Abstract
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Pancreatic Adenocarcinoma discuss the workup and management of tumors of the exocrine pancreas. These NCCN Guidelines Insights provide a summary and explanation of major changes to the 2012 NCCN Guidelines for Pancreatic Adenocarcinoma. The panel made 3 significant updates to the guidelines: 1) more detail was added regarding multiphase CT techniques for diagnosis and staging of pancreatic cancer, and pancreas protocol MRI was added as an emerging alternative to CT; 2) the use of a fluoropyrimidine plus oxaliplatin (e.g., 5-FU/leucovorin/oxaliplatin or capecitabine/oxaliplatin) was added as an acceptable chemotherapy combination for patients with advanced or metastatic disease and good performance status as a category 2B recommendation; and 3) the panel developed new recommendations concerning surgical technique and pathologic analysis and reporting.
Overview
In 2012, an estimated 43,920 people will be diagnosed with pancreatic cancer, and approximately 37,390 people will die of pancreatic cancer in the United States.1 This disease is the fourth most common cause of cancer-related death among U.S. men (after lung, prostate, and colorectal cancer) and women (after lung, breast, and colorectal cancer).1 Its peak incidence occurs in the seventh and eighth decades of life.2 Although incidence is roughly equal in both sexes, African Americans seem to have a higher incidence of pancreatic cancer than white Americans.3 Furthermore, the incidence and mortality rates of pancreatic cancer in the United States have remained approximately the same over the past 2 decades.4
Multiphase Diagnostic Imaging Techniques
Margin-negative surgical resection is the only potentially curative technique for pancreatic cancer. Patients with margin-positive resections; with visceral, peritoneal, or pleural metastases; or with metastases to nodes beyond the field of resection derive no benefit from resection.5–7 Accurate determination of resectability is therefore critical for the optimal management of pancreatic cancer. Unlike many other cancers, imaging is the primary means through which the stage of pancreatic cancer is determined. Therefore, high-quality multiphase diagnostic imaging, which can help to preoperatively distinguish between patients eligible for resection with curative intent and those with unresectable disease, is essential.
Pancreatic protocol CT is the most widely available and best-validated imaging modality for staging patients with pancreatic cancer.8,9 Studies have shown that 70% to 85% of patients determined to have resectable tumors through CT were able to undergo re-section.8,10–14 During the institutional review of the NCCN Guidelines, a reviewer suggested that pancreas protocol MRI should also be listed as an option for diagnostic staging. Panelists confirmed that pancreas protocol MRI is emerging as an equivalent alternative to CT (see PANC-1). Some NCCN Member Institutions, in fact, now prefer MRI over CT imaging because of concerns regarding radiation dose over time with CT scans. Most NCCN Member Institutions, however, use CT and MRI interchangeably. In fact, comparisons show that the 2 modalities are similar in their ability to predict vessel and node involvement.15–18 MRI may be superior to CT for detecting small hepatic and peritoneal metastases,18,19 and therefore may also be a helpful adjunct to CT in high-risk patients if CT is initially performed and no metastases are found.
The institutional review also included a request for more details regarding optimal multiphase imaging techniques. The discussion that ensued centered on the thickness at which images are captured and rendered. The consensus among the panelists was that cuts should be thin, at 3 mm or less. The panelists agreed that optimal multiphase imaging technique (CT or MRI) includes a noncontrast phase plus arterial, pancreatic parenchymal, and portal venous phases of contrast enhancement with thin cuts (≤ 3 mm) through the abdomen. This technique allows precise visualization of the relationship of the primary tumor to the mesenteric vasculature and detection of metastatic deposits as small as 3 to 5 mm (see PANC-A).8,12,20,21
The difference in contrast enhancement between the parenchyma and adenocarcinoma is highest during the late arterial phase, thereby providing a clear distinction between a hypodense lesion in the pancreas and the rest of the organ. A multiphasic pancreatic protocol allows for selective visualization of important arterial (e.g., celiac axis, superior mesenteric artery, and peripancreatic arteries) and venous structures (e.g., superior mesenteric vein, splenic vein, and portal vein), thereby providing an assessment of vascular invasion by the tumor.
