification and programming of robotic equipment and Bruce Wiltermood for helpful discussions regarding automation. The authors also thank the Purdue University College of Pharmacy Dean’s office for providing the NIH clinical collections I and II.
Abbreviations
- A23187
5-(methylamino)-2-[[2R,3R,6S,8S,9R,11R)-3,9,11-trimethyl-8-[(1S)-1-methyl-2-oxo-2-(1H-pyrrol-2-yl)-ethyl]-1,7-dioxaspiro[5.5]undec-2-yl]methyl]-4-benzoxazolecarboxylic acid
- AC
adenylyl cyclase
- AraAde
Adenine 9-β-D-Arabinofuranoside
- BisI
bisindolylmaleimide I, 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide
- BODIPY
boron-dipyrromethene
- 2′5′-ddAD
2′5′-dideoxyadenosine
- DMEM
Dulbecco’s modified Eagle’s medium
- hBSMCs
human bronchial smooth muscle cells
- HBSS
Hanks’ balanced salt solution
- HEK293
human embryonic kidney 293 cells
- HTS
high-throughput screening
- IBMX
3-isobutyl-1-methylxanthine
- IL-6
interleukin 6
- MDL-12330A
(±)-N-[(1R*,2R*)-2-phenylcyclopentyl]-azacyclotridec-1-en-2-amine hydrochloride
- NB001
5-[[2-(6-Amino-9H-purin-9-yl)ethyl]amino]-1-pentanol
- NET
neuroendocrine tumor
- NKY80
2-Amino-7-(furanyl)-7,8-dihydro-5(6H)-quinazolinone
- PGE2
prostaglandin E2
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PMC-6
1R,4R-3-(6-aminopurin-9-yl)-cyclopentanecarboxylic acid hydroxamide
- Sf9
Spodoptera frugiperda cell line
- (±)-SKF-83566
8-bromo-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepin-7-ol hydrobromide
- SQ22,536
[9-(tetrahydro-2-furanyl)-9H-purin-6-amine]
- TCA
tricholoroacetic acid
- TR-FRET
time-resolved fluorescence resonance energy transfer
Authorship Contributions
Participated in research design: Conley, Dessauer, Ostrom, Watts.
Conducted experiments: Conley, Brand, Bogard.
Contributed new reagents or analytical tools: Pratt, Xu, Hockerman.
Performed data analysis: Conley, Brand, Bogard, Dessauer, Ostrom, Watts.
Wrote or contributed to the writing of the manuscript: Conley, Dessauer, Ostrom, Xu, Hockerman, Watts.
Footnotes
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH060397]; and the Brain and Behavior Research Foundation (formerly NARSAD).
Portions of this work were previously presented in abstract form: Conley JM, Bogard AS, Brand CS, Dessauer CW, Ostrom RS, Watts VJ (2013) Identification and characterization of novel small molecule modulators of adenylyl cyclase. Experimental Biology. 2013 Apr 20–25, Boston, MA.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Bender AT, Beavo JA. (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520 [DOI] [PubMed] [Google Scholar]
- Berdeaux R, Stewart R. (2012) cAMP signaling in skeletal muscle adaptation: hypertrophy, metabolism, and regeneration. Am J Physiol Endocrinol Metab 303:E1–E17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard AS, Adris P, Ostrom RS. (2012) Adenylyl cyclase 2 selectively couples to E prostanoid type 2 receptors, whereas adenylyl cyclase 3 is not receptor-regulated in airway smooth muscle. J Pharmacol Exp Ther 342:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard AS, Xu CF, Ostrom RS. (2011) Human bronchial smooth muscle cells express adenylyl cyclase isoforms 2, 4, and 6 in distinct membrane microdomains. J Pharmacol Exp Ther 337:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeunig JH, Schweda F, Han PL, Seifert R. (2013) Similarly potent inhibition of adenylyl cyclase by P-site inhibitors in hearts from wild type and AC5 knockout mice. PLoS ONE 8:e68009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumbay MG, Watts VJ. (2001) Heterologous sensitization of recombinant adenylate cyclases by activation of D(2) dopamine receptors. J Pharmacol Exp Ther 297:1201–1209 [PubMed] [Google Scholar]
- Degorce F, Card A, Soh S, Trinquet E, Knapik GP and Xie B (2009) HTRF: A technology tailored for drug discovery—a review of theoretical aspects and recent applications. Curr Chem Genomics 3:22–32. [DOI] [PMC free article] [PubMed]
- Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. (1997) Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem 272:19125–19132 [DOI] [PubMed] [Google Scholar]
