Abstract
Background
Recent evidence suggests that Rho‐kinase (ROCK) plays an important role in the pathogenesis of atherosclerosis and a marker of atherosclerotic burden. Polyvascular disease with concomitant peripheral arterial disease (PAD) and coronary artery disease (CAD) is common and associated with a worse prognosis. The aim of this study was to evaluate ROCK activity as a marker of polyvascular disease.
Hypothesis
Methods
We retrospectively analyzed patients undergoing coronary angiography at our institution between February 2009 and May 2009. Patients with only CAD (n = 40) defined by coronary artery stenosis of ≥50% by angiography, only PAD (n = 40) defined by an ankle brachial index (ABI) <0.9, and combined CAD/PAD (n = 40) were matched by age and sex to control patients (n = 40) without CAD or PAD. ROCK activity was determined by phosphorylation of the myosin binding subunit in leukocytes and then compared between each group. Multivariate analysis was used to determine independent predictors of polyvascular disease. Discriminative ability of elevated ROCK activity was assessed using receiver operator characteristics (ROC) curves.
Results
Patients (age 68 ± 12 years, 79% male) with CAD, PAD, and CAD/PAD had a mean ABI of 1.08, 0.62, and 0.65, respectively, compared to 1.08 in the control group. There was an incremental increase in ROCK activity in patients with CAD (4.61 ± 2.11), PAD (4.27 ± 1.39), and CAD/PAD (5.96 ± 1.94) compared to control (2.40 ± 0.43) (all P < 0.05). ROCK activity (odds ratio: 4.53, 95% confidence interval: 1.26‐6.30) was an independent predictor of polyvascular disease. The ROCK cutoff value of 4.85 had a sensitivity of 72.7% and a specificity of 65.7%, with an area under ROC curve of 0.71 for polyvascular disease.
Conclusions
Patients with concomitant peripheral and coronary arterial disease are associated with increased Rho‐kinase activity. Rho‐kinase activity may be a potential marker of atherosclerotic burden for patients with polyvascular disease.
Introduction
Atherosclerosis is a systemic disease that can affect the coronary artery, peripheral arteries, and cerebrovascular arteries.1 Coronary artery disease (CAD) is prevalent in patients with peripheral arterial disease (PAD), and detection of PAD in patients with CAD is important for several reasons.1 Concomitant CAD and PAD is associated with a 2‐ to 3‐fold increased risk of cardiovascular disease (CVD) mortality.1 Patients with PAD have more severe CAD manifested by higher frequency of left‐main and multivessel CAD, suggesting a greater burden of atherosclerosis and myocardial ischemia.2 Patients with PAD are associated with physical function impairment and worse quality of life, which often exceed that observed in patients with other forms of CVD.3 Early diagnosis of PAD in patients with CAD should prompt aggressive risk factor modification to slow progression of atherosclerosis, and prevent premature deaths, heart attack, and stroke.
Rho‐kinase (ROCK) is 1 of the effectors of the small guanine nucleotide‐binding protein Rho, which regulates a wide range of fundamental cell functions and is emerging as a potential therapeutic target in CVD.4 Growing evidence suggests that the Rho/ROCK system may play an important role in the pathogenesis of atherosclerosis involving vascular smooth muscle cells contraction, platelet aggregation and activation, regulation of endothelial nitric oxide synthase synthesis,5 and other important steps in the inflammatory process.4 The aim of this study was to assess the discriminative ability of increased ROCK activity in detecting patients with increasing atherosclerotic burden among patients with only or concomitant PAD or CAD.
