Abstract
Background
Rho-associated kinases (ROCKs) play an important role in Ca2+ sensitization and vascular resistance. Activation of ROCKs is associated with hypertension. The purpose of this study was to evaluate the effect of the calcium channel blocker amlodipine on ROCKs activity in patients with hypertension.
Methods
We evaluated ROCK activity in peripheral leukocytes by Western blot analysis in 651 patients with hypertension treated with antihypertensive agents, 28 untreated hypertensive patients and 28 healthy subjects, and the effects of treatment with amlodipine or losartan for 12 weeks on ROCK activity in 28 untreated hypertensive patients who were randomly divided into an amlodipine group (n=14) and a losartan group (n=14). ROCK activity was defined as the ratio of phospho myosin-binding subunit (MBS) on myosin light-chain phosphatase to total-MBS.
Results
Leukocyte ROCK activity was greater in untreated patients with essential hypertension than in the healthy subjects (0.84±0.24 vs. 0.61±0.18, P=0.03). In 651 patients with hypertension treated with antihypertensive agents, ROCK activity was significantly lower in the calcium channel blocker-treated group than in the groups treated with renin-angiotensin system inhibitors, diuretics, and β blockers (0.70±0.24 vs. 0.85±0.29, 0.83±0.24, and 0.86±0.31, P<0.05, respectively). ROCK activity after 4 weeks and 12 weeks of treatment was significantly decreased in the amlodipine group (0 weeks: 0.85±0.25, 4 weeks: 0.66±0.16, 12 weeks: 0.64±0.15, P<0.05, respectively) but not in the losartan group, while the antihypertensive effects were similar in the two groups.
Conclusion
These findings suggest that calcium channel blocker amlodipine inhibits ROCK activity in patients with hypertension.
Introduction
Rho-associated kinases (ROCKs), one of the first downstream targets of the small GTP-binding protein Rho A, mediate various cellular physiologic functions, such as cell proliferation, migration, adhesion, apoptosis and contraction,1-4 involved in the pathogenesis of atherosclerosis. Activation of ROCKs induces an increase in vascular smooth muscle cell (VSMC) contraction. Increase in peripheral artery resistance and remodeling of arteries induced by activation of ROCKs may play an important role in the maintenance and development of hypertension. Indeed, the Rho/ROCK pathway is activated in experimental models of hypertension5-7 and patients with hypertension.8 In addition, it is well known that activation of calcium channels contributes to increased peripheral artery resistance through an increase in intracellular calcium concentration, while the Rho-ROCK pathway regulates the contraction of VSMCs through subsequent phosphorylation of myosin light chain independent of intracellular calcium concentration. Both inhibition of calcium channels and ROCK activities reduces VSMC contraction, leading to decreased peripheral artery resistance, which results in reduction in blood pressure. Antihypertensive agents other than calcium channels blockers also have antihypertensive effects through several mechanisms. It is thought that treatment of hypertension with antihypertensive agents inhibits ROCK activity. However, there is no information on the effects of antihypertensive agents on ROCK activity in patients with hypertension.
In this study, we therefore evaluated 1) leukocyte ROCK activity in patients with hypertension and healthy subjects, 2) leukocyte ROCK activity in patients with hypertension treated with antihypertensive drugs, and 3) the effect of the calcium channel blocker amlodipine on leukocyte ROCK activity before and after 4 and 12 weeks of treatment in patients with essential hypertension.
Methods
Study protocol 1. ROCK activity in patients with essential hypertension
We studied 28 untreated patients with essential hypertension (21 men and 7 women; mean age, 53±9 years) and 28 healthy subjects (21 men and 7 women; mean age, 51±10 years). Hypertension was defined as systolic blood pressure of more than 140 mm Hg and/or diastolic blood pressure of more than 90 mm Hg, in a sitting position, on at least three different occasions. Patients with secondary forms of hypertension were excluded on the basis of complete history, physical examination, radiological and ultrasound examinations, urinalysis, plasma renin activity, plasma aldosterone and norepinephrine concentrations, serum creatinine, potassium, calcium, and free thyroxine concentrations, and 24-hour urinary excretion of 17-hydroxycorticosteroids, 17-ketogenic steroids, and vanillymandelic acid. None of the patients had a history of cardiovascular or cerebrovascular disease, dyslipidemia, diabetes mellitus, liver disease, or renal disease. Normotension was defined as a systolic blood pressure of less than 130 mm Hg and a diastolic blood pressure 80 mm Hg. The healthy control subjects had no history of serious disease and took no medication for at least 4 weeks before the study. The study protocol was approved by the Ethics Committee of Hiroshima University Graduate School of Biomedical Sciences. Written informed consent was obtained from all subjects before participation.