All of this information can improve the prediction of resectability. Software allowing for 3-dimensional reconstruction of imaging data can provide additional valuable information on the anatomic relationship between the pancreatic tumor and the surrounding blood vessels and organs, although the panelists agreed that further development of this technology is needed before it is routinely integrated into clinical practice.13
Patients commonly present to the oncologist with a non–pancreas protocol CT already performed. The panelists agreed that if the CT scan is of high quality, it can be sufficient. If not, a pancreas protocol CT or MRI is recommended.22
Fluoropyrimidine Plus Oxaliplatin for Patients With Advanced or Metastatic Disease
For the 2012 version of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Pancreatic Adenocarcinoma, the combination of a fluoropyrimidine (5-FU/leucovorin or capecitabine) with oxaliplatin was added as a possible first-line treatment for metastatic or locally advanced disease as a category 2B recommendation (see PANC-G; to view the most recent version to these guidelines, visit NCCN.org). During the review of the 2011 guidelines that preceded the 2012 panel meeting, a reviewer commented that FOLFOX (5-FU/leucovorin/oxaliplatin) and FOLFIRI (5-FU/leucovorin/irinotecan) regimens should be listed as acceptable initial chemotherapy regimens for patients with advanced or metastatic disease, based on the strong level 1 evidence for FOLFIRINOX (5-FU/leucovorin/oxaliplatin/irinotecan) in the metastatic setting.23 The panel, however, was not comfortable listing FOLFIRI as an option, because of the lack of strong phase II data on FOLFIRI in advanced or metastatic pancreatic cancer.24,25 In contrast, the panel cited the randomized phase III CONKO-003 trial (5-FU/leucovorin/oxaliplatin vs. best supportive care) and a phase II study (CapeOx) to justify a recommendation for a fluoropyrimidine with oxaliplatin.26,27 Both of these studies only enrolled patients who had received 1 prior chemotherapy regimen, but the panel unanimously agreed that an extrapolation to first-line therapy is appropriate. However, because the recommendation is based on lower-level evidence, they listed it as category 2B.
Principles of Surgical Technique and Pathologic Analysis
The panel discussed the fact that there have been no standardized recommendations on how to ink, orient, or slice a pancreatic ductal adenocarcinoma surgical specimen nor on what margins should be routinely assessed and reported. A subcommittee consisting of pathologists and surgeons from NCCN Member Institutions was formed to discuss these issues and develop recommendations. Although the subcommittee members agreed that the data and information in the pathology report should be standardized and mandated, many felt strongly that how the information is obtained should be left to the discretion of individual surgeons and pathologists. Surgical techniques and dissection approaches can differ on a case-by-case basis depending on the size, shape, orientation, and location of the tumor.
The subcommittee agreed that standardized pathologic assessment and reporting would allow prospective data to be obtained regarding how the status of each margin relates to local, regional, and distant recurrences. What constitutes an adequate margin in pancreatic carcinoma specimens is currently unknown. A standardized definition would allow better stratification of patients into adjuvant regimens after surgical extirpation. For instance, if less than 1 mm clearance is associated with an unacceptably high incidence of local recurrence then strong consideration for postoperative radiation therapy might be indicated if not received preoperatively. The subcommittee and panel strongly recommend reporting tumor clearance in millimeters for all margins to allow prospective accumulation of this important data for future analysis. Because margin definitions and uniformity of nomenclature are also critical to accurate and standardized reporting, the subcommittee established definitions for the various margins of pancreatic surgical specimens (see PANC-D, 1 and 2 of 4). The subcommittee also discussed the use of the pathology synoptic reports from the College of American Pathologists (CAP),28 and listed an abbreviated minimum analysis of pancreatic cancer specimens from the CAP recommendations in the NCCN Guidelines (see PANC-D, 3 of 4).
The subcommittee also developed the “Principles of Surgical Technique” and “Principles of Pathologic Analysis” sections of the guidelines to provide general information and guiding principles. The information that the subcommittee developed is further discussed here.
Principles of Surgical Technique
The goal of surgery is to achieve a margin-negative (R0) resection. The nature and extent of surgery for resectable tumors depend on the location and size of the tumor. Because tumors of the body and tail cause symptoms late in their development, they are usually advanced at diagnosis and are rarely resectable. When tumors in the pancreatic tail are resectable, distal pancreatectomy is commonly performed, in which the surgeon removes the tail and body of the pancreas and spleen. Patients with tumors in the head of the pancreas, who usually present because of jaundice, are treated with open or laparoscopic pancreatoduodenectomy (i.e., the Whipple procedure).29 If the cancer diffusely involves the pancreas or is present at multiple sites within the pancreas, a total pancreatectomy may be required, in which the surgeon removes the entire pancreas, part of the small intestine, a portion of the stomach, the common bile duct, the gallbladder, the spleen, and nearby lymph nodes.
