Abstract
UDP-glucuronosyltransferase (UGT) 1A1 is the sole enzyme that can metabolize bilirubin. Human infants physiologically develop hyperbilirubinemia as the result of inadequate expression of UGT1A1 in the liver. Although phototherapy using blue light is effective in preventing jaundice, sunlight has also been suggested, but without conclusive evidence, to reduce serum bilirubin levels. We investigated the mRNA expression pattern of human UGT1A1 in human skin, human skin keratinocyte (HaCaT) cells, and skin of humanized UGT1 mice. The effects of UVB irradiation on the expression of UGT1A1 in the HaCaT cells were also examined. Multiple UGT1A isoforms, including UGT1A1, were expressed in human skin and HaCaT cells. When HaCaT cells were treated with UVB-exposed tryptophan, UGT1A1 mRNA and activity were significantly induced. Treatment of the HaCaT cells with 6-formylindolo[3,2-b]carbazole, which is one of the tryptophan derivatives formed by UVB, resulted in an induction of UGT1A1 mRNA and activity. In neonates, the expression of UGT1A1 was greater in the skin; in adults, UGT1A1 was expressed mainly in the liver. Treatment of humanized UGT1 mice with UVB resulted in a reduction of serum bilirubin levels, along with increased UGT1A1 expression and activity in the skin. Our data revealed a protective role of UGT1A1 expressed in the skin against neonatal hyperbilirubinemia. Sunlight, a natural and free source of light, makes it possible to treat neonatal jaundice while allowing mothers to breast-feed neonates.
Introduction
UDP glucuronosyltransferases (UGTs; EC 2.4.1.17) are a family of membrane-bound enzymes that catalyze the transfer of the glucuronic acid moiety of UDP-glucuronic acid to a large number of endogenous and exogenous compounds (Dutton, 1980). Human UGTs are divided into two distinct families, UGT1 and UGT2, on the basis of evolutionary divergence and homology (Mackenzie et al., 2005). The UGT1 gene is located on chromosome 2q37 and produces nine functional enzymes (UGT1A1, UGT1A3–UGT1A10) by exon sharing (Ritter et al., 1992). The UGT2A and UGT2B genes are located on chromosome 4q13, encoding three and seven functional proteins, respectively. The UGT2A1 and UGT2A2 are formed by differential splicing of variable first exons and common exons 2–6, likely the UGT1A gene (Mackenzie et al., 2005). Meanwhile, UGT2A3 and each UGT2B are encoded by individual genes (Mackenzie et al., 2005). Each UGT enzyme expresses in a tissue-specific manner and exhibits substrate specificity (Tukey and Strassburg, 2000).
Bilirubin is an end product of heme catabolism, formed by the breakdown of red blood cells. Bilirubin is bound to serum proteins and is taken up by the liver, where it is conjugated by UGT1A1 with glucuronic acid. The conjugated bilirubin is excreted into the small intestine via the bile duct (Kamisako et al., 2000). Because UGT1A1 is the sole bilirubin conjugating enzyme (Bosma et al., 1994), its genetic polymorphism or inhibition of UGT1A1 activity by agents such as coadministered drugs can cause increased serum levels of unconjugated bilirubin (Mackenzie at al., 2000; Danoff et al., 2004). The unconjugated bilirubin crosses the blood-brain barrier and accumulates in the brain, causing neurotoxicity. Newborn infants commonly develop mild hyperbilirubinemia, which is called physiologic jaundice. Although jaundice usually disappears within a few weeks, severe hyperbilirubinemia can be severe and cause kernicterus (Gourley, 1997). To prevent occurrences of kernicterus, infants who develop severe hyperbilirubinemia are often treated with phototherapy to reduce plasma bilirubin levels directly by isomerizing bilirubin. However, the limitation with phototherapy is that it requires mothers not to breast-feed; additionally, it requires conventional phototherapy units, which are not available in some countries.
Sunlight has been suggested as an alternative treatment of neonatal jaundice (Salih, 2001). It was demonstrated that sunlight is more effective in isomerizing bilirubin than phototherapy is (Salih, 2001). Furthermore, it was shown that UVB in sunlight photo-oxidizes l-tryptophan, activating the aryl hydrocarbon receptor (AhR) in the skin (Wincent et al., 2009). Because the expression of human UGT1A1 is regulated by various nuclear receptors, including AhR (Yueh et al., 2003), it is hypothesized that UGT1A1 expressed in the skin might play an important role in sunlight-induced reduction of serum bilirubin, since the skin covers a surface area of approximately 1.7 m2 in an average adult body and 0.2 m2 in a 3-kg newborn infant, and it receives about one-third of the circulating blood. However, little is known about the expression pattern of UGT1A1 in human skin. To investigate the protective role of UGT1A1 in the skin against neonatal hyperbilirubinemia, we examined mRNA expression patterns of human UGT1 family enzymes in human skin, human skin keratinocyte (HaCaT) cells, and in recently developed humanized UGT1 (hUGT1) mice (Fujiwara et al., 2010). We also examined the effects of UVB irradiation on the expression of human UGT1 in HaCaT cells.
Materials and Methods
Chemicals and Reagents.
We purchased l-tryptophan from Sigma-Aldrich (St. Louis, MO), 6-formylindolo[3,2-b]carbazole (FICZ) and 7-ethoxyresorufin from BIOMOL (Enzo Life Sciences, Plymouth Meeting, PA), and HaCaT cells from CLS Cell Lines Service (Eppelheim, Germany). The human total skin and liver RNA was purchased from Agilent Technologies (Santa Clara, CA) and Applied StemCell (Sunnyvale, CA). Primers were commercially synthesized at Invitrogen (Carlsbad, CA). PrimeSTAR DNA polymerase was purchased from TaKaRa Bio (Shiga, Japan), and Ssofast Evagreen Supermix was obtained from Bio-Rad (Hercules, CA). UGT1A1 supersomes and human liver microsomes were purchased from BD Gentest (Woburn, MA). Anti-human UGT1A antibody (B-4) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals and solvents were of analytical grade or the highest grade commercially available.
Cell Cultures and Chemical Treatments.
HaCaT cells have differentiation characteristics similar to those of normal human keratinocytes even though they are immortal and genetically abnormal (Schoop et al., 1999). HaCaT cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 1 mM sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mM nonessential amino acids, 2 mM Gluta-MAX-I, and 10% fetal bovine serum with 5% CO2 at 37°C. Before the chemical treatment, HaCaT cells (passages 40–45) were seeded into six-well plates at 1 × 106 cells/well. After 72 hours, the culture medium was changed to a Dulbecco’s modified Eagle’s medium containing 0.1–50 nM FICZ, irradiated tryptophan, or vehicle; subsequently, cells were maintained for 6 hours until harvesting. FICZ was dissolved in dimethylsulfoxide with a final concentration in the medium of less than 0.1%.
Animals and Treatments.
Tg(UGT1A1*28)Ugt1−/− (hUGT1) mice were developed previously in a C57BL/6 background (Fujiwara et al., 2010). All animals received food and water ad libitum, and mouse handling and experimental procedures were conducted in accordance with our animal care protocol, which was approved by Kitasato University. A day after 60-minute irradiation of 3-day-old male hUGT1 mice to UVB using a UV lamp (irradiation wavelength: 302 nm, 3UV-38 3UV Lamp; UVP, Inc., Upland, CA) at 15-cm distance from skin surface, blood and tissues were collected. For tissue collections, male mice were anesthetized by diethyl ether inhalation, and the liver was perfused with ice-cold 1.15% KCl. The skin and liver were rinsed in cold 1.15% KCl and stored at −80°C.