- Dessauer CW. (2002) Kinetic analysis of the action of P-site analogs. Methods Enzymol 345:112–126 [DOI] [PubMed] [Google Scholar]
- Drozdov I, Svejda B, Gustafsson BI, Mane S, Pfragner R, Kidd M, Modlin IM. (2011) Gene network inference and biochemical assessment delineates GPCR pathways and CREB targets in small intestinal neuroendocrine neoplasia (Abstract). PLoS ONE 6:e22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GI, Severson DL. (1979) Cyclic nucleotides and cardiac function. Circ Res 44:145–153 [DOI] [PubMed] [Google Scholar]
- Duerr EM, Mizukami Y, Ng A, Xavier RJ, Kikuchi H, Deshpande V, Warshaw AL, Glickman J, Kulke MH, Chung DC. (2008) Defining molecular classifications and targets in gastroenteropancreatic neuroendocrine tumors through DNA microarray analysis. Endocr Relat Cancer 15:243–256 [DOI] [PubMed] [Google Scholar]
- Erdorf M, Mou T-C, Seifert R. (2011) Impact of divalent metal ions on regulation of adenylyl cyclase isoforms by forskolin analogs. Biochem Pharmacol 82:1673–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Defer N. (2001) Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41:145–174 [DOI] [PubMed] [Google Scholar]
- Hellevuo K, Yoshimura M, Kao M, Hoffman PL, Cooper DMF, Tabakoff B. (1993) A novel adenylyl cyclase sequence cloned from the human erythroleukemia cell line. Biochem Biophys Res Commun 192:311–318 [DOI] [PubMed] [Google Scholar]
- Iwatsubo K, Bravo C, Uechi M, Baljinnyam E, Nakamura T, Umemura M, Lai L, Gao SM, Yan L, Zhao X, et al. (2012) Prevention of heart failure in mice by an antiviral agent that inhibits type 5 cardiac adenylyl cyclase. Am J Physiol Heart Circ Physiol 302:H2622–H2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo K, Minamisawa S, Tsunematsu T, Nakagome M, Toya Y, Tomlinson JE, Umemura S, Scarborough RM, Levy DE, Ishikawa Y. (2004) Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J Biol Chem 279:40938–40945 [DOI] [PubMed] [Google Scholar]
- Jacobowitz O, Iyengar R. (1994) Phorbol ester-induced stimulation and phosphorylation of adenylyl cyclase 2. Proc Natl Acad Sci USA 91:10630–10634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038 [DOI] [PubMed] [Google Scholar]
- Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, Nestler EJ, Han PL. (2006) Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci USA 103:3908–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lee ML, Bruchas MR, Chan GC, Storm DR, Chavkin C. (2006) Calmodulin-stimulated adenylyl cyclase gene deletion affects morphine responses. Mol Pharmacol 70:1742–1749 [DOI] [PubMed] [Google Scholar]
- Lin H, Sassano MF, Roth BL, Shoichet BK. (2013) A pharmacological organization of G protein-coupled receptors. Nat Methods 10:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig MG, Seuwen K. (2002) Characterization of the human adenylyl cyclase gene family: cDNA, gene structure, and tissue distribution of the nine isoforms. J Recept Signal Transduct Res 22:79–110 [DOI] [PubMed] [Google Scholar]
- Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY, Irvin CG, Kaminsky DA, Rincon M. (2010) Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res 11:28 DOI: 10.1186/1465-9921-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein EH, Berkowitz BA. (1985) SCH 23390 and SK&F 83566 are antagonists at vascular dopamine and serotonin receptors. Eur J Pharmacol 108:205–208 [DOI] [PubMed] [Google Scholar]
- Okumura S, Kawabe J, Yatani A, Takagi G, Lee MC, Hong C, Liu J, Takagi I, Sadoshima J, Vatner DE, et al. (2003) Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ Res 93:364–371 [DOI] [PubMed] [Google Scholar]
- Patel TB, Du ZY, Pierre S, Cartin L, Scholich K. (2001) Molecular biological approaches to unravel adenylyl cyclase signaling and function. Gene 269:13–25 [DOI] [PubMed] [Google Scholar]
- Pavan B, Biondi C, Dalpiaz A. (2009) Adenylyl cyclases as innovative therapeutic goals. Drug Discov Today 14:982–991 [DOI] [PubMed] [Google Scholar]
- Pieroni JP, Harry A, Chen JQ, Jacobowitz O, Magnusson RP, Iyengar R. (1995) Distinct characteristics of the basal activities of adenylyl cyclases 2 and 6. J Biol Chem 270:21368–21373 [DOI] [PubMed] [Google Scholar]