Subjects fasted the previous night for at least 12 hours. Thirty minutes after remaining in the supine position, basal ROCK activity, fasting serum concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides, creatinine, insulin, glucose, and electrolytes, and plasma renin activity were measured.
Study protocol 2. ROCK activity in hypertensive patients treated with antihypertensive agents
To evaluate the effects of antihypertensive agents on ROCK activity, we measured leukocyte ROCK activity in 651 patients with hypertension treated with antihypertensive agents: calcium channel blockers (n=261; 169 men and 92 women; mean age, 62.3±12.6 years), angiotensin type I receptor blockers (ARBs, n=201; 131 men and 70 women; mean age, 63.3±13.8 years), angiotensin-converting enzyme inhibitors (ACEIs, n=52; 37 men and 15 women; mean age, 61.3±12.9 years), β blockers (n=58; 40 men and 18 women; mean age, 61.9±12.7 years), and diuretics (n=79; 56 men and 23 women; mean age, 64.1±14.8 years). This was a cross-sectional study design. To avoid effects of acute reactive change on ROCK activity, we excluded patients with acute myocardial infarction, unstable angina pectoris, acute thromboembolism, acute inflammatory disease, and acute heart failure. Consecutive patients receiving monotherapy with either calcium channel blockers, ARBs, ACEIs, β blockers and diuretics were enrolled. All patients receiving antihypertensive drugs were treated for more than 24 weeks. Written informed consent was obtained from all subjects before participation.
Study protocol 3. Effect of the calcium channel blocker amlodipine on ROCK activity in patients with hypertension
This was a double-blind, randomized, study with parallel group design. Twenty-eight patients with essential hypertension enrolled in study protocol 1 were randomized to double-blind treatment with either amlodipine (n=14, Pfizer Pharmaceutical Co.) at a dose of 5 mg or losartan potassium (n=14, Banyu Pharmaceutical Co.) at a dose of 100 mg once daily in the morning during the 12-week active treatment period. None of the patients had a history of antihypertensive treatment before the study. After a 4-week run-in period, the pretreatment value in the amlodipine group was compared to that in the losartan group. Active treatment was then performed for 12 weeks and the time courses of the effects of amlodipine and losartan were evaluated.
Measurements of leukocyte ROCK activity were performed at the beginning of treatment (0 weeks) and after 4 and 12 weeks of treatment.
Measurement of ROCK activity
ROCK activity was assayed in peripheral blood leukocytes as the amount of phospho-Thr853 in the myosin binding subunit (MBS) of myosin light-chain phosphatase.9,10 Blood was collected at room temperature in heparinized tubes (20 U/mL). After adding an equal volume of 2% dextran, the sample was kept at room temperature for 30 minutes. The supernatant was spun at 1450 rpm for 10 minutes. Red blood cells in the resulting cell pellet were lysed with the addition of water and spun at 1450 rpm for 10 minutes after the addition of Hank’s balanced salt solution (Hyclone, Logan, Utah). The resulting leukocyte pellet was resuspended in medium 199 (Sigma Chemical Co) and leukocytes were counted using a hematocytometer. Cells were fixed in 10% trichloroacetic acid and 10 mmol/L dichlorodiphenyltrichloroethane. After centrifugation, the cell pellets were stored at -80°C for Western blot analysis. Cells pellets were dissolved in 10 μL of 1 mol/L Tris base and then mixed with 100 μL of extraction buffer (8 mol/L urea, 2% sodium dodecyl sulfate, 5% sucrose, and 5% 2-mercaptoethanol). Equal amounts of cell extracts were subjected to 7.5% SDS-PAGE and transferred to nitrocellulose membranes. NIH 3T3 cell lysates were used as a positive control and to standardize the results of Western blot analyses from several membranes. After serum starvation for 20 hours, confluent cells were stimulated with 10 μmol/L lysophosphatidic acid for 10 minutes and then subsequently fixed and harvested in 10% trichloroacetic acid and 10 mmol/L dichlorodiphenyltrichloroethane. Following centrifugation at 1450 rpm for 10 minutes at 4°C, precipitates were dissolved in 10 μL of 1 mol/L Tris base and mixed with 100 μL of extraction buffer. An equal volume of positive control cell lysate was used for each gel. Membranes were incubated with rabbit anti–phospho-specific Thr853–MBS polyclonal antibody (Biosource Invitrogen), rabbit anti-MBS polyclonal antibody (Covance Laboratories), or antiactin monoclonal antibody (Sigma). Bands were visualized using the ECL system (Amersham-Pharmacia Co.). Images were captured using Adobe Photoshop, and the band intensities were quantified using National Institutes of Health (NIH) Image 1.61. ROCK activity was expressed as the ratio of phospho-Thr853–MBS in each sample to phosphoThr853–MBS in each positive control divided by MBS in each sample per MBS in each positive control.