Bilirubin Measurements.
Blood was obtained from the submandibular vein and centrifuged at 2000g for 5 minutes. Serum samples (10 μl) were measured for total serum bilirubin using a Bilirubinometer (B-105N; Erma, Tokyo, Japan). For each serum sample, the bilirubin value was measured three times, and the mean value was used for data analysis. To avoid hemolysis of the serum and photolysis of the bilirubin, the serum samples were analyzed promptly after the collection of blood.
Semiquantitative and Quantitative Reverse-Transcription Polymerase Chain Reaction.
Total RNA was extracted from HaCaT cells with Trizol reagent (Invitrogen). The cDNA was synthesized from total RNA using ReverTra Ace (TOYOBO, Osaka, Japan) according to the manufacturer’s protocol. A 1-µl portion of the reverse-transcribed (RT) mixture was added to polymerase chain reaction (PCR) mixtures (25 μl). The amplification was performed by denaturation at 98°C for 10 seconds, annealing at 60°C for 10 seconds, and extension at 72°C for 20 seconds for 20 cycles for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and for 35 cycles for UGTs. The sequences of primers used in the present study are as follows: CYP1A1-forward: 5′-GGC TGG CCT ATG TGG TCT AA-3′; reverse: 5′-ATG TGG CCC TGT TTT ACC TG-3′. Primers for human UGTs and GAPDH were developed previously (Nakamura et al., 2008). The PCR products (20 µl) were analyzed by electrophoresis with 2% agarose gel and visualized by ethidium bromide staining. Expression of GAPDH mRNA was used as an internal control for the cDNA quantity and quality. Quantitative RT-PCR (qRT-PCR) was performed with Ssofast Evagreen Supermix, and the reactions were run in a CFX96 Real-Time PCR Detection System (Bio-Rad).
Irradiation of l-Tryptophan.
Aqueous solutions of l-tryptophan (100 ml of 80 µM) were irradiated with UVB lamps (BLE-1T158; Spectronics Corp., Westbury, NY) for 5–60 minutes at a distance of 30 cm (1.4 mW/cm2) at room temperature. To eliminate UVC wavelengths, a UVC filter (CLAREX S-0; Nitto Jushi Kogyo, Tokyo, Japan) was used while l-tryptophan was being irradiated. After irradiation, the tryptophan solutes were protected from light and were used immediately for the cell treatment. HaCaT cells were treated for 6 hours with Irradiated l-tryptophan by adding a 1-ml portion of irradiated l-tryptophan to the culture medium.
7-Ethoxy-Resorufin-O-Deethylase Activity.
7-Ethoxy-resorufin-O-deethylase (EROD) activity in the HaCaT cells was determined as follows. Nontreatment or chemically treated HaCaT cells in six-well plates were washed twice with 1 ml of Hanks’ balanced salt solution (HBSS; 145 mM NaCl, 3 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 5 mM d-glucose, 5 mM HEPES pH 7.4). After the cells were preincubated with 1.5 ml of HBSS for 10 minutes at 37°C, the HBSS solution was replaced with 2 ml of HBSS containing 7-ethoxy-resorufin at a final concentration of 2 µM, and cells were then incubated for 30 to 120 minutes at 37°C. The reaction was stopped by adding 2 ml of ice-cold methanol. The cells were then sonicated for 5 minutes. After the sonication, the cell solution was centrifuged at 10,000g for 10 minutes at 4°C, and a 50 µl-portion of the supernatant was subjected to high-performance liquid chromatography (HPLC) to quantitate the formation of resorufin.
HPLC Conditions.
The formation of resorufin was determined by the HPLC system using an LC-10AD pump (Shimadzu, Kyoto, Japan), an FP-2020 fluorescence detector (JASCO, Tokyo, Japan), an SIL-10A autosampler (Shimadzu), an SLC-10A system controller (Shimadzu), and a Mightysil RP-18 GP column (4.6 × 150 mm, 5 μm; Kanto Chemical, Tokyo, Japan). The mobile phase was 25 mM phosphate buffer (pH 7.0)-methanol (64:36, v/v), and the flow rate was 0.8 ml/min. Detection was accomplished with a fluorescence detector at 560-nm excitation and 585-nm emission. Quantification of resorufin was carried out by comparing the HPLC peak area to that of the authentic standard.
Estradiol 3-O-Glucuronidation.
Skin microsomes were prepared using the following procedure. Skin was homogenized in three volumes of Tris-buffered saline [25 mM Tris-HCl buffer (pH 7.4), 138 mM NaCl, and 2.7 mM KCl]. The homogenate was centrifuged at 10,000g for 30 minutes at 4°C, and the supernatant was collected. The supernatant was centrifuged at 105,000g for 60 minutes at 4°C, and the pellet was suspended in the same buffer and used as the microsomal fraction. Protein concentrations of microsomal fractions were measured by the Bradford method using bovine serum albumin as a standard (Bradford, 1976). Estradiol 3-O-glucuronide formation was determined according to the method of Fujiwara et al. (2007) with slight modifications. Briefly, a typical incubation mixture (200 μl of total volume) contained 50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 2 mM uridine-diphosphoglucuronic acid, 50 μg/ml alamethicin, 0.25–2.0 mg/ml microsomes, and 10 μM estradiol. The reaction was initiated by the addition of uridine-disphosphoglucuronic acid after a 3-minute preincubation at 37°C. After incubation at 37°C for 90 minutes, the reaction was terminated by the addition of 200 μl of cold methanol. After removal of the protein by centrifugation at 12,000g for 5 minutes, a 50-μl portion of the sample was subjected to HPLC. The condition of HPLC analysis was described previously (Fujiwara et al., 2007).
SDS-PAGE and Immunoblotting.
Microsomal fractions were prepared as described; 10–150 μg of microsomal protein was separated on 4–12% NuPAGE Bis-Tris polyacrylamide gels (Invitrogen). Immunoblots with antibodies to UGT1A were performed as previously outlined (Fujiwara et al., 2010).
Statistical Analyses.
Statistical significances were determined by analysis of variance followed by Dunnett’s test or unpaired Student’s t test. A value of P < 0.05 was considered statistically significant.
Results
Identification of UGT Isoforms Expressed in Human Skin.