Methods
Study Subjects and Study Design
We retrospectively analyzed patients who underwent coronary angiography at our institution between February 2009 and May 2009. Patients with only CAD (n = 40), PAD (n = 40), and combined CAD/PAD (n = 40) were identified. CAD was defined by the presence of ≥50% stenosis in at least 1 major epicardial coronary artery on angiography. The presence of PAD was defined by an ankle brachial index (ABI) ≤0.9 using the Omron VP‐2000 Non‐Invasive Vascular Profile Device (Omron Healthcare, Inc. Bannockburn, IL). The ABI (the ratio of the systolic blood pressure at the ankle to the higher systolic blood pressure at the arm) was calculated for both limbs. We used the lower ABI in either leg to determine the presence of PAD. Coronary angiograms were reviewed by an experienced cardiologist blinded to the individual's ABI. An age‐ and sex‐matched control group (n = 40) were identified without CAD or PAD. Brachial ankle pulse wave velocity (ba‐PWV) was also measured using the Omron VP‐2000 for each lower limb, and the higher ba‐PWV was recorded. Physical function was measured using the 6‐minute walk test. Following a standardized protocol, participants walk up and down a hallway for 6 minutes after instructions to cover as much distance as possible. The distance completed during the 6‐minutes was recorded. All the additional tests were done in the clinic before the patients were selected into this study. Only the patients with suitable ABI and coronary angiography were recruited into the study.
Laboratory
All blood samples were collected during admission for coronary angiography. All samples were collected into Ethylene Diamine Tetraacetic Acid‐Na tubes without stasis and were centrifuged at 2200g for 20 minutes within 30 minutes of sample collection. Plasma and serum samples were collected into aliquots and stored at −80°C until batch analysis. Plasma samples were used for measurement of B‐type natriuretic peptide (BNP), using with Triage Meters, a fluorescence immunoassay (Triage BNP; Biosite Diagnostics, San Diego, CA). The high‐sensitivity C‐reactive protein (hs‐CRP) enzyme‐linked immunosorbent assay (DSL‐10‐42100UsCRP ELISA KIT, Diagnostic Systems, Laboratories, Inc, US) kit was used for hs‐CRP testing.
Assay for Leukocyte Rho‐Kinase Activity
Leukocytes were isolated from 10 mL peripheral blood following a validated and standardized protocol.6 The leukocytes were frozen and stored at −80°C until all samples were collected. The ROCK assays were performed on all leukocyte samples at the same time. The samples were analyzed by Western blotting for the phosphorylation of the myosin‐binding subunit (MBS) of myosin light‐chain phosphatase with an antibody that specifically recognizes phosphorylated Thr853 MBS.6 Interexperimental results were standardized to lysophosphatidic acid‐induced MBS phosphorylation (positive control).
Statistical Analysis
All statistical analyses were conducted with the SPSS statistical package for Vista version 15.0 (IBM, Armonk, NY). One‐way analysis of variance was used for comparing mean values of continuous variables among groups, and post hoc analysis was performed by Scheffe test to examine for intergroup differences. Univariate linear regression (Pearson and Spearman correlation) models were used to assess the relation between parametric clinical variables and ROCK activity. Logistic regression analysis using the enter method was used to identify independent predictors of CAD/PAD. Receiver operating characteristics (ROC) analysis was performed to determine the best cutoff value of ROCK activity to polyvascular disease in the patient cohort. Data were expressed as mean ± standard deviation, and a 2‐sided P value of <0.05 was considered statistically significant.
Results
Clinical Characteristics
The mean age of patients was 68 ± 12 years, and the patients were predominantly male (79%). Smoking, hypertension, hypercholesterolemia, and type 2 diabetes were matched in each subgroup (CAD, PAD, CAD/PAD). Physical function capacity assessed by a 6‐minute walk test showed a progressive and predictable decline in walking distance in patients with CAD, PAD, and CAD/PAD compared to control (P < 0.01). ba‐PWV was significantly lower in the control group than in the diseased groups. Both hs‐CRP and ROCK activity were significantly higher in the diseased groups than the control group (Figure 1).
ROCK Activity
ROCK activity in the CAD/PAD group (5.96 ± 1.94) was significantly higher than that of the other 3 groups (control: 2.4 ± 0.43, CAD: 4.61 ± 2.11, PAD: 4.27 ± 1.39) (all P < 0.05) (Table 2). Similarly, ROCK activity in the PAD and CAD groups was higher than that of the control group (P < 0.05). There was no significant difference in ROCK activity between the PAD and CAD groups (Figure 1).
Table 2.