Statistical analysis
Results are presented as mean±SD. Values of P<0.05 were considered to indicate statistical significance. The Mann-Whilney U test was used to evaluate differences between the hypertensive patients and healthy subjects. Comparisons of time course curves of parameters were analyzed by two-way analysis of variance (ANOVA) for repeated measures on one factor followed by Bonferroni correction for multiple-paired comparisons. Multigroup comparisons of variables were carried out by one-way ANOVA followed by Bonferroni correction. The data were processed using the software package Stat View V (SAS Institute).
Results
Study protocol 1. ROCK activity in patients with essential hypertension
Baseline clinical characteristics of the 28 patients with essential hypertension and 28 healthy subjects are summarized in Table 1. Systolic and diastolic blood pressures were significantly higher in patients with hypertension than in healthy subjects. All other variables were similar in the hypertensive patients and healthy subjects.
Table 1.
Clinical Characteristics of the Healthy Subjects and Patients with Essential Hypertension
| Variable | Healthy subjects (n=28) |
Hypertensive patients (n=28) |
|---|---|---|
| Body mass index (kg/m2) | 23.6 ± 2.7 | 24.2 ± 2.8 |
| Systolic blood pressure (mm Hg) | 115.9 ± 10.3 | 158.2 ± 13.2* |
| Diastolic blood pressure (mm Hg) | 69.1 ± 8.6 | 96.8 ± 10.1* |
| Heart rate (bpm) | 68.6 ± 7.9 | 70.3 ± 8.8 |
| Total cholesterol (mmol/L) | 4.81 ± 0.69 | 4.98 ± 0.77 |
| Triglycerides (mmol/L) | 1.11 ± 0.38 | 1.17 ± 0.37 |
| HDL cholesterol (mmol/L) | 1.45 ± 0.31 | 1.42 ± 0.25 |
| LDL cholesterol (mmol/L) | 3.17 ± 0.57 | 3.36 ± 0.69 |
| Glucose (mmol/L) | 4.7 ± 0.4 | 4.9 ± 0.3 |
| Insulin (pmol/L) | 46.3 ± 17.1 | 43.7 ± 11.8 |
| Creatinine (μmol/L) | 81.1 ± 10.3 | 88.7 ± 16.4 |
| Sodium (mmol/L) | 140.1 ± 1.4 | 140.8 ± 1.6 |
| Potassium (mmol/L) | 4.2 ± 0.2 | 4.1 ± 0.2 |
| Plasma renin activity (ng/mL/hr) | 2.0 ± 1.5 | 1.6 ± 1.3 |
| Smoker (%) | 25 | 25 |
HDL indicates high density lipoprotein; LDL, low density lipoproteine.
P<.05 versus normotensive subjects. Without statistical significant differences for the rest parameters.
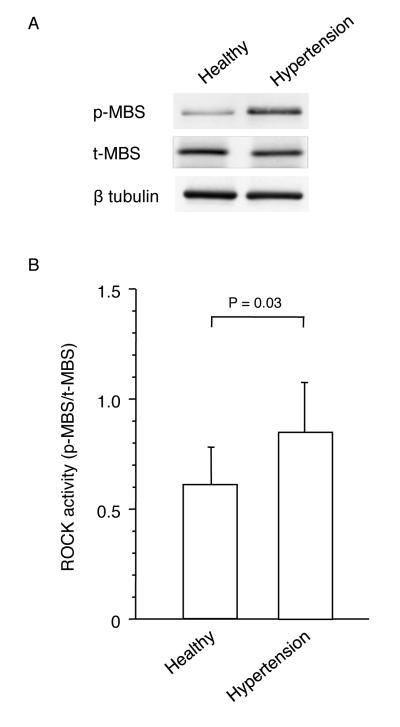
Leukocyte ROCK activity was significantly greater in the hypertensive patients group than in the healthy subjects group (0.84±0.24 vs. 0.61±0.18, P=0.03) (Figure 1). Protein expression levels of MBS were similar in the patients with hypertension and healthy subjects.