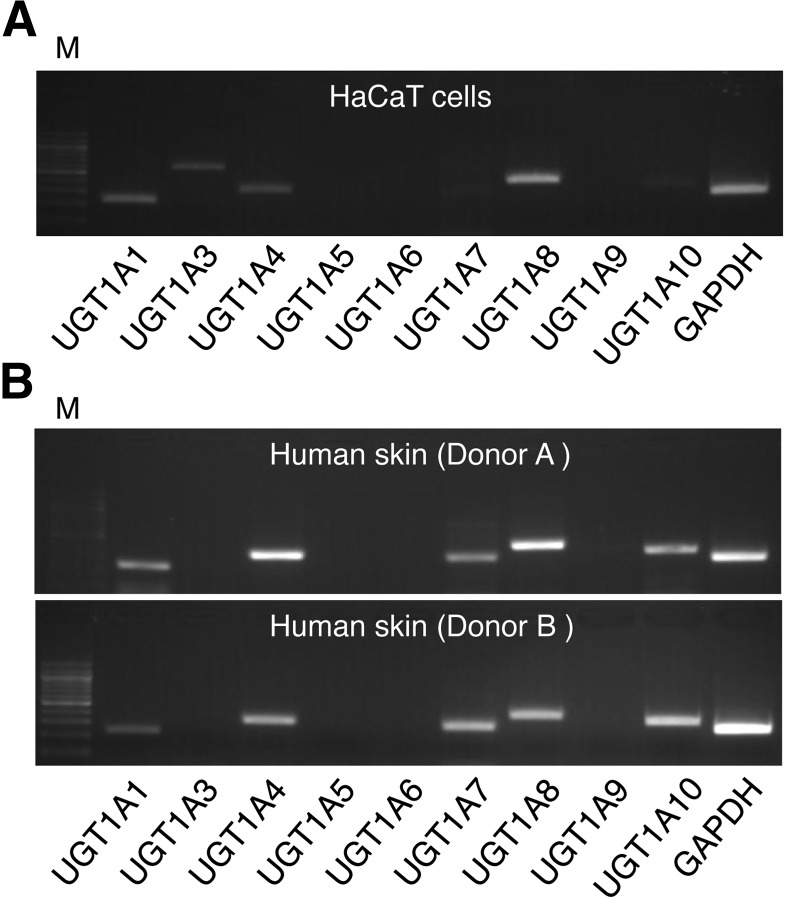
The skin is one of the body’s largest primary barriers to the environment, but the expression pattern of detoxification enzymes such as the UGTs in human skin has not yet been quantitated. In this study, the expression levels of human UGT1A1, UGT1A3, UGT1A4, UGT1A5, UGT1A6, UGT1A7, UGT1A8, UGT1A9, and UGT1A10 in human keratinocyte HaCaT cells were examined using human UGT1A isoform–specific primers (Nakamura et al., 2008). The semiquantitative RT-PCR revealed that a wide variety of UGT1A isoforms, UGT1A1, UGT1A3, UGT1A4, and UGT1A8, were expressed in the HaCaT cells (Fig. 1A). We further determined the expression patterns of human UGT1A mRNA in human skin from two different donor samples. In addition to the expression of UGT1A1, UGT1A3, UGT1A4, and UGT1A8, we observed that UGT1A7 and UGT1A10 were highly expressed in human skin (Fig. 1B).
Fig. 1.
The expression pattern of human UGT1A family enzymes in human skin. A 1-µl portion of complementary DNA reverse-transcribed from HaCaT total RNA (A) or human skin RNA (B) was added to PCR mixtures (25 μl). The PCR products (20 µl) were analyzed by electrophoresis with 2% agarose gel and visualized by ethidium bromide staining. M, 100-bp ladder marker.
Induction of UGT1A1, UGT1A8, and CYP1A1 by Irradiated l-Tryptophan in HaCaT Cells.
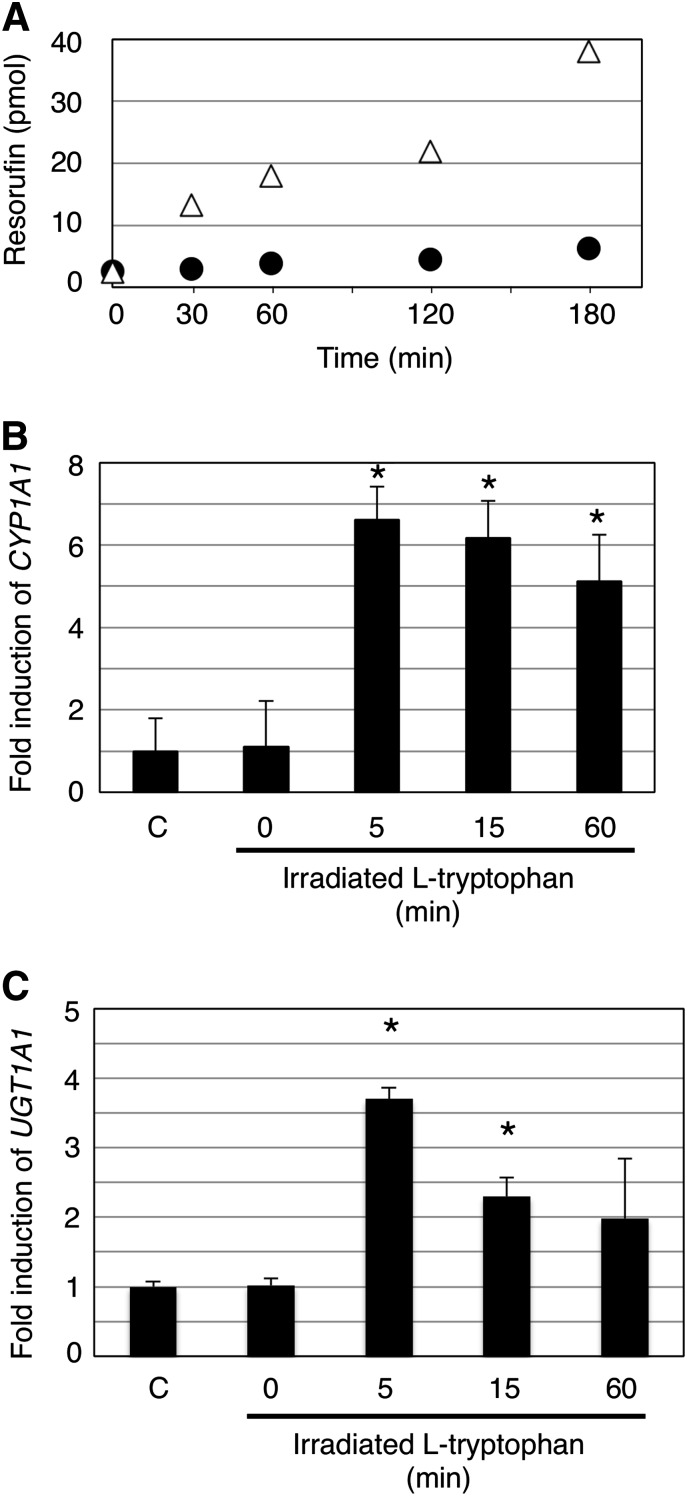
It has been reported that UVB irradiation photo-oxidizes tryptophan in the skin and produces FICZ, which is an agonist of AhR (Wincent et al., 2009). To understand whether UVB irradiation of l-tryptophan could result in an activation of AhR in the HaCaT cells, we irradiated 80 µM of l-tryptophan, comparable to the concentration of tryptophan in human blood (Suzuki et al., 2010), with UVB for up to 60 minutes, followed by treatment of HaCaT cells. Because induced EROD activity reflects AhR-dependent induction of CYP1A1 activity, we first determined EROD activity in control and irradiated l-tryptophan–treated HaCaT cells by quantitating the formation of resorufin in the culture medium. HaCaT cells were exposed for 6 hours to irradiated l-tryptophan for 60 minutes. The amount of formed resorufin was approximately 4–5 times greater in the irradiated l-tryptophan–treated HaCaT cells (CLint = 1.34 µl/min per milligram of protein) than of that in control cells (CLint = 0.28 µl/min per milligram of protein) (Fig. 2A), indicating that irradiated l-tryptophan does induce CYP1A1. To understand the effects of irradiation time on the inducibility of l-tryptophan for CYP1A1, HaCaT cells were exposed for 6 hours to l-tryptophan that had been irradiated for 5, 15, and 60 minutes, respectively, and the expression level of CYP1A1 mRNA was determined by qRT-PCR. CYP1A1 was induced 5-fold when HaCaT cells were treated with l-tryptophan that had been irradiated for 60 minutes (Fig. 2B), which was comparable to the induced EROD activity (Fig. 2A). The level of CYP1A1 induction was even higher when HaCaT cells were treated with l-tryptophan that was irradiated for 5 minutes (Fig. 2B). Since UGT1A1 and UGT1A8 play important roles in the detoxification of neurotoxic bilirubin and carcinogens, we next investigated the inducibility of UVB-irradiated l-tryptophan on UGT1A1 and UGT1A8 in human skin HaCaT cells. The induction pattern of UGT1A1 in HaCaT cells with UVB-irradiated l-tryptophan was very similar to that of CYP1A1 (Fig. 2C). With 5-minute UVB-irradiated l-tryptophan, UGT1A1 was induced approximately 4-fold, whereas it resulted in a 10-fold induction of UGT1A8 (Supplemental Fig. 1).