Laboratory Testing of PAD, CAD/PAD, and Control Groups
| CAD, n = 30 | PAD, n = 30 | CAD/PAD, n = 30 | Control, n = 35 | ANOVA P | |
|---|---|---|---|---|---|
| ROCK activity | 4.61 ± 2.11a | 4.27 ± 1.39a | 5.96 ± 1.94ab | 2.4 ± 0.43 | <0.001 |
| eGFR, mL/min | 65.6 ± 20.6 | 53.7 ± 20.6a | 62.6 ± 30.1 | 76.4 ± 20.1 | 0.029 |
| hs‐CRP, mg/L | 3.10 ± 4.37a | 3.58 ± 2.64a | 3.11 ± 2.05a | 2.05 ± 1.65 | 0.938 |
| BNP, pg/mL | 92.9 ± 83.5 | 112.6 ± 158.0 | 139.2 ± 189.1 | 33.2 ± 28.3 | 0.265 |
| Urea, mmol/L | 6.89 ± 3.07 | 8.33 ± 3.19 | 8.5 ± 4.64 | 6.0 ± 2.21 | 0.127 |
| Total cholesterol, mg/dL | 3.9 ± 0.8b | 4.7 ± 1.3 | 4.3 ± 0.7 | 4.9 ± 1.0 | 0.022 |
| Triglycerides, mg/dL | 1.7 ± 1.0 | 1.5 ± 0.6 | 1.4 ± 0.6 | 1.4 ± 1.1 | 0.644 |
| LDL cholesterol, mg/dL | 2.0 ± 0.7b | 2.8 ± 1.2 | 2.4 ± 0.6 | 2.9 ± 0.8 | 0.018 |
| HDL cholesterol, mg/dL | 1.2 ± 0.3 | 1.2 ± 0.5 | 1.3 ± 0.3 | 1.5 ± 0.4 | 0.114 |
| Fasting glucose, mg/dL | 5.9 ± 1.0 | 6.5 ± 2.3 | 6.8 ± 3.5 | 6.5 ± 1.8 | 0.635 |
Abbreviations: ANOVA, analysis of various; BNP, B‐type natriuretic peptide; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; PAD, peripheral arterial disease; ROCK, Rho‐kinase.
P < 0.05 vs control group.
P < 0.05 vs CAD and PAD groups.
P < 0.05 vs PAD and control groups.
Figure 1.
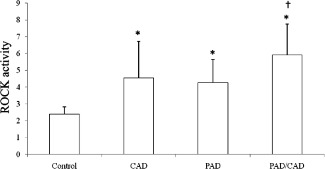
Comparison of Rho‐kinase (ROCK) activity in the control, coronary artery disease (CAD), peripheral arterial disease (PAD), and CAD/PAD groups. *P < 0.05 vs control group. †P < 0.05 vs coronary artery disease group and peripheral arterial disease group.
Table 1.
Clinical Characteristics of PAD, CAD/PAD, and Control Groups
| CAD, n = 40 | PAD, n = 40 | CAD/PAD, n = 40 | Control, n = 40 | ANOVA P | |
|---|---|---|---|---|---|
| Male sex, n (%) | 32 (80) | 33 (82.5) | 30 (75) | 30 (75) | 0.786 |
| Age, y | 66.2 ± 11.9 | 71.4 ± 11.2 | 70.2 ± 9.6 | 68.1 ± 8.1 | 0.654 |
| SBP, mm Hg | 134.2 ±4.5 | 149.3 ± 24.7 | 142.8 ± 37.2 | 143.6 ± 23.6 | 0.632 |
| DBP, mm Hg | 80.0 ± 12.7 | 78.2 ± 11.5 | 77.6 ± 17.4 | 78.8 ± 9.1 | 0.986 |
| ΔP, mm Hg | 57.7 ± 18.1 | 71.2 ± 21.4 | 65.1 ± 26.8 | 64.7 ± 21.2 | 0.658 |
| Heart rate, bpm | 62.2 ± 7.1 | 71.5 ± 10.1 | 71.6 ± 11.1 | 67.7 ± 9.8 | 0.896 |
| Body mass index, kg/m2 | 24.3 ± 3.5 | 22.9 ± 3.2 | 21.8 ± 5.3 | 25.7 ± 7.4 | 0.365 |
| ABI | 1.08 ± 0.1 | 0.62 ± 0.1a | 0.65 ± 0.2a | 1.08 ± 0.1 | <0.001 |
| Pulse wave velocity, m/s | 15.8 ± 2.3 | 16.0 ± 4.7 | 15.4 ± 4.3 | 11.3 ± 4.0b | 0.005 |
| 6‐minute meter | 317.0 ± 91.2 | 286.6 ± 97.7 | 251.8±75.5 | 394±105.5b | 0.003 |
| Smoking (current), n (%) | 4 (10) | 4 (10) | 3 (8) | 4 (10) | 0.841 |
| Hyperlipidemia, n (%) | 5 (13) | 9 (23) | 9 (23) | 6 (15) | 0.851 |
| Hypertension, n (%) | 9 (23) | 11 (28) | 12 (30) | 18 (45) | 0.746 |
| Diabetes mellitus, n (%) | 12 (30) | 11 (28) | 12 (30) | 2 (5)b | 0.022 |
Abbreviations: ABI, ankle brachial index; ANOVA, analysis of various; CAD, coronary artery disease; DBP, diastolic blood pressure; PAD, peripheral arterial disease; SDP, systolic blood pressure.