Figure 1.
Rho-associated kinase (ROCK) activity in patients with essential hypertension and healthy subjects. A, Western blot analysis for phospho-myosin-binding subunit (p-MBS), total-myosin-binding subunit (t-MBS), and β tublin in healthy subject and patient with essential hypertension. B, ROCK activity (p-MBS/t-MBS) in patients with essential hypertension and normotensive subjects.
Study protocol 2. ROCK activity in hypertensive patients treated with antihypertensive agents
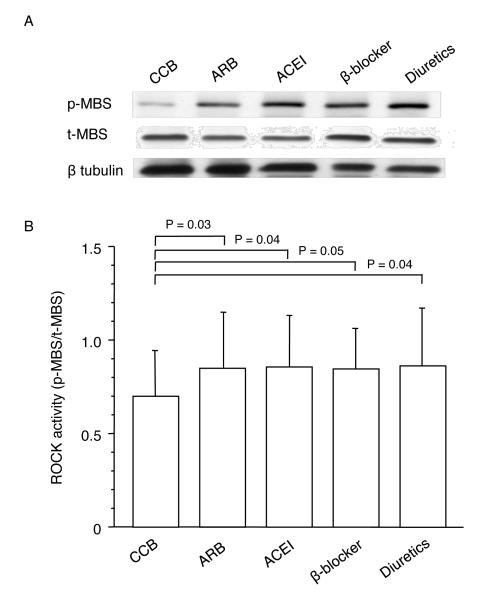
Baseline clinical characteristics of the each group are summarized in Table 2. There were no significant differences in parameters among the five groups. ROCK activity was significantly lower in the calcium channel blocker-treated group than in the groups treated with ARBs, ACEIs, diuretics, and β blockers (0.70±0.24 vs. 0.84±0.30, 0.85±0.27, 0.83±0.24, and 0.86±0.31, P=0.03, P=0.04, P=0.05, and P=0.04, respectively), but ROCK activities were similar among the last four groups (Figure 2). The protein expression of MBS was similar among the five groups. There was no significant difference in ROCK activity between the calcium channel blocker-treated group and healthy subject group in study protocol 1 (0.70±0.24 vs. 0.61±0.18, P=0.10). ROCK activity was significantly lower in the healthy subject group than in the groups treated with ARBs, ACEIs, diuretics, and β blockers (P=0.03, P=0.04, P=0.04, and P=0.04, respectively). The protein expression levels of MBS were similar among the healthy subject group and the other five treated groups.
Table 2.
Clinical Characteristics of the Patients with Essential Hypertension Treated with Calcium Channel Blockers, ARBs, ACEIs, β-blockers, and Diuretics
| Variable | Calcium channel blockers (n=261) |
ARBs (n=201) |
ACEIs (n=52) |
β -blockers (n=58) |
Diuretics (n=79) |
|---|---|---|---|---|---|
| Body mass index (kg/m2) | 23.9 ± 2.7 | 23.8 ± 3.1 | 23.7 ± 3.0 | 23.8 ± 2.8 | 23.4 ± 2.9 |
| Systolic blood pressure (mm Hg) | 140.5 ± 14.1 | 143.2 ± 13.8 | 144.1 ± 13.9 | 142.3 ± 13.5 | 144.3 ± 12.8 |
| Diastolic blood pressure (mm Hg) | 82.3 ± 10.7 | 84.5 ± 10.5 | 84.7 ± 11.4 | 83.9 ± 9.8 | 85.2 ± 11.2 |
| Heart rate (bpm) | 71.2 ± 10.1 | 69.7 ± 8.9 | 68.6 ± 9.3 | 66.2 ± 8.2 | 70.2 ± 9.6 |
| Total cholesterol (mmol/L) | 4.98 ± 0.63 | 4.79 ± 0.65 | 4.78 ± 0.72 | 5.01 ± 0.74 | 5.09 ± 0.79 |