Fig. 2.
The effect of UVB-irradiated l-tryptophan on CYP1A1 and UGT expression in HaCaT cells. A 1-ml portion of UVB-irradiated l-tryptophan was added to the cell culture medium, and cells were treated for 6 hours. EROD activity in the UVB-irradiated l-tryptophan–treated HaCaT cells was determined by incubating the cells with 7-ethoxy-resorufin for 30, 60, 120, and 180 minutes. Formed resorufin was quantified by HPLC. Filled circles and open triangles refer to formed resorufin in nontreatment cells and in UVB-irradiated L-tryptophan–treated cells (A). The HaCaT cells were treated for 6 hours with l-tryptophan that had been irradiated for 0, 5, 15, and 60 minutes with UVB. Total RNA was isolated from the HaCaT cells, and qRT-PCR was carried out for CYP1A1 (B) and UGT1A1 (C). C, control. *P < 0.05, compared with the expression in the control HaCaT cells.
Induction of CYP1A1, UGT1A1, and UGT1A8 in the FICZ-Treated HaCaT Cells.
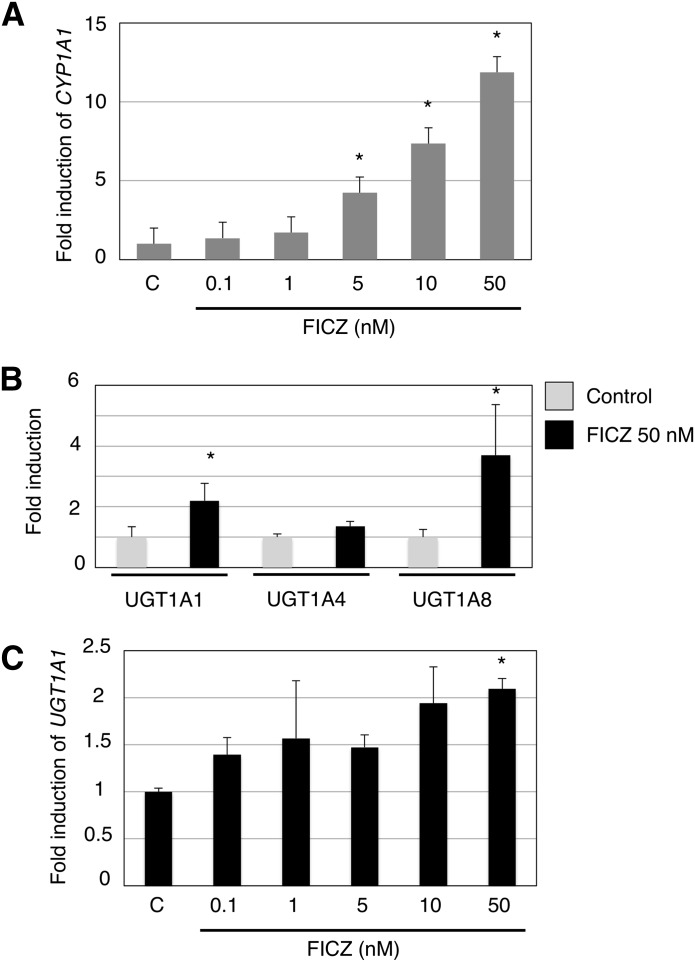
To investigate the direct effects of FICZ on the expression of UGT1A1 and UGT1A8, as well as CYP1A1, HaCaT cells were treated with FICZ and RT-PCR was carried out for these genes. The FICZ treatment of HaCaT cells in our study showed a dose-dependent induction of CYP1A1 (Fig. 3A). Our semiquantitative RT-PCR revealed that UGT1A1 and UGT1A8 were induced by 50 nM FICZ in the HaCaT cells (Supplemental Fig. 2). Although UGT1A7 and UGT1A10 were slightly expressed in nontreated HaCaT cells (Fig. 1A), clearer bands of those isoforms were observed in the FICZ-treated HaCaT cells (Supplemental Fig. 2). We further conducted a qRT-PCR analysis of UGT1A1, UGT1A4, and UGT1A8 in control and FICZ-treated HaCaT cells. In the FICZ treatment, UGT1A1 and UGT1A8 were induced 2- and 3.7-fold, respectively, compared with control cells (Fig. 3B). Similar to CYP1A1, UGT1A1 was also induced by FICZ dose dependently (Fig. 3C). On the other hand, UGT1A4 was not induced by 50 nM FICZ (Fig. 3B). The data indicate that not only CYP1A1 but also multiple UGT1A isoforms, especially UGT1A1 and UGT1A8, were induced by FICZ in the human skin cells.
Fig. 3.
The effect of FICZ treatment on CYP1A1 and UGT expression in HaCaT cells. Total RNA was isolated from HaCaT cells that were treated with FICZ for 6 hours. Complementary DNA was synthesized, and then qRT-PCR was carried out for human CYP1A1 (A) and UGT (B). Concentration-dependent effects of FICZ on UGT1A1 expression was also analyzed (C). Each column is the mean ± S.D. of three independent determinations. C, control. *P < 0.05, compared with the expression in the control HaCaT cells.
Induction of Estradiol 3-O-Glucuronidation Activities by UVB and FICZ in HaCaT Cells.
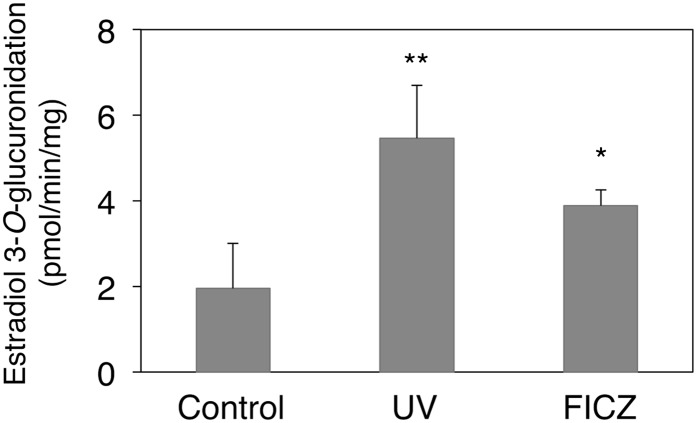
We further investigated whether the treatment of the HaCaT cells with UVB-exposed tryptophan and FICZ results in an increase in UGT activities. To obtain a sufficiently large amount of the microsome fraction from HaCaT cells to conduct UGT enzyme assays, HaCaT cells were cultured in 15 (150-mm) dishes for each group. After the cells were treated for 24 hours with UVB-exposed tryptophan or 50 nM FICZ, the cells were harvested and the microsomes were prepared using the method described earlier. Estradiol 3-O-glucuronidation is one of the metabolic reactions selectively metabolized by UGT1A1. The estradiol 3-O-glucuronidation activity was 1.9 pmol/min per milligram in the microsomes prepared from the control HaCaT cells (Fig. 4). Treatment of the cells with UVB-exposed tryptophan and FICZ increased microsomal activity to 5.5 pmol/min per milligram and 3.9 pmol/min per milligram, respectively. The fold induction of the UGT activity was correlated with the induction level of UGT1A1 mRNA (Figs. 2 and 3), indicating that UVB or FICZ similarly induced both UGT1A1 mRNA and activity in the HaCaT cells.