Newly delineated details of pancreatoduodenectomy and distal pancreatectomy are included in these NCCN Guidelines Insights. The discussion section of the full guidelines contains additional information regarding portal vein resection, pylorus preservation, pancreatic anastomosis, and extended lymphadenectomy (to view the full guidelines, visit NCCN.org).
Pancreatoduodenectomy (Whipple Procedure)
Achievement of a margin-negative resection must focus on meticulous perivascular dissection of the lesion in resectional procedures, the need for vascular resection and/or reconstruction, and the potential need for extrapancreatic organ resection. The biology of the cancer, however, might not allow for an R0 resection, even with the most meticulous surgery.
Medial dissection of pancreatic head lesions is best achieved through complete mobilization of the portal and superior mesenteric veins from the uncinate process (assuming no evidence of tumor infiltration). Further, skeletonization of the lateral, posterior, and anterior borders of the superior mesenteric artery down to the level of the adventitia will maximize uncinate yield and radial margin.30–32 Optimal dissection and skeletonization of the superior mesenteric artery can be achieved using ultrasonic or thermal dissectors (Harmonic scalpel or LigaSure). Division of the retroperitoneal tissues between the uncinate process and the superior mesenteric artery with a stapler or a clamp and cut technique may leave up to 43% of the soft tissue between the uncinate process and the SMA in situ, and results in suboptimal clearance and increases the risk of an R1 resection.33,34
In the absence of frank venous occlusion noted on preoperative imaging, the need for lateral venorrhaphy or complete portal or superior mesenteric vein resection and reconstruction to achieve an R0 resection may be suggested, but it is often not known until division of the pancreatic neck has occurred. Tethering of the carcinoma to the lateral wall of the portal vein is not uncommon and requires careful dissection to free the vein from the pancreatic head, if in fact it is possible to do so. Differentiation of tumor infiltration into the vein wall from tumor-related desmoplasia is frequently impossible to ascertain. The liberal use of partial or complete vein resection when vein infiltration is suspected during Whipple procedures has been studied.35–37 On evaluation of excised vein specimens, only 60% to 70% had histologic evidence of frank tumor involvement, and R0 resections were still not obtainable in 10% to 30% of patients despite increasing the magnitude of the operative procedure. However, if an R0 resection is obtained with vein excision, longevity seems similar to those with R0 resections without venous involvement, with no significant increase in morbidity and mortality. These data support an aggressive approach to partial or complete vein excision if tumor infiltration is suspected, although acceptance of this concept (particularly with respect to vein resection) is not universal.
Although numbers are more limited, similar findings have been noted with respect to hepatic arterial resection and reconstruction.37,38 Although further data with respect to arterial resection are clearly needed, the subcommittee states that judicious use of this technique would seem reasonable in very select populations.
Distal Pancreatectomy
The goals of left-sided resection are similar to those of pancreatoduodenectomy, although they are often more difficult to achieve because of the advanced stage at which most of these cancers are discovered. An R0 distal pancreatectomy for adenocarcinoma mandates en bloc organ removal beyond that of the spleen alone in up to 40% of patients.39,40 In addition, similar to the Whipple procedure, lateral venorrhaphy, vein excision and reconstruction, and dissection to the level of the celiac axis and superior mesenteric artery adventitia should be performed if complete tumor clearance can be achieved.40,41 Use of these radical resections is associated with an increase in blood loss, transfusion requirements, operating time, length of stay, and morbidity, but mortality remains rare.39–41 Encouragingly, tumor clearance (R0 resection) has been reported in up to 72% to 91% of patients, with long-term survival equivalent to those having standard resection for more localized disease.40,41 Local recurrence, however, remains problematic even with pathologically negative margins.41
Principles of Pathologic Analysis
The panel agrees that progress in treating pancreatic adenocarcinoma is encumbered by a lack of uniformity in pathologic analysis and reporting.42 A more standardized approach in this area could maximize the chances of a more complete and consistent pathology report that is similar among pathologists in the same institution and among institutions around the world. Ultimately, a more consistent approach to patient assessment, surgical technique, and pathologic evaluation of the resected pancreatic specimen from gross examination to pathologic report will provide better communication among the various treating physicians. It will also provide a clear and specific understanding of the individual patient's malignancy, including critical margin status, which will then allow a more accurate comparison of the existing and evolving treatment regimens for this lethal disease.