P < 0.05 vs CAD and control groups.
P < 0.05 vs CAD, PAD, and CAD/PAD groups.
Independent Predictors for Concomitant PAD and CAD
After adjusting for hs‐CRP, diabetes mellitus, and smoking status, ROCK activity (odds ratio [OR]: 4.53, 95% confidence interval [CI]: 1.26‐6.30) and ABI (OR: 0.723, 95% CI: 0.578‐0.857) were identified as independent predictors for concomitant CAD/PAD (Table 3). A ROCK cutoff value of 4.85 had a sensitivity of 72.7% and a specificity of 65.7%, with an area under ROC curve of 0.71 for polyvascular disease.
Table 3.
Logistic Regression Analyses of Peripheral Arterial Disease Plus Coronary Artery Disease in the Cohort of All Patients (P < 0.05)
| Multivariate (Enter) | |||
|---|---|---|---|
| OR | P | 95% CI | |
| Age | 1.129 | 0.351 | 0.141‐2.142 |
| Sex | 2.347 | 0.749 | 0.873‐4.101 |
| Hypertension | 2.498 | 0.672 | 0.283‐3.291 |
| ROCK activity | 4.526 | 0.021 | 1.257‐6.295 |
| ABI | 0.723 | 0.003 | 0.578‐0.857 |
| hs‐CRP | 1.165 | 0.898 | 0.564‐1.652 |
| Renal function (eGFR) | 3.987 | 0.217 | 0.035‐7.219 |
| Diabetes mellitus | 5.532 | 0.532 | 0.012‐9.761 |
| Smoker | 2.180 | 0.280 | 0.498‐4.118 |
| Hyperlipidemia | 2.561 | 0.451 | 0.039‐5.783 |
Abbreviations: ABI, ankle brachial index; CI, confidence interval; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; OR, odds ratio; ROCK, Rho‐kinase.
Discussion
In this study, we demonstrated that ROCK activity was increased in patients with CAD and PAD. Patients with polyvascular disease involving both CAD and PAD had significantly higher ROCK activity than patients with disease involving 1 vascular territory. ROCK activity may be useful as a marker of atherosclerotic and inflammation burden of polyvascular disease.