Fig. 4.
Effects of UVB and FICZ treatments on the UGT1A1 activity in the HaCaT cells. Microsomes were prepared from the HaCaT cells, and estradiol 3-O-glucuronide formation was measured. Error bars show S.D., n = 3. **P < 0.01; *P < 0.05.
Comparison of UGT1A1 Expression in the Skin and Liver.
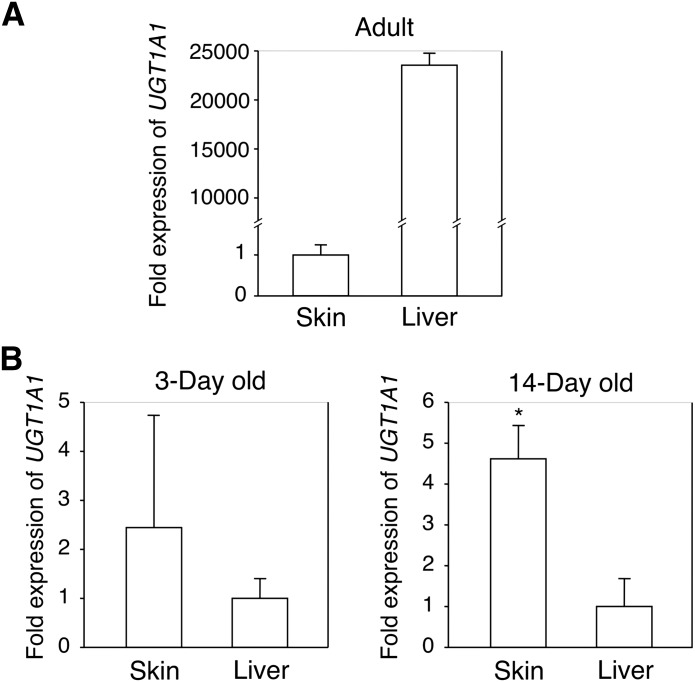
It is known that UGT1A1 is expressed in the liver. Therefore, the liver has been considered the main tissue for bilirubin metabolism in adults. In human infants, however, the liver expresses inadequate UGT1A1 (Coughtrie et al., 1988), causing the development of physiologic hyperbilirubinemia. In agreement with previous reports, human adult UGT1A1 is highly expressed in the liver: 20,000-fold higher than in skin (Fig. 5A). To understand the importance of UGT1A1 expressed in the skin in neonatal hyperbilirubinemia, we determined and compared the UGT1A1 expression levels in the skin and liver of neonatal hUGT1 mice. Whereas in adults, UGT1A1 was mainly expressed in the liver (Fig. 5A), in neonates, the expression of UGT1A1 was greater in the skin (Fig. 5B). At 3 days after birth, the expression of UGT1A1 in the skin was 2.5-fold higher than that in the liver. At 14 days after birth, which is when hUGT1 mice develop severe jaundice (Fujiwara et al., 2010), the expression of UGT1A1 in the skin was 4.5-fold greater than that in the liver. These results indicate that in neonatal life, UGT1A1 expressed in the skin might be important in reducing serum bilirubin to prevent the onset of kernicterus.
Fig. 5.
The expression of UGT1A1 in the skin and liver. Complementary DNA was synthesized from total skin and liver RNA from adult human (A) or 3- and 14-day-old hUGT1 mice (B), and qRT-PCR was carried out for human UGT1A1. Each column is the mean ± S.D. of three independent determinations. *P < 0.05.
Effects of UVB Irradiation on UGT1A1 Expression and Activity in Neonatal hUGT1 Mice.
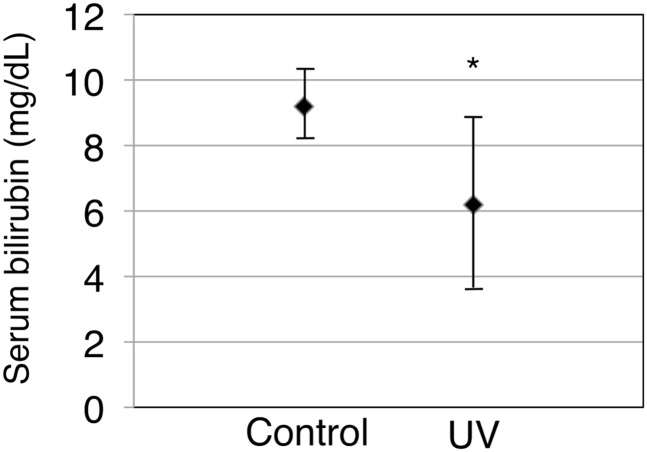
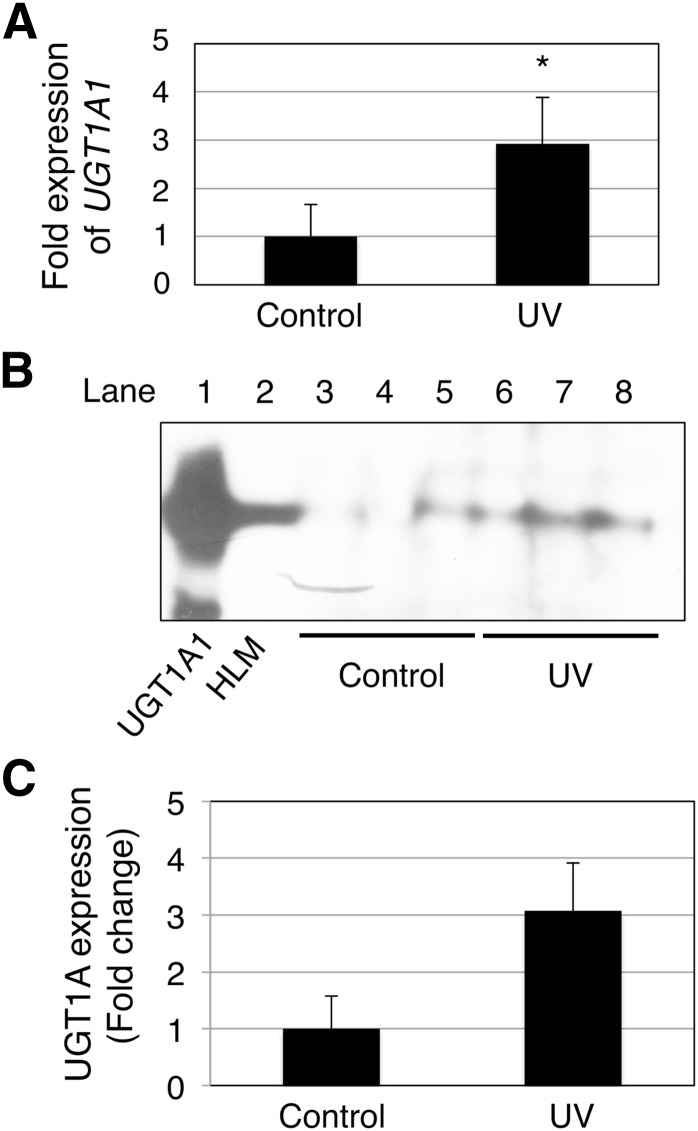
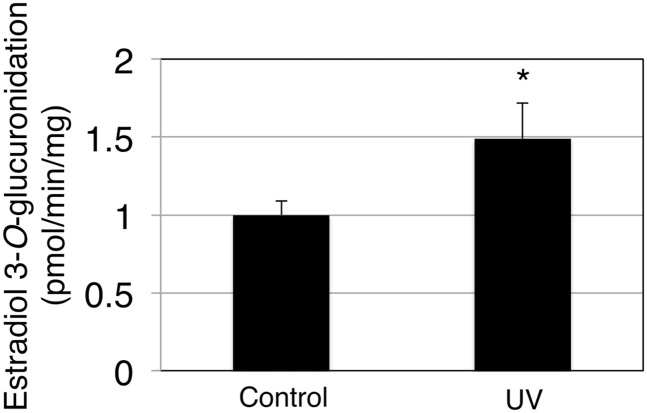
To investigate further whether UVB irradiation results in an induction of skin UGT1A1 in vivo, 3-day-old hUGT1 mice were exposed to UVB, and then serum bilirubin levels were determined, as well as UGT1A1 mRNA, protein, and activity in the skin. At 4 days after birth, hUGT1 mice had on average 9.2 mg/dl total serum bilirubin. When 3-day-old hUGT1 mice were exposed to UVB for 60 minutes, their total serum bilirubin levels decreased to 6.2 mg/dl at 4 days after birth, which was 24 hours after the UVB treatment (Fig. 6). Because bilirubin is metabolized solely by UGT1A1, this result suggests that the UVB might have induced UGT1A1 expression in the skin to accelerate bilirubin metabolism. qRT-PCR analysis revealed that the UGT1A1 level in the skin of UVB-treated mice was 3-fold higher than the level in the skin of nontreated mice (Fig. 7A). To examine the effect of the UVB treatment on UGT1A1 protein expression in the skin, skin microsomes were prepared from the control and UVB-treated mice and subjected to immunoblot analysis. Although faint, the expression of UGT1A protein in the skin from hUGT1 mice was observed in the immunoblot analysis (Fig. 7B, lanes 3–5). With the 60-minute UVB treatment of the mice, UGT1A protein expressions increased (Fig. 7B, lanes 6–8), and the level was more than 3-fold compared with the level in control mice (Fig. 7C). UGT1A1 activities in the skin microsomes were further assessed to examine whether the induced UGT1A1 mRNA/protein expression resulted in an increase in functional UGT1A1. The estradiol 3-O-glucuronide formation rate was 1.0 pmol/min per milligram and 1.5 pmol/min per milligram in the skin microsomes from control and UVB-treated hUGT1 mice, respectively (Fig. 8), indicating that UGT1A1 activities in the skin were induced with UVB. It was further demonstrated that the UVB treatment of the mice induced UGT1A1 only in the skin (Supplemental Fig. 3). This finding indicates that the reduction in serum bilirubin levels in the UVB-treated mice can be attributed mainly to the increased expression of UGT1A1 in the skin.
Fig. 6.
Serum bilirubin levels of control and UVB-treated hUGT1 mice. Blood was collected from 4-day-old hUGT1 mice, and serum bilirubin levels were measured. Error bars show S.D., n = 8. *P < 0.05.
Fig. 7.
Effects of UVB treatments on the expression of UGT1A1 in the skin of hUGT1 mice. RNA was isolated from the skin of control and UVB-treated hUGT1 mice, and qRT-PCR was carried out for human UGT1A1 (A). Microsomes from the skin were prepared and subjected to immunoblot analysis with anti-UGT1A antibodies. As the positive control, UGT1A1 supersomes (10 µg) and human liver microsomes (10 µg) were also subjected to immunoblotting (B). The density of the bands was quantified using ImageJ Software (Wayne Rasband, NIH) (C). Error bars show S.D., n = 3. *P < 0.05.
Fig. 8.