Specimen Orientation, Sectioning, Pathologic Analysis, and Reporting
The primary purpose of pathologic analysis of the pancreatic specimen is to determine the pathologic stage of the tumor by evaluating the type, grade, size, and extent of the cancer. Pathology synoptic reports (protocols) are useful for reporting results from examinations of surgical specimens; these reports assist pathologists in providing clinically useful and relevant information. On January 1, 2004, the Commission on Cancer (COC) of the American College of Surgeons mandated the use of specific checklist elements of the protocols as part of its Cancer Program Standards for Approved Cancer Programs. The pathology synoptic reports from CAP comply with the COC requirements, and the latest revisions to the CAP pancreatic (exocrine) protocol were issued in February 2011.28 The NCCN Pancreatic Adenocarcinoma Panel supports the CAP pathology synoptic reports. The proposal included in the guidelines (see PANC-D, 3 of 4) is an abbreviated minimum analysis of pancreatic cancer specimens from the CAP recommendations. In addition to the standard TNM staging, other variables are included, all of which have prognostic implications in the evolution of this disease.43,44
Whipple Specimen
Specimen orientation and inking involves both pathologist and surgeon, because this will help to ensure accurate assessment of the size and extent of the tumor. Either direct communication should occur between the surgeon and pathologist for proper orientation and margin identification, or the surgeon should identify the important margins with a clearly understood and documented method (i.e., written on the pathology requisition); for example, a stitch can be placed on the posterior margin and a safety pin on the retroperitoneal/uncinate margin.
One impediment to data comparison across institutions is the variability in the names given to various margins. Definitions of the margins and uniformity of nomenclature are critical to accurate reporting. The panel's recommended definitions are included on PANC-D. Some of these margins can be visualized on Figure 1. Other margins analyzed in Whipple specimens include the proximal and distal enteric margins (en face sections) and the anterior surface (closest representative). The anterior surface is not a true margin, but identification and reporting of this surface when positive may portend a risk of local recurrence, and therefore should be reported in all cases.42,45–47 Collectively, these pancreatic tissue surfaces constitute the circumferential transection margin. Designating the various specific margins with different colored inks will allow recognition on microscopy.
Figure 1.
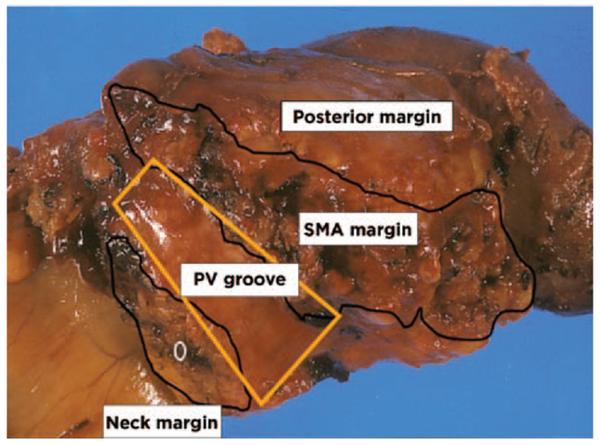
Whipple specimen with labeled margins. Courtesy of N. Volkan Adsay, MD, Atlanta, GA.
The approach to histologic sectioning of a Whipple specimen is determined by the unique characteristics of the tumor, but is also influenced by institutional preferences, expertise, and experience. Options include axial, bivalve, or multivalve slicing, and perpendicular sliding.42 Some experts in the field bisect the pancreas along probes placed in the bile and pancreatic ducts and then serially section along each half of the pancreas. Axial slicing provides an overall assessment of the epicenter of the tumor relative to the ampulla, bile duct, duodenum, pancreas, and all of the pancreatic circumferential tissue margins.42 There is no one correct way to dissect a Whipple specimen. The most important aspects of dissection are clear and accurate assessment of the margins. Attached organs resected with the specimen en bloc require serial sectioning to assess not only direct extension but also metastatic deposits.
Distal Pancreatectomy Specimen
In left-sided resections, the peripancreatic soft tissue margins and the pancreatic neck are assessed.47 Additionally, involvement of the splenic vessels should be documented and invasion of the spleen is important to determine, because direct tumor invasion constitutes a pT4 pathologic stage. Frozen section analysis of the pancreatic neck is recommended. Definitions of the proximal pancreatic (transection) margin, the anterior (cephalad) peripancreatic (peripheral) surface, and the posterior (caudad) peripancreatic (peripheral) margin are listed on PANC-D.
Summary of Changes to the 2012 NCCN Guidelines for Pancreatic Adenocarcinoma
Resection remains the only chance for a cure of pancreatic adenocarcinoma, and resectable patients should undergo surgery without delay, followed by adjuvant therapy. Borderline resectable patients can undergo neoadjuvant therapy (category 2B) in the hopes of improving the chances for an R0 resection, or can undergo immediate surgery. Patients with unresectable disease (locally advanced or metastatic) undergo chemotherapy and chemoradiation but should be spared the morbidities of surgical resection.