| Triglycerides (mmol/L) | 1.14 ± 0.43 | 1.09 ± 0.45 | 1.13 ± 0.52 | 1.15 ± 0.49 | 1.21 ± 0.55 |
| HDL cholesterol (mmol/L) | 1.46 ± 0.33 | 1.39 ± 0.32 | 1.39 ± 0.32 | 1.42 ± 0.35 | 1.49 ± 0.42 |
| LDL cholesterol (mmol/L) | 3.29 ± 0.67 | 3.17 ± 0.59 | 3.34 ± 0.69 | 3.39 ± 0.58 | 3.48 ± 0.87 |
| Glucose (mmol/L) | 5.0 ± 0.2 | 4.9 ± 0.3 | 4.8 ± 0.3 | 4.9 ± 0.2 | 5.0 ± 0.4 |
| Insulin (pmol/L) | 42.1 ± 9.8 | 43.2 ± 11.3 | 44.3 ± 13.9 | 40.4 ± 12.7 | 39.9 ± 14.1 |
| Creatinine (μmol/L) | 88.1 ± 17.2 | 84.2 ± 13.9 | 82.3 ± 14.5 | 89.5 ± 18.3 | 91.2 ± 18.7 |
| Sodium (mmol/L) | 140.3 ± 1.1 | 141.0 ± 1.5 | 140.9 ± 1.4 | 140.1 ± 1.4 | 140.0 ± 1.6 |
| Potassium (mmol/L) | 4.1 ± 0.1 | 4.2 ± 0.3 | 4.2 ± 0.2 | 4.2 ± 0.2 | 4.0 ± 0.1 |
| Statin treatment (%) | 15 | 18 | 17 | 14 | 18 |
| Smoker (%) | 27 | 29 | 31 | 30 | 27 |
ARBs indicates angiotensin type I receptor blockers; ACEIs, angiotensin-converting enzyme inhibitor; HDL, high density lipoprotein; LDL, low density lipoprotein.
Without statistical significant differences for the rest parameters
Figure 2.
Effects of antihypertensive drugs, calcium channel blocker (CCB), angiotensin type I receptor blocker (ARB), angiotensin-converting enzyme inhibitor (ACEI), β blocker and diuretics on Rho-associated kinase (ROCK) activity in patients with hypertension. A, Western blot analysis for phospho-myosin-binding subunit (p-MBS), total-myosin-binding subunit (t-MBS), and β tublin in calcium channel blocker, ARB, ACEI, β blocker and diuretic groups. B, ROCK activity (p-MBS/t-MBS) in the CCB, ARB, ACEI, β blocker and diuretics groups.
Study protocol 3. Effect of calcium channel blocker amlodipine on ROCK activity in patients with hypertension
The effects of amlodipine and losartan on baseline parameters are shown in Table 3. Both amlodipine and losartan significantly reduced blood pressures after 4 weeks of treatment compared to baseline values (0 weeks). The blood pressure lowering effect of amlodipine was maintained throughout the 12-week treatment period. Heart rate was not significantly modified by treatment in each treatment period. Serum levels of lipids and glucose were similar in all treatment periods in both groups.
Table 3.
Clinical Characteristics of the Amlodipine Group and the Losartan Group
| Amlodipine group (n=14) |
Losartan group (n=14) |
|||||
|---|---|---|---|---|---|---|
| Variable | 0 weeks | 4 weeks | 12 weeks | 0 weeks | 4 weeks | 12 weeks |