Effects of UVB treatments on the UGT1A1 activity in the skin of hUGT1 mice. Microsomes from the skin were prepared, and estradiol 3-O-glucuronide formation was measured. Error bars show S.D., n = 3. *P < 0.05.
Discussion
Because the liver plays a crucial role in the metabolism of endogenous and exogenous compounds such as drugs, environmental carcinogens, and hormones, the expression pattern of phase I and phase II drug-metabolizing enzymes in the liver has been widely analyzed (Shimada et al., 1994; Tukey and Strassburg, 2000). Meanwhile, extrahepatic tissues such as the skin, the small and large intestine, the kidneys, and the lungs also contribute to the metabolic process of a wide variety of chemicals. Because the skin covers a surface of approximately 1.7 m2 in an average adult and receives about one-third of the blood supply, it plays an important role in the metabolic clearance and metabolic activation of endogenous and exogenous compounds. Although UGT is involved in the detoxification of various compounds, a previous report indicated that UGT RNA could not be identified in human skin (Ritter et al., 1992), even though Peters et al. (1987) originally determined the presence of the bilirubin-UGT isoform and 4-nitrophenol-UGT isoform(s) in human skin. However, in vitro and in vivo analyses subsequently identified the expression of UGT in human skin (Ademola et al., 1993; Vecchini et al., 1995; Moss et al., 2000). A recent publication by Götz et al. (2012) also showed glucuronidation activity toward 4-methylumbelliferone in human skin microsomes, which indicates the presence of functional UGT enzymes in the skin. Whereas the expression pattern of human cytochromes in the skin has already been determined (Bergström et al., 2007), the expression pattern of human UGTs in the skin was not fully examined. In the present study, we performed RT-PCR and real-time RT-PCR from HaCaT cells and human skin, finding that various UGT1A family enzymes, including UGT1A1, UGT1A3, UGT1A4, UGT1A7, UGT1A8, and UGT1A10, were expressed (Fig. 1). This finding indicates that although the HaCaT cell line is a spontaneously immortalized human keratinocyte cell line, the UGT1 gene expression pattern in HaCaT cells is very similar to that in actual human skin.
Neonatal jaundice is a condition marked by high levels of serum bilirubin. Although it is generally treatable with phototherapy or phenobarbital treatment, it has a risk of causing permanent brain damage, kernicterus. The metabolic pathway of bilirubin has been reported as follows: 1) transport of bilirubin into the liver by organic anion-transporting polypeptide (Oatp1b2 in mice and OATP1B1 and 1B3 in humans) or by diffusion; 2) conjugation with a glucuronic acid by UGT1A1 in the liver; 3) transport of the glucuronide into bile by multidrug resistance–associated protein 2; 4) excretion into intestine; 5) excretion into feces as parent compound or intestinal metabolites (Kamisako et al., 2000; Kikuchi et al., 2002). However, previous studies suggest the contribution of extrahepatic tissues to bilirubin metabolism in humans (Medley et al., 1995). More recently, using hUGT1 mice, where the expression of hepatic UGT1A1 is low, extrahepatic expression of UGT1A1 in the gastrointestinal tract was shown to prevent the onset of kernicterus (Fujiwara et al., 2012). During neonatal development, UGT1A1 gene expression was greater in the skin than the level in the liver (Fig. 5). UGT1A1 expression and activity were even induced by UVB exposure of HaCaT cells, possibly by activating AhR (Figs. 3 and 4). Our in vivo study with hUGT1 mice also showed that UVB exposure of the mice caused increased UGT1A1 expression in the skin, resulting in reduction of serum bilirubin levels (Figs. 6–8). Although almost 100% of UVC (190–280 nm) is filtered by the ozone layer and does not reach the earth, a certain amount of UVB (280–315 nm) and about 95% of UVA (315–400 nm) penetrate the ozone layer, reaching the surface of the earth. Therefore, the expression of UGT1A1 and its induction by UVB might be the mechanism underlying sunlight-induced reduction of serum bilirubin in human infants. Interestingly, the UV treatment induced only UGT1A1 in the skin (Supplemental Fig. 3), which is in contrast to a finding that a treatment of hUGT1 mice with 2,3,7,8,-tetrachlorodibenzo-p-dioxin, a ligand of AhR, resulted in an increase of UGT1A1 in the liver and small intestine (Fujiwara et al., 2010). At 3 to 5 days after birth, the serum bilirubin level in humanized UGT1 mice is on average 10 mg/dl lower than that in Ugt1a1 knockout mice (Fujiwara et al., 2010), indicating that UGT1A1 expressed in hUGT1 mice can clear 10 mg/dl serum bilirubin from the body. In the current study, the serum bilirubin level in UVB-treated hUGT1 mice was 3 mg/dl lower than that in control hUGT1 mice, which indicates that clearance capacity was increased by 30% with UVB exposure.