Because prediction of resectability critically informs management decisions in this disease, high-quality radiologic imaging is vital. The panel thus updated its recommendations for multiphase imaging techniques.
Because the prognosis for patients with locally advanced or metastatic disease is dismal, the panel continuously assesses additional treatment options that can be considered for this population. Therefore, fluoropyrimidine plus oxaliplatin was added as a category 2B recommendation.
Because few data exist regarding prognostic factors predicting the frequent recurrences that occur after resection of pancreatic adenocarcinoma, the panel added a strong recommendation for standardized pathologic reporting that can provide future prospective data. They also detailed possible surgical techniques and pathologic assessment procedures and defined margins that should be assessed.
PANC-1.
NCCN Categories of Evidence and Consensus
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
-
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
PANC-A.
PRINCIPLES OF DIAGNOSIS AND STAGING
-
#1
Decisions about diagnostic management and resectability should involve multidisciplinary consultation with reference to appropriate imaging studies to evaluate the extent of disease. Resections should be done at institutions that perform a large number (15–20) of pancreatic resections annually.
-
#2
Imaging should include specialized pancreatic CT or MRI. CT should be performed according to a defined pancreas protocol such as triphasic cross-sectional imaging and thin slices. Optimal multi-phase imaging technique includes a non-contrast phase plus arterial, pancreatic parenchymal and portal venous phases of contrast enhancement with thin cuts (3mm) through the abdomen. This technique allows precise visualization of the relationship of the primary tumor to the mesenteric vasculature as well as detection of metastatic deposits as small as 3–5 mm. Pancreas protocol MRI is emerging as an alternative to CT for patients.
-
#3
The role of PET/CT scan remains unclear. PET/CT scan may be considered after formal pancreatic CT protocol in “high-risk” patients to detect extra pancreatic metastases. It is not a substitute for high-quality, contrast enhanced CT.
-
#4
Endoscopic ultrasound (EUS) may be complementary to CT for staging.
-
#5
EUS-directed FNA biopsy is preferable to a CT-guided FNA in patients with resectable disease because of better diagnostic yield, safety, and potentially lower risk of peritoneal seeding with EUS FNA when compared with the percutaneous approach. Biopsy proof of malignancy is not required before surgical resection and a non-diagnostic biopsy should not delay surgical resection when the clinical suspicion for pancreatic cancer is high.
-
#6
Diagnostic staging laparoscopy to rule out subradiologic metastases (especially for body and tail lesions) is used routinely in some institutions prior to surgery or chemoradiation, or selectively in patients who are at higher risk for disseminated disease (borderline resectable disease, markedly elevated CA 19–9, large primary tumors, or large regional lymph nodes).
-
#7
Positive cytology from washings obtained at laparoscopy or laparotomy is equivalent to M1 disease. If resection has been done for such a patient, he or she should be treated for M1 disease.
Version 2.2012 © National Comprehensive Cancer Network, Inc. 2012, All rights reserved. The NCCN Guidelines and this illustration may not be reproduced in any form without the express written permission of NCCN®.
PANC-D (1 of 4).
PATHOLOGICAL ANALYSIS: SPECIMEN ORIENTATION, HISTOLOGICAL SECTIONS AND REPORTING
The primary purpose of pathological analysis of the pancreatic specimen is to determine the pathological stage of the tumor by evaluating the type, grade, size and extent of the cancer.
Whipple Specimen
- Specimen orientation
-
➤Specimen orientation and inking involves both pathologist and surgeon as this will help to ensure accurate assessment of the size and extent of the tumor. There should be either direct communication between the surgeon and pathologist for proper orientation and margin identification, or the surgeon should identify the important margins with a clearly understood and documented method [e.g. written on the pathology requisition]; for example: stitch on posterior margin, safety pin on the retroperitoneal/uncinate margin.
-
➤
- Margins
-
➤Definitions of the margins and uniformity of nomenclature are critical to accurate reporting
-
◇SMA (Retroperitoneal/uncinate) Margin: The most important margin is the soft tissue directly adjacent to the proximal 3–4 cm of the superior mesenteric artery. This margin is often referred to as the “retroperitoneal margin” or “posterior margin”, but has also been referred to as the “uncinate margin” or “mesenteric margin”. More recently, this margin has been referred to as the “SMA margin” to correlate with its location on the specimen. Radial rather than en face sections of this margin will more clearly demonstrate how closely this margin is approached by tumor. The simple step of palpating the specimen can help guide the pathologist as to the best spot along the SMA margin to select for sampling.