| Body mass index (kg/m2) | 24.2 ± 2.8 | 24.8 ± 2.8 | 24.1 ± 2.7 | 24.2 ± 2.9 | 24.2 ± 2.8 | 24.2 ± 2.7 |
| Systolic blood pressure (mm Hg) | 158.4 ± 14.1 | 132.9 ± 11.3* | 133.1 ± 12.2* | 157.9 ± 13.9 | 134.9 ± 11.7* | 134.4 ± 10.8* |
| Diastolic blood pressure (mm Hg) | 96.9 ± 10.8 | 81.5 ± 7.1* | 82.2 ± 7.4* | 96.7 ± 10.3 | 83.4 ± 7.5* | 83.1 ± 7.7* |
| Heart rate (bpm) | 71.4 ± 9.3 | 70.4 ± 8.8 | 72.3 ± 9.5 | 69.4 ± 9.1 | 70.1 ± 9.4 | 71.1 ± 8.9 |
| Total cholesterol (mmol/L) | 5.04 ± 0.81 | 5.05 ± 0.92 | 4.99 ± 0.78 | 4.97 ± 0.74 | 5.01 ± 0.79 | 4.96 ± 0.68 |
| Triglycerides (mmol/L) | 1.20 ± 0.41 | 1.21 ± 0.43 | 1.18 ± 0.34 | 1.14 ± 0.32 | 1.18 ± 0.39 | 1.15 ± 0.41 |
| HDL cholesterol (mmol/L) | 1.41 ± 0.25 | 1.42 ± 0.29 | 1.40 ± 0.30 | 1.43 ± 0.28 | 1.42 ± 0.26 | 1.44 ± 0.39 |
| LDL cholesterol (mmol/L) | 3.41 ± 0.72 | 3.39 ± 0.68 | 3.40 ± 0.73 | 3.31 ± 0.68 | 3.35 ± 0.71 | 3.34 ± 0.67 |
| Glucose (mmol/L) | 4.9 ± 0.3 | 4.9 ± 0.4 | 4.9 ± 0.3 | 5.0 ± 0.3 | 4.9 ± 0.3 | 4.9 ± 0.3 |
| Insulin (pmol/L) | 44.5 ± 13.2 | 45.3 ± 14.1 | 44.9 ± 12.8 | 42.6 ± 12.3 | 41.9 ± 14.2 | 42.1 ± 12.7 |
| Creatinine (μmol/L) | 88.6 ± 18.4 | 85.2 ± 16.7 | 87.3 ± 17.4 | 89.4 ± 17.5 | 84.8 ± 16.5 | 85.1 ± 17.7 |
| Sodium (mmol/L) | 140.6 ± 1.8 | 140.7 ± 1.7 | 140.5 ± 1.5 | 141.0 ± 1.6 | 140.8 ± 1.7 | 140.7 ± 1.6 |
| Potassium (mmol/L) | 4.1 ± 0.2 | 4.1 ± 0.3 | 4.1 ± 0.2 | 4.1 ± 0.2 | 4.2 ± 0.3 | 4.2 ± 0.3 |
| Smoker (%) | 25 | 25 | ||||
HDL indicates high density lipoprotein; LDL, low density lipoproteine.
P<0.05 versus 0 weeks in the same group. Without statistical significant differences for the rest parameters.
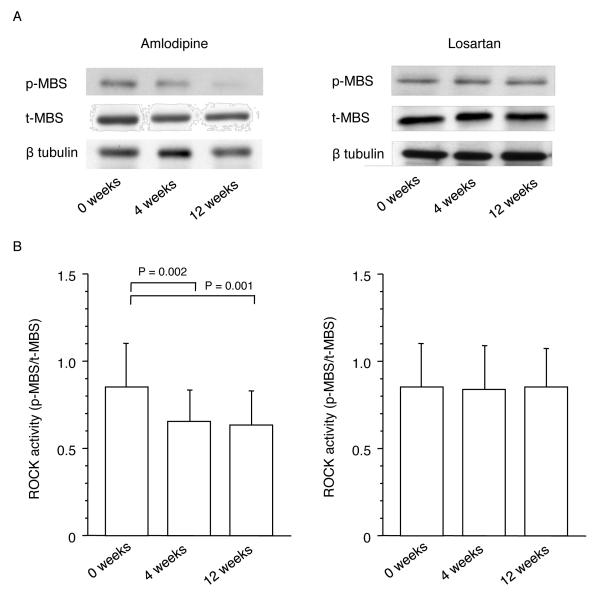
Effects of amlodipine and losartan on ROCK activity before and after 4 and 12 weeks of treatment in patients with essential hypertension are shown in Figure 3. Amlodipine significantly reduced ROCK activity after 4 weeks of treatment in comparison with the pretreatment value (0.85±0.25 vs. 0.66±0.16, P=0.002). The ability of amlodipine to decrease ROCK activity was maintained throughout the 12-week treatment period (0.64±0.15, P=0.001 vs. 0 weeks). ROCK activities after 4 weeks and 12 weeks of treatment were similar. Losartan did not alter ROCK activity after 4 weeks and 12 weeks of treatment in comparison with the pretreatment value (0.84±0.27, 0.83±0.26, and 0.84±0.24). Protein expression levels of MBS were similar in all treatment periods in the 2 groups.
Figure 3.