It has been demonstrated that CYP1A1 does oxidize bilirubin, contributing to metabolizing bilirubin (Zaccaro et al., 2001). To investigate the role of CYP1A1 in the skin in contributing to the elimination of bilirubin after UV irradiation of skin, bilirubin was incubated with skin microsomes from the UV-treated hUGT1 mice in the presence and absence of the NADPH generating system. The residual concentration of bilirubin in the incubation mixture was similar between the two samples (data not shown), indicating that cytochromes P450 did not metabolize bilirubin that much in the in vitro experimental condition with skin microsomes. However, this does not eliminate the possibility that CYP1A1 contributes to the elimination of bilirubin after UV irradiation of skin in human neonates. Whereas a more complete quantification of serum bilirubin conjugation and degradation products will further support our conclusions, there is some technical difficulty in conducting such experiments, as we can only obtain up to 10 µl of serum from neonatal mice, which allows us to conduct one bilirubin assay with a bilirubinometer. We have tried to quantitate direct and indirect bilirubin contents in the serum on a small scale; however, it did not produce reproducible values as a result of the inadequate volume of serums used in the assay, in which 150 µl of serum was required by the manufacturer’s protocol.
Clinical intervention to treat episodes of severe hyperbilirubinemia calls for extended phototherapy treatment or even blood transfusion. Although exchange transfusions using the umbilical vein are now becoming extinct, phototherapy also has disadvantages. Increased insensible water loss, skin rashes, pyrexia, decreased maternal-infant interaction, and lack of visual sensory input are potential disadvantages of this treatment (Tan, 1977; Wananukul and Praisuwanna, 2002; Kumar et al., 2010). Breast-feeding has been implicated in short- and long-term health benefits to growing children, including reduced risks of infectious diarrhea, necrotizing enterocolitis (Agostoni et al., 2009), type 1 and type 2 diabetes (Mayer et al., 1988; Pettitt et al., 1997), reduced frequency of food allergies (Saarinen and Kajosaari, 1995), and a protective effect on the development of early onset inflammatory bowel disease (Barclay et al., 2009), although breast milk can also potentially induce the risk for kernicterus (Newman and Gross, 1963). Therefore, treatment of newborn infants with sunlight might be the ideal therapeutic method because it allows continuous breast-feeding. Clinical data will be needed to confirm the protective role of skin UGT1A1 against human neonatal hyperbilirubinemia in vivo.
We also observed the expression of UGT1A8 in human skin and its induction by UVB in HaCaT cells (Fig. 1; Supplemental Figs. 1 and 2). Whereas CYP1A1 is involved in the activation of benzo[a]pyrene (Nelson et al., 2004), which is one of the polycyclic aromatic hydrocarbon compounds and is involved in the UVB-associated development of skin cancer, UGT1A8 is involved in the detoxification of benzo[a]pyrene (Fang et al., 2002; Zheng et al., 2002). Therefore, UGT1A8 expressed in the skin might play a protective role against the development of skin cancer by accelerating the metabolism of benzo[a]pyrene. AhR is the sole transcription factor that can regulate the expression of CYP1A1. On the other hand, multiple transcription factors, such as pregnane X receptor and constitutive androstane receptor, and the peroxisome proliferator-activated receptor-α, in addition to AhR, can regulate human UGT gene expression (Yueh et al., 2003; Chen et al., 2005; Sugatani et al., 2005; Senekeo-Effenberger et al., 2007). Therefore, upregulation of benzo[a]pyrene-metabolizing UGTs by activation of those UGT-specific transcription factors with natural substances such as coumarins and flavonoids might result in a decrease in the development of skin cancer (Chlouchi et al., 2007).
In conclusion, this is the first study to determine the expression pattern of human UGT1A family enzymes in human skin. Our data revealed a protective role of UGT1A1 expressed in the skin against neonatal hyperbilirubinemia. Sunlight, a natural and free source of light, makes it possible to treat neonatal jaundice without phototherapy units while allowing mothers to breast-feed neonates.
Supplementary Material
Abbreviations
- AhR
aryl hydrocarbon receptor
- EROD
7-ethoxy-resorufin-O-deethylase
- FICZ
6-formylindolo[3,2-b]carbazole
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HaCaT
human skin keratinocyte
- HBSS
Hanks’ balanced salt solution
- HPLC
high-performance liquid chromatography
- hUGT1
humanized UGT1
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- RT-PCR
reverse-transcription polymerase chain reaction
- UGT
UDP-glucuronosyltransferase
Authorship Contributions
Participated in research design: Itoh, Tukey, Fujiwara.
Conducted experiments: Sumida, Kawana, Kouno, Takano, Narawa, Fujiwara.
Performed data analysis: Sumida, Kawana, Fujiwara.
Wrote or contributed to the writing of the manuscript: Itoh, Tukey, Fujiwara.