-
◇Posterior Margin: This margin is from the posterior caudad aspect of the pancreatic head that merges with the uncinate margin and that appears to be covered by loose connective tissue. Radial rather than en face sections of this margin will more clearly demonstrate whether it is involved by tumor. In some instances this margin can be included in the same section as the SMA margin section.
-
◇Portal Vein Groove Margin: This is the smooth-surfaced groove on the posterior-medial surface of the pancreatic head that rests over the portal vein. Radial rather than en face sections of this margin will more clearly demonstrate whether it is involved by tumor and also will provide the distance of the tumor from the margin. As is true for the posterior margin, in some instances this margin can be included in the same section as the SMA margin section.
-
◇Portal Vein Margins: If an en bloc partial or complete vein resection is added to the surgical specimen it should be marked separately. En face proximal and distal end margins of the vein should be separately submitted as Proximal Portal Vein Margin and Distal Portal Vein Margin. A section documenting tumor invasion into the vein wall should also be submitted. If feasible, this section should be a full thickness of the vein wall demonstrating the depth of tumor invasion as this has been shown to have prognostic value.8
-
◇Pancreatic Neck (transection) Margin: This is the en face section of the transected pancreatic neck. The section should be placed into the cassette with true margin facing up so that the initial section into the block represents the true surgical margin.
-
◇Bile Duct Margin: This is the en face section of the bile duct end. The section should be removed from the unopened duct and placed into the cassette with true margin facing up so that the initial section into the block represents the true surgical margin.
-
◇
-
➤Other margins analyzed in Whipple specimens include the proximal and distal enteric margins (en face sections) and anterior surface (closest representative). The anterior surface is not a true margin, but identification and reporting of this surface when positive may portend a risk of local recurrence, and so should be reported in all cases.9–12
-
➤Collectively, these pancreatic tissue surfaces constitute the circumferential transection margin. Designating the various specific margins with different colored inks will allow recognition on microscopy.
-
➤
Version 2.2012 © National Comprehensive Cancer Network, Inc. 2012, All rights reserved. The NCCN Guidelines and this illustration may not be reproduced in any form without the express written permission of NCCN®.
PANC-D (2 of 4).
PATHOLOGICAL ANALYSIS: SPECIMEN ORIENTATION, HISTOLOGICAL SECTIONS AND REPORTING
- Histological sectioning
-
➤The approach to histological sectioning is determined by the unique characteristics of the tumor, but is also influenced by institutional preferences, expertise and experience. Options include axial, bi- or multi-valve slicing and perpendicular sliding. Some experts in the field bisect the pancreas along probes placed in the bile and pancreatic ducts and then serially section along each half of the pancreas.
-
➤Axial slicing provides an overall assessment of the epicenter of the tumor relative to the ampulla, bile duct, duodenum and pancreas, and all of the pancreatic circumferential tissue margins mentioned above.
-
➤There is no one correct way to dissect a Whipple specimen. The most important aspects of dissection are clear and accurate assessment of the margins.
-
➤It is currently unknown what constitutes an adequate margin in pancreatic carcinoma resection specimens. A standardized definition of this would allow better stratification of patients into adjuvant regimens following surgical extirpation. For instance, if less than 1mm clearance is associated with an unacceptably high incidence of local recurrence then strong consideration for post-operative radiation therapy might be indicated if not received pre-operatively. Tumor clearance should be reported in millimeters for the SMA margin described above to allow prospective accumulation of this important data for future analysis.
-
➤Attached organs resected with the specimen en bloc require serial sectioning to assess not only direct extension, but metastatic deposits as well. One section that demonstrates direct invasion of the organ and/or a separate metastatic deposit is required.
-
➤
Distal Pancreatectomy
In left sided resections the peripancreatic soft tissue margins and the pancreatic neck are assessed. Additionally, involvement of the splenic vessels should be documented and invasion of the spleen is important to determine, as direct tumor invasion constitutes a pT4 pathological stage.
Frozen section analysis of the pancreatic neck is recommended.
- Margins definitions are as follows:
-
◇Proximal pancreatic (transection) margin: A full en face section of the pancreatic body along the plane of transection. The section should be placed into the cassette with true margin facing up so that the initial section into the block represents the true surgical margin. More than one block may be needed.