- Pierre S, Eschenhagen T, Geisslinger G, Scholich K. (2009) Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov 8:321–335 [DOI] [PubMed] [Google Scholar]
- Pinto C, Papa D, Hübner M, Mou TC, Lushington GH, Seifert R. (2008) Activation and inhibition of adenylyl cyclase isoforms by forskolin analogs. J Pharmacol Exp Ther 325:27–36 [DOI] [PubMed] [Google Scholar]
- Przybyla JA, Watts VJ. (2010) Ligand-induced regulation and localization of cannabinoid CB1 and dopamine D2L receptor heterodimers. J Pharmacol Exp Ther 332:710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Irvin CG. (2012) Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 8:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison GA, Butcher RW, Sutherland EW. (1968) Cyclic AMP. Annu Rev Biochem 37:149–174 [DOI] [PubMed] [Google Scholar]
- Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK. (1999) Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation 99:3099–3102 [DOI] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Lushington GH, Mou TC, Gille A, Sprang SR. (2012) Inhibitors of membranous adenylyl cyclases. Trends Pharmacol Sci 33:64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. (2008) Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Shen TS, Poyard M, Best-Belpomme M, Hanoune J, Defer N. (1998) Expression of adenylyl cyclase mRNAs in the denervated and in the developing mouse skeletal muscle. Am J Physiol 274:C1674–C1685 [DOI] [PubMed] [Google Scholar]
- Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JXJ, Roth DM, Hammond HK. (2008) Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117:61–69 [DOI] [PubMed] [Google Scholar]
- Taussig R, Tang W-J, and Gilman AG(1994) Expression and purification of recombinant adenylyl cyclases in Sf9 cells, in Methods in Enzymology; Heterotrimeric G-protein effectors (Iyengar R ed) pp 95–108, Academic Press, Inc., San Diego, CA. [DOI] [PubMed] [Google Scholar]
- Tepe NM, Lorenz JN, Yatani A, Dash R, Kranias EG, Dorn GW, 2nd, Liggett SB. (1999) Altering the receptor-effector ratio by transgenic overexpression of type V adenylyl cyclase: enhanced basal catalytic activity and function without increased cardiomyocyte beta-adrenergic signalling. Biochemistry 38:16706–16713 [DOI] [PubMed] [Google Scholar]
- Torgan CE, Kraus WE. (1996) Regulation of type II adenylyl cyclase mRNA in rabbit skeletal muscle by chronic motor nerve pacing. Am J Physiol 271:E253–E260 [DOI] [PubMed] [Google Scholar]
- Vadakkan KI, Wang H, Ko SW, Zastepa E, Petrovic MJ, Sluka KA, Zhuo M. (2006) Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated adenylyl cyclases. Mol Pain 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu H, Wu L-J, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, et al. (2011) Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain (Abstract). Sci Transl Med 3:ra3. [DOI] [PubMed] [Google Scholar]
- Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ, et al. (2002) Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 36:713–726 [DOI] [PubMed] [Google Scholar]
- Whitfield JF, Boynton AL, Macmanus JP, Sikorska M, Tsang BK. (1979) Regulation of Cell-Proliferation by Calcium and Cyclic-AMP. Molecular and Cellular Biochemistry 27(3):155–179 [DOI] [PubMed] [Google Scholar]
- Willoughby D, Baillie GS, Lynch MJ, Ciruela A, Houslay MD, Cooper DMF. (2007) Dynamic regulation, desensitization, and cross-talk in discrete subcellular microdomains during beta2-adrenoceptor and prostanoid receptor cAMP signaling. J Biol Chem 282:34235–34249 [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. (2007) Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130:247–258 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Cooper DMF. (1993) Type-specific stimulation of adenylylcyclase by protein kinase C. J Biol Chem 268:4604–4607 [PubMed] [Google Scholar]
- Yu SJ, Yu JK, Ge WT, Hu HG, Yuan Y, Zheng S. (2011) SPARCL1, Shp2, MSH2, E-cadherin, p53, ADCY-2 and MAPK are prognosis-related in colorectal cancer. World J Gastroenterol 17:2028–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Chung TDY, Oldenburg KR. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.