To our knowledge, this is the first study to explore the expression of ROCK in PAD and its diagnostic value in polyvascular arterial diseases. Recently, elevated ROCK activity was proven to involve in cerebral and coronary vasospasm, hypertension, pulmonary hypertension, atherosclerosis, and metabolic syndrome.4 In humans, ROCK activity was increased in current smokers, and there was a positive relationship between ROCK activity and endothelial dysfunction.7 In healthy male subjects, ROCK activity was significantly associated with arterial stiffness assessed by carotid femoral pulse wave velocity.8
In a study by Feske et al., ROCK activity was increased in acute stroke patients, suggesting its pathogenesis role in acute ischemia.9 Accumulating evidence suggests that ROCK is involved in the vascular effects of various vasoactive factors, including angiotensin II, thrombin, endothelin‐1, and serotonin.4 On the other hand, within fibroblasts and inflammatory cells, ROCK upregulates proinflammatory molecules including activator protein‐1, Nuclear Factor‐KappaB (NF‐κB),10 nicotinamide‐adenine dinucleotide phosphate (NAD(P)H),11 interleukin‐6,12 monocyte chemoattractant protein‐1,13 macrophage migration inhibitory factor,14 and interferon‐γ,14 all of which were involved in the pathogenesis of atherosclerosis. Thus, Rho‐kinase may play an important role in the pathogenesis of arteriosclerosis. Furthermore, long‐term inhibition of ROCK has been shown to cause marked regression of coronary arteriosclerosis and disappearance of coronary vasospastic activities in vivo in a pig model.15 Rho/ROCK signaling inhibition by 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitors (statins) offers a potential mechanism for some of the pleotropic effects of these agents.16 Importantly, Nohria et al. demonstrated that statins inhibit ROCK activity and improve endothelial function in patients with stable atherosclerosis.17 In this study, ROCK activity increased in CAD or PAD when compared with normal control. Similar findings were found in 2 recent studies by Kikuchi et al18 and Hung et al.19 The treatment with antispastic agents substantially reduced the level of leukocyte ROCK activity.19 Interestingly, there was no significant difference of ROCK activity between CAD and PAD in our study.
Identification of patients with PAD among CAD or CAD among PAD provides valuable prognostic information for risk stratification ABI testing as an accurate noninvasive index for diagnosis of PAD for most patients. However, it can underestimate the presence of PAD among high‐risk patients with calcified lower limb vessels such as the elderly, diabetics, and dialysis patients because of a falsely high lower limb systolic pressure reading. Thus, finding a biomarker to improve the diagnostic accuracy of ABI among these high‐risk patients would be clinically useful. Smoking, hypertension, hypercholesterolemia, and type 2 diabetes were proven the majority of risks associated with the development of clinically significant PAD. Thus, the percentage of medical risk factors of each subgroup (CAD, PAD, CAD/PAD) was matched to minimize the effect of different cardiovascular risk factors, which could affect ROCK activity. However, although in this study the ROC curve of ROCK activity for polyvascular disease was statistically good, we may consider ROCK activity useful for evaluating severity of atherosclerosis but not an independent predictor of polyvascular disease. Other biomarkers associated with atherosclerosis burden have been identified, such as D dimer, plasminogen, prothrombin fragment 1t2, plasminogen activator inhibitor, and thrombomodulin.20 Many of them were either an inflammatory or atherosclerosis marker, but none appear to measure atherosclerotic burden. Previously, a 4‐biomarker panel comprising β2M, cystatin C, hs‐CRP, and glucose was associated with the presence of PAD independent of traditional risk factors.21 There was no significant difference in hs‐CRP among CAD, PAD, and CAD/PAD groups in our study despite significantly higher ROCK activity in the CAD/PAD group. Inflammation has been shown to play a key role in the pathogenesis of atherosclerosis, but increased levels of inflammation may not reflect the severity of disease or atherosclerotic burden.22 Increase in leucocyte ROCK activity has been shown to be associated with both inflammation and oxidative stress.23 Therefore, increased ROCK activity may reflect an increased systemic reactive oxygen species in patients with CAD/PAD and a potential marker of atherosclerotic burden.
Limitations
The main limitation of this study was the small sample size. It would be interesting to study the changes of ROCK activity in the patients of atherosclerotic disease (CAD, PAD, or both) on drug treatment (eg, statins). It would also be interesting to study if ROCK activity help to predict long‐term cardiovascular endpoints.
Conclusion
Our study demonstrated that ROCK activity was associated with inflammation and atherosclerotic burden. Patients with polyvascular disease involving both coronary and peripheral arteries had high ROCK activity compared to patients with arterial diseases involving 1 vascular territory such as CAD and PAD. Increased ROCK activity may be a potential marker for polyvascular disease.