Rho-associated kinase (ROCK) activity in the amlodipine group and losartan group at the beginning of treatment (0 weeks) and after 4 and 12 weeks of treatment. A, Western blot analysis for phospho-myosin-binding subunit (p-MBS), total-myosin-binding subunit (t-MBS) and β tublin in the amlodipine group and losartan group at the beginning of treatment (0 weeks) and after 4 and 12 weeks of treatment. B, ROCK activity (p-MBS/t-MBS) in the amlodipine group and losartan group at the beginning of treatment (0 weeks) and after 4 and 12 weeks of treatment.
Discussion
In this study, we demonstrated that leukocyte ROCK activity was greater in patients with essential hypertension than in healthy subjects and that ROCK activity was significantly decreased in the calcium channel blocker-treated group but not in the groups treated with RAS inhibitors, ß blockers, and diuretics. In addition, we confirmed that treatment with the calcium channel blocker amlodipine for 12 weeks inhibited ROCK activity in patients with hypertension.
It has been reported that ROCKs are involved in the pathogenesis of hypertension through, at least in part, causing vascular smooth muscle cell contraction.2,11 The ROCK inhibitor Y-27632 inhibited focal adhesion and stress fiber in cultured vascular smooth muscle cells not only by ROCK overexpression but also by Rho activation and agonist-induced vasoconstriction.2 In addition, Y-27632 decreased blood pressure in a dose-dependent manner in spontaneous hypertensive rats.2 In the present study, hypertension was associated with increased leukocyte ROCK activity. It has been shown that intra-arterial infusion of the ROCK inhibitor fasudil decreases forearm vascular resistance in patients with hypertension.8 These findings suggest that ROCK activity is elevated in hypertension and that ROCK activity plays an important pathophysiological role in the development of hypertension.
In a cross-sectional study, leukocyte ROCK activity was significantly lower in the calcium channel blocker-treated group than in the group treated with RAS inhibitors, ß blockers, and diuretics, while the antihypertensive effects were similar among the groups. In addition, in a double-blind, randomized, parallel group study, the calcium channel blocker amlodipine, but not losartan, also decreased leukocyte ROCK activity in patients with hypertension. It has been shown that the calcium channel blocker benidipine attenuates vasodilation induced by Y-27632 in spontaneous hypertensive rats.12 These finding suggest that calcium channel blockers inhibit ROCK activity in hypertension. Although the precise mechanism involved in the decrease in ROCK activity by calcium channel blockers remains unclear, we have proposed a few hypotheses for how a calcium channel blocker could inhibit ROCK activity in hypertensive patients. It is unlikely that decrease in blood pressures by antihypertensive agents is directly involved in the reduction in ROCK activity. Nevertheless, we cannot completely deny the possibility that such a calcium channel blocker has a stronger effect on blood pressures that results in the decrease in ROCK activity. Also, it is well known that calcium channel blockers such as amlodipine can improve endothelial function. Improved endothelial function by regular intake of a calcium channel blocker means increases in release and accumulation of cyclic guanosine monophosphate, which could inhibit ROCK activity.11 However, we could not find decreases in leukocyte ROCK activity in the groups treated with both RAS inhibitors and diuretics in the present cross-sectional study; hence, mechanisms by which ROCK activity is reduced by calcium channel blockers might be different from those for other agents. In addition, the administrated doses of RAS inhibitors and diuretics in this study might have been too low to decrease leukocyte ROCK activity. Accordingly, it is thought that calcium channel blockers reduce vascular resistance through inhibition of the Rho/ROCK pathway. Inhibition of ROCK activity may be a potential therapeutic target for hypertension.
ROCK inhibitors such as fasudil and Y-27632 have been used in previous studies to assess ROCK activity in vitro and in vivo.13-16 In human studies, intra-arterial or intravenous infusion of fasudil has been used.8,17-21 These methods are burdensome and time-consuming for subjects. Recently, studies using a specific antibody to phospho-MBS on myosin light-chain phosphatase, which is a downstream target of ROCK, have clearly demonstrated that ROCK activity in peripheral leukocytes is enhanced in patients with metabolic syndrome and patients with coronary artery disease.9,10,22 In the present study, we confirmed that ROCK activity was increased in hypertension using a noninvasive method for measurement of ROCK activity in peripheral blood leukocytes. It is well known that peripheral blood leukocytes per se play an important role in the cause, maintenance, and development of atherosclerosis. Indeed, leukocyte ROCK has been shown to increase leukocyte attachment and subsequent infiltration into the vascular wall, which result in the progression of atherosclerosis. Therefore, our findings suggest that the enhancement of ROCK activity by hypertension may cause leukocyte activation and enhanced leukocyte infiltration into the vascular wall, leading to progression of atherosclerosis. It is clinically important to estimate the degree of ROCK activity. A noninvasive method for measuring leukocyte ROCK activity would also be useful for assessing ROCK activity. Leukocyte ROCK activity may become a novel biomarker of atherosclerosis.