-
◇Anterior (cephalad) peripancreatic (peripheral) surface: This surface demonstrates the relationship between the tumor and the anterior or cephalad peripancreatic soft tissue and can be representative if grossly positive. Several such sections should be taken closest to the tumor to document absence of involvement; the exact number is dependent on the degree of ambiguity of involvement grossly.
-
◇Posterior (caudad) peripancreatic (peripheral) margin: This margin demonstrates the relationship between the tumor and the posterior or caudad peripancreatic soft tissue and can be representative if grossly positive. Several such sections should be taken closest to the tumor to document absence of involvement; the exact number is dependent on the degree of ambiguity of involvement grossly.
-
◇
Version 2.2012 © National Comprehensive Cancer Network, Inc. 2012, All rights reserved. The NCCN Guidelines and this illustration may not be reproduced in any form without the express written permission of NCCN®.
PANC-D (3 of 4).
PATHOLOGICAL ANALYSIS: SPECIMEN ORIENTATION, HISTOLOGICAL SECTIONS AND REPORTING
Reporting
The NCCN Pancreatic Cancer Panel currently supports pathology synoptic reports from the College of American Pathologists (CAP). The proposal included herein is an abbreviated minimum analysis of pancreatic cancer specimens from the CAP recommendations. In addition to the standard TNM staging, other variables are included all of which have prognostic implications in the evolution of this disease.13,14
Specimen type
Tumor size (obtained from careful gross measurement of the largest dimension of the tumor in cm.
Histologic grade (G (x-4))
Primary tumor extent of invasion (T (x-4))
- Regional lymph nodes (N (x-1))
-
➤# Nodes recovered
-
➤# Nodes involved
-
➤
Metastases (M (x-1))
- Margins: [Involvement should be defined and surgical clearance measured in mm]
-
➤Whipple Resection:
-
◇SMA (Retroperitoneal/uncinate) Margin
-
◇Posterior Margin
-
◇Portal Vein Groove Margin
-
◇Pancreatic Neck (transection) Margin
-
◇Bile Duct Margin
-
◇Enteric Margins
-
◇Anterior surface
-
◇
-
➤Distal pancreatectomy:
-
◇Proximal pancreatic (transection) margin
-
◇Anterior (cephalad) peripancreatic (peripheral) surface
-
◇Posterior (caudad) peripancreatic (peripheral) margin
-
◇
-
➤
Lymphatic (small vessel) invasion (L)
Vascular (large vessel) invasion (V)
Perineural invasion (P)
-
Additional pathologic findings
-
➤Pancreatic intraepithelial neoplasia
-
➤Chronic pancreatitis
Final Stage: G, T, N, M, L, V, P
-
➤
Version 2.2012 © National Comprehensive Cancer Network, Inc. 2012, All rights reserved. The NCCN Guidelines and this illustration may not be reproduced in any form without the express written permission of NCCN®.
PANC-G (1 of 3).
PRINCIPLES OF CHEMOTHERAPY (1 of 3)
Systemic therapy is used in the neoadjuvant or adjuvant setting and in the management of locally advanced unresectable and metastatic disease
Goals of systemic therapy should be discussed with patients prior to initiation of therapy, and enrollment in a clinical trial is strongly encouraged.
Close follow-up of patients undergoing chemotherapy is indicated.
Metastatic
- Acceptable monotherapy options include:
-
➤Gemcitabine at 1000 mg/m2 over 30 minutes, weekly for 3 weeks every 28 days (category 1).
-
➤Fixed-dose rate gemcitabine (10 mg/m2/minute) may substitute for standard infusion of gemcitabine over 30 minutes (category 2B).
-
➤Capecitabine (category 2B)
-
➤
- Acceptable chemotherapy combinations (for patients with good performance status):
-
➤Gemcitabine + erlotinib1 (category 1)
-
➤FOLFIRINOX2 (category 1)
-
➤Gemcitabine + capecitabine3
-
➤Gemcitabine + cisplatin (especially for patients with possible hereditary cancers)4
-
➤Fixed-dose rate gemcitabine, docetaxel, capecitabine (GTX regimen) (category 2B)5
-
➤Gemcitabine + nab-paclitaxel6 (category 2B)
-
➤Fluoropyrimidine + oxaliplatin (category 2B) (eg, 5-FU/leucovorin/oxaliplatin7 or CapeOx8)
-
➤
Second-line therapy may consist of gemcitabine for those patients not previously treated with the drug. Other options include capecitabine (1000 mg/m2 PO twice daily, days 1–14 every 21 days) or 5-FU/leucovorin/oxaliplatin7 or CapeOx.8 Results of the CONKO 003 trial demonstrated a significant improvement in overall survival with the addition of oxaliplatin to 5-FU /leucovorin.7
1Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960–1966.