Ming Dong PhD and Xin Jiang PhD are co‐first authors.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Steg PG, Bhatt DL, Wilson PW, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 2. Moussa ID, Jaff MR, Mehran R, et al. Prevalence and prediction of previously unrecognized peripheral arterial disease in patients with coronary artery disease: the Peripheral Arterial Disease in Interventional Patients Study. Catheter Cardiovasc Interv. 2009;73:719–724. [DOI] [PubMed] [Google Scholar]
- 3. Wilson WR, Fitridge RA, Weekes AJ, et al. Quality of life of patients with peripheral arterial disease and chronic stable angina. Angiology. 2012;63:223–228. [DOI] [PubMed] [Google Scholar]
- 4. Dong M, Yan BP, Liao JK, et al. Rho‐kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today. 2010;15:622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bussemaker E, Pistrosch F, Forster S, et al. Rho kinase contributes to basal vascular tone in humans: role of endothelium‐derived nitric oxide. Am J Physiol Heart Circ Physiol. 2007;293:H541–H547. [DOI] [PubMed] [Google Scholar]
- 6. Liu PY, Liao JK. A method for measuring Rho kinase activity in tissues and cells. Methods Enzymol. 2008;439:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noma K, Goto C, Nishioka K, et al. Smoking, endothelial function, and Rho‐kinase in humans. Arterioscler Thromb Vasc Biol. 2005;25:2630–2635. [DOI] [PubMed] [Google Scholar]
- 8. Noma K, Goto C, Nishioka K, et al. Roles of rho‐associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feske SK, Sorond FA, Henderson GV, et al. Increased leukocyte ROCK activity in patients after acute ischemic stroke. Brain Res. 2009;1257:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montaner S, Perona R, Saniger L, et al. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. [DOI] [PubMed] [Google Scholar]
- 11. Higashi M, Shimokawa H, Hattori T, et al. Long‐term inhibition of Rho‐kinase suppresses angiotensin II‐induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. [DOI] [PubMed] [Google Scholar]
- 12. Radeff JM, Nagy Z, Stern PH. Rho and Rho kinase are involved in parathyroid hormone‐stimulated protein kinase C alpha translocation and IL‐6 promoter activity in osteoblastic cells. J Bone Miner Res. 2004;19:1882–1891. [DOI] [PubMed] [Google Scholar]
- 13. Funakoshi Y, Ichiki T, Shimokawa H, et al. Rho‐kinase mediates angiotensin II‐induced monocyte chemoattractant protein‐1 expression in rat vascular smooth muscle cells. Hypertension. 2001;38:100–104. [DOI] [PubMed] [Google Scholar]
- 14. Hattori T, Shimokawa H, Higashi M, et al. Long‐term treatment with a specific Rho‐kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. [DOI] [PubMed] [Google Scholar]
- 15. Morishige K, Shimokawa H, Eto Y, et al. Adenovirus‐mediated transfer of dominant‐negative rho‐kinase induces a regression of coronary arteriosclerosis in pigs in vivo. Arterioscler Thromb Vasc Biol. 2001;21:548–554. [DOI] [PubMed] [Google Scholar]
- 16. Hernandez‐Perera O, Perez‐Sala D, Soria E, et al. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin‐1 gene expression by simvastatin in vascular endothelial cells. Circ Res. 2000;87:616–622. [DOI] [PubMed] [Google Scholar]
- 17. Nohria A, Prsic A, Liu PY, et al. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kikuchi Y, Yasuda S, Aizawa K, et al. Enhanced Rho‐kinase activity in circulating neutrophils of patients with vasospastic angina: a possible biomarker for diagnosis and disease activity assessment. J Am Coll Cardiol. 2011;58:1231–1237. [DOI] [PubMed] [Google Scholar]
- 19. Hung MJ, Cherng WJ, Hung MY, et al. Increased leukocyte Rho‐associated coiled‐coil containing protein kinase activity predicts the presence and severity of coronary vasospastic angina. Atherosclerosis. 2012;221:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mota AP, de Castro Santos ME, Lima e Silva FC, et al. Hypercoagulability markers in patients with peripheral arterial disease: association to ankle‐brachial index. Angiology. 2009;60:529–535. [DOI] [PubMed] [Google Scholar]
- 21. Fung ET, Wilson AM, Zhang F, et al. A biomarker panel for peripheral arterial disease. Vasc Med. 2008;13:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krause KJ, Williams DS, White N. Baseline distribution and correlation analysis of hsCRP in an insurance applicant population. J Insur Med. 2008;40:124–129. [PubMed] [Google Scholar]
- 23. Noma K, Rikitake Y, Oyama N, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]