In conclusion, the present study showed an increase in ROCK activity in peripheral leukocytes in patients with hypertension. Leukocyte ROCK activity may be useful as a surrogate biological marker for atherosclerosis, including hypertension. It is important to select an appropriate intervention that is effective for inhibiting ROCK activity. Calcium channel blockers should be one of the factors that inhibit ROCK activity. Lifestyle modifications and pharmacological interventions have the potential for inhibiting ROCK activity as treatment for cardiovascular disease associated with increase in ROCK activity. Further studies are needed to understand more clearly the biology of ROCK activity in a clinical setting.
Acknowledgement
The authors thank Megumi Wakisaka and Satoko Michiyama for an excellent secretarial assistance. This work was carried out at the Analysis Center of Life Science, Hiroshima University.
Funding Sources This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (1859081500 and 21590898) and the National Institutes of Health (HL052233 and HL080187).
Footnotes
Conflict of Interest Dr. James K Liao is a consultant for Asahi-Kasei Pharmaceutical, Inc. There are no other conflicts of interests for other the authors.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amano M, Chihara K, Kimura K, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 2.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 3.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 4.Kimura K, Fukata Y, Matsuoka Y, et al. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- 5.Seasholtz TM, Zhang T, Morissette MR, Howes AL, Yang AH, Brown JH. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ Res. 2001;89:488–495. doi: 10.1161/hh1801.096337. [DOI] [PubMed] [Google Scholar]
- 6.Wehrwein EA, Northcott CA, Loberg RD, Watts SW. Rho/Rho kinase and phosphoinositide 3-kinase are parallel pathways in the development of spontaneous arterial tone in deoxycorticosterone acetate-salt hypertension. J Pharmacol Exp Ther. 2004;309:1011–1019. doi: 10.1124/jpet.103.062265. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Y, Zhuang S, Gloddek J, Tseng CC, Boss GR, Pilz RB. Regulation of cGMP-dependent protein kinase expression by Rho and Kruppel-like transcription factor-4. J Biol Chem. 2006;281:16951–16961. doi: 10.1074/jbc.M602099200. [DOI] [PubMed] [Google Scholar]
- 8.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 9.Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49:1619–1624. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 12.Kitayama J, Kitazono T, Ooboshi H, Takada J, Fujishima M, Ibayashi S. Long-term effects of benidipine on cerebral vasoreactivity in hypertensive rats. Eur J Pharmacol. 2002;438:153–158. doi: 10.1016/s0014-2999(02)01311-0. [DOI] [PubMed] [Google Scholar]
- 13.Breitenlechner C, Gassel M, Hidaka H, et al. Protein kinase A in complex with Rho-kinase inhibitors Y-27632, Fasudil, and H-1152P: structural basis of selectivity. Structure. 2003;11:1595–1607. doi: 10.1016/j.str.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Jarajapu YP, Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H1917–1922. doi: 10.1152/ajpheart.01012.2004. [DOI] [PubMed] [Google Scholar]
- 15.Savoia C, Tabet F, Yao G, Schiffrin EL, Touyz RM. Negative regulation of RhoA/Rho kinase by angiotensin II type 2 receptor in vascular smooth muscle cells: role in angiotensin II-induced vasodilation in stroke-prone spontaneously hypertensive rats. J Hypertens. 2005;23:1037–1045. doi: 10.1097/01.hjh.0000166845.49850.39. [DOI] [PubMed] [Google Scholar]
- 16.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 17.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 18.Noma K, Rikitake Y, Oyama N, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 20.Fukumoto Y, Mohri M, Inokuchi K, et al. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. J Cardiovasc Pharmacol. 2007;49:117–121. doi: 10.1097/FJC.0b013e31802ef532. [DOI] [PubMed] [Google Scholar]
- 21.Noma K, Goto C, Nishioka K, et al. Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49:698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nohria A, Prsic A, Liu PY, et al. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205:517–521. doi: 10.1016/j.atherosclerosis.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]