Footnotes
This work was supported by the US Public Health Service [Grants P42ES010337 and GM100481]; a Grant-in-Aid for Research Activity Start-up [24890224]; and a Kitasato University Research Grant for Young Researchers.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Ademola JI, Wester RC, Maibach HI. (1993) Metabolism of 3-indolylacetic acid during percutaneous absorption in human skin. J Pharm Sci 82:150–154 [DOI] [PubMed] [Google Scholar]
- Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, Michaelsen KF, Mihatsch W, Moreno LA, Puntis J, Shamir R, et al. ESPGHAN Committee on Nutrition (2009) Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 49:112–125 [DOI] [PubMed] [Google Scholar]
- Barclay AR, Russell RK, Wilson ML, Gilmour WH, Satsangi J, Wilson DC. (2009) Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J Pediatr 155:421–426 [DOI] [PubMed] [Google Scholar]
- Bergström MA, Ott H, Carlsson A, Neis M, Zwadlo-Klarwasser G, Jonsson CA, Merk HF, Karlberg AT, Baron JM. (2007) A skin-like cytochrome P450 cocktail activates prohaptens to contact allergenic metabolites. J Invest Dermatol 127:1145–1153 [DOI] [PubMed] [Google Scholar]
- Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL. (1994) Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem 269:17960–17964 [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Chen S, Beaton D, Nguyen N, Senekeo-Effenberger K, Brace-Sinnokrak E, Argikar U, Remmel RP, Trottier J, Barbier O, Ritter JK, et al. (2005) Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J Biol Chem 280:37547–37557 [DOI] [PubMed] [Google Scholar]
- Chlouchi A, Girard C, Bonet A, Viollon-Abadie C, Heyd B, Mantion G, Martin H, Richert L. (2007) Effect of chrysin and natural coumarins on UGT1A1 and 1A6 activities in rat and human hepatocytes in primary culture. Planta Med 73:742–747 [DOI] [PubMed] [Google Scholar]
- Coughtrie MW, Burchell B, Leakey JE, Hume R. (1988) The inadequacy of perinatal glucuronidation: immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol Pharmacol 34:729–735 [PubMed] [Google Scholar]
- Danoff TM, Campbell DA, McCarthy LC, Lewis KF, Repasch MH, Saunders AM, Spurr NK, Purvis IJ, Roses AD, Xu CF. (2004) A Gilbert’s syndrome UGT1A1 variant confers susceptibility to tranilast-induced hyperbilirubinemia. Pharmacogenomics J 4:49–53 [DOI] [PubMed] [Google Scholar]
- Dutton GJ. (1980) Acceptor substrates of UDP glucuronosyltransferase and their assay, in Glucuronidation of Drugs and Other Compounds (Dutton GJ. ed) pp 69–78, CRC Press, Boca Raton, FL [Google Scholar]
- Fang JL, Beland FA, Doerge DR, Wiener D, Guillemette C, Marques MM, Lazarus P. (2002) Characterization of benzo(a)pyrene-trans-7,8-dihydrodiol glucuronidation by human tissue microsomes and overexpressed UDP-glucuronosyltransferase enzymes. Cancer Res 62:1978–1986 [PubMed] [Google Scholar]
- Fujiwara R, Chen S, Karin M, Tukey RH. (2012) Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-κB leads to hyperbilirubinemia. Gastroenterology 142:109–118, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R, Nakajima M, Yamanaka H, Nakamura A, Katoh M, Ikushiro S, Sakaki T, Yokoi T. (2007) Effects of coexpression of UGT1A9 on enzymatic activities of human UGT1A isoforms. Drug Metab Dispos 35:747–757 [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nguyen N, Chen S, Tukey RH. (2010) Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci USA 107:5024–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz C, Pfeiffer R, Tigges J, Ruwiedel K, Hübenthal U, Merk HF, Krutmann J, Edwards RJ, Abel J, Pease C, et al. (2012) Xenobiotic metabolism capacities of human skin in comparison with a 3D-epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: phase II enzymes. Exp Dermatol 21:364–369 [DOI] [PubMed] [Google Scholar]
- Gourley GR. (1997) Bilirubin metabolism and kernicterus. Adv Pediatr 44:173–229 [PubMed] [Google Scholar]
- Kamisako T, Kobayashi Y, Takeuchi K, Ishihara T, Higuchi K, Tanaka Y, Gabazza EC, Adachi Y. (2000) Recent advances in bilirubin metabolism research: the molecular mechanism of hepatocyte bilirubin transport and its clinical relevance. J Gastroenterol 35:659–664 [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, Yonemura S, Yamagishi H, Keppler D, Tsukita S, et al. (2002) Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet 31:320–325 [DOI] [PubMed] [Google Scholar]
- Kumar P, Murki S, Malik GK, Chawla D, Deorari AK, Karthi N, Subramanian S, Sravanthi J, Gaddam P, Singh SN. (2010) Light emitting diodes versus compact fluorescent tubes for phototherapy in neonatal jaundice: a multi center randomized controlled trial. Indian Pediatr 47:131–137 [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15:677–685 [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Miners JO, McKinnon RA. (2000) Polymorphisms in UDP glucuronosyltransferase genes: functional consequences and clinical relevance. Clin Chem Lab Med 38:889–892 [DOI] [PubMed] [Google Scholar]
- Mayer EJ, Hamman RF, Gay EC, Lezotte DC, Savitz DA, Klingensmith GJ. (1988) Reduced risk of IDDM among breast-fed children: the Colorado IDDM Registry. Diabetes 37:1625–1632 [DOI] [PubMed] [Google Scholar]
- Medley MM, Hooker RL, Rabinowitz S, Holton R, Jaffe BM. (1995) Correction of congenital indirect hyperbilirubinemia by small intestinal transplantation. Am J Surg 169:20–27 [DOI] [PubMed] [Google Scholar]
- Moss T, Howes D, Williams FM. (2000) Percutaneous penetration and dermal metabolism of triclosan (2,4, 4′-trichloro-2′-hydroxydiphenyl ether). Food Chem Toxicol 38:361–370 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. (2008) Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Dispos 36:1461–1464 [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14:1–18 [DOI] [PubMed] [Google Scholar]
- Newman AJ, Gross S. (1963) Hyperbilirubinemia in breast-fed infants. Pediatrics 32:995–1001 [PubMed] [Google Scholar]
- Peters WH, Allebes WA, Jansen PL, Poels LG, Capel PJ. (1987) Characterization and tissue specificity of a monoclonal antibody against human uridine 5′-diphosphate-glucuronosyltransferase. Gastroenterology 93:162–169 [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Forman MR, Hanson RL, Knowler WC, Bennett PH. (1997) Breastfeeding and incidence of non-insulin-dependent diabetes mellitus in Pima Indians. Lancet 350:166–168 [DOI] [PubMed] [Google Scholar]
- Ritter JK, Chen F, Sheen YY, Tran HM, Kimura S, Yeatman MT, Owens IS. (1992) A novel complex locus UGT1 encodes human bilirubin, phenol, and other UDP-glucuronosyltransferase isozymes with identical carboxyl termini. J Biol Chem 267:3257–3261 [PubMed] [Google Scholar]
- Saarinen UM, Kajosaari M. (1995) Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet 346:1065–1069 [DOI] [PubMed] [Google Scholar]
- Salih FM. (2001) Can sunlight replace phototherapy units in the treatment of neonatal jaundice? An in vitro study. Photodermatol Photoimmunol Photomed 17:272–277 [DOI] [PubMed] [Google Scholar]
- Schoop VM, Mirancea N, Fusenig NE. (1999) Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J Invest Dermatol 112:343–353 [DOI] [PubMed] [Google Scholar]
- Senekeo-Effenberger K, Chen S, Brace-Sinnokrak E, Bonzo JA, Yueh MF, Argikar U, Kaeding J, Trottier J, Remmel RP, Ritter JK, et al. (2007) Expression of the human UGT1 locus in transgenic mice by 4-chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic acid (WY-14643) and implications on drug metabolism through peroxisome proliferator-activated receptor alpha activation. Drug Metab Dispos 35:419–427 [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. (1994) Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270:414–423 [PubMed] [Google Scholar]
- Sugatani J, Nishitani S, Yamakawa K, Yoshinari K, Sueyoshi T, Negishi M, Miwa M. (2005) Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol Pharmacol 67:845–855 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H, Chida K. (2010) Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 67:361–365 [DOI] [PubMed] [Google Scholar]
- Tan KL. (1977) The nature of the dose-response relationship of phototherapy for neonatal hyperbilirubinemia. J Pediatr 90:448–452 [DOI] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616 [DOI] [PubMed] [Google Scholar]
- Vecchini F, Mace K, Magdalou J, Mahe Y, Bernard BA, Shroot B. (1995) Constitutive and inducible expression of drug metabolizing enzymes in cultured human keratinocytes. Br J Dermatol 132:14–21 [DOI] [PubMed] [Google Scholar]
- Wananukul S, Praisuwanna P. (2002) Clear topical ointment decreases transepidermal water loss in jaundiced preterm infants receiving phototherapy. J Med Assoc Thai 85:102–106 [PubMed] [Google Scholar]
- Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. (2009) The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem 284:2690–2696 [DOI] [PubMed] [Google Scholar]
- Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. (2003) Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J Biol Chem 278:15001–15006 [DOI] [PubMed] [Google Scholar]
- Zaccaro C, Sweitzer S, Pipino S, Gorman N, Sinclair PR, Sinclair JF, Nebert DW, De Matteis F. (2001) Role of cytochrome P450 1A2 in bilirubin degradation Studies in Cyp1a2 (-/-) mutant mice. Biochem Pharmacol 61:843–849 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Fang JL, Lazarus P. (2002) Glucuronidation: an important mechanism for detoxification of benzo[a]pyrene metabolites in aerodigestive tract tissues. Drug Metab Dispos 30:397–403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.