2Conroy T, Desseigne F, Ychou M, et al. Randomized phase III trial comparing FOLFIRINOX versus gemcitabine as first-line treatment for metastatic pancreatic adenocarcinoma: Preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial [abstract]. J Clin Oncol 2010;28(suppl_7s):Abstract 4010.
3Cunningham D, Chau I, Stocken D D, et al. Phase III randomized comparison of gemcitabine (GEM) versus gemcitabine plus capecitabine (GEM-CAP) in patients with advanced pancreatic cancer. J Clin ONcol. 2009; 27:5513–5518.
4Oliver GR, Sugar E, Laheru D, et al. Family history of cancer and sensitivity to platinum chemotherapy in pancreatic adenocarcinoma [abstract]. Presented at: 2010 ASCO Gastrointestinal Cancers Symposium; January 22–24, 2010; Orlando, Florida. Abstract 180.
5Fine RL, Fogelman DR, Schreibman SM, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol 2008;61:167–175.
6Van Hoff DD, Ramanathan R, Borad M, et al. SPARC correlation with response to gemcitabine plus nab-paclitaxel in patients with advanced metastatic pancreatic cancer: A phase I/II study [abstract]. J Clin Oncol 2009;27(suppl_15s):Abstract 4525.
7Pelzer U, Kubica K, et al. A randomized trial in patients with gemcitiabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol 26: 2008 (May 20 suppl) Abstract 4508.
8Xiong HQ, Varadhachary GR, Blais JC, et al. A phase ll trial of oxaliplatin plus capecitabine (xelox) as second-line therapy for patients with advanced pancreatic cancer. Cancer 2008; 1 13:2046–2052.
Version 2.2012 © National Comprehensive Cancer Network, Inc. 2012, All rights reserved. The NCCN Guidelines and this illustration may not be reproduced in any form without the express written permission of NCCN®.
Footnotes
Disclosures for the NCCN Pancreatic Adenocarcinoma Panel Individual disclosures of potential conflicts of interest for the NCCN Pancreatic Adenocarcinoma Panel can be found online at NCCN.org.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. The NCCN Guidelines® Insights highlight important changes in the NCCN Guidelines® recommendations from previous versions. Colored markings in the algorithm show changes and the discussion aims to further understanding of these changes by summarizing salient portions of the panel's discussion, including the literature reviewed.
The NCCN Guidelines Insights do not represent the full NCCN Guidelines; further, the National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding the content, use, or application of the NCCN Guidelines and NCCN Guidelines Insights and disclaims any responsibility for their applications or use in any way.
The full and most current version of these NCCN Guidelines is available at NCCN.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LD, Patel AV, Yan Y, et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev. 2009;18:2397–2405. doi: 10.1158/1055-9965.EPI-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.StatBite U.S. pancreatic cancer rates. J Natl Cancer Inst. 2010;102:1822. doi: 10.1093/jnci/djq517. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Talamonti MS, Sener SF, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207:510–519. doi: 10.1016/j.jamcollsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Zervos EE, Rosemurgy AS, Al-Saif O, Durkin AJ. Surgical management of early-stage pancreatic cancer. Cancer Control. 2004;11:23–31. doi: 10.1177/107327480401100104. [DOI] [PubMed] [Google Scholar]
- 8.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 9.Wong JC, Lu DSK. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol. 2008;6:1301–1308. doi: 10.1016/j.cgh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrman GM, Charnsangavej C, Abbruzzese JL, et al. Thin-section contrast-enhanced computed tomography accurately predicts the resectability of malignant pancreatic neoplasms. Am J Surg. 1994;167:104–111. doi: 10.1016/0002-9610(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 11.Horton KM, Fishman EK. Adenocarcinoma of the pancreas: CT imaging. Radiol Clin North Am. 2002;40:1263–1272. doi: 10.1016/s0033-8389(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 12.House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg. 2004;8:280–288. doi: 10.1016/j.gassur.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Klauss M, Schobinger M, Wolf I, et al. Value of three-dimensional reconstructions in pancreatic carcinoma using multidetector CT: initial results. World J Gastroenterol. 2009;15:5827–5832. doi: 10.3748/wjg.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNulty NJ, Francis IR, Platt JF, et al. Multi-detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 15.Gabata T, Matsui O, Kadoya M, et al. Small pancreatic adenocarcinomas: efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology. 1994;193:683–688. doi: 10.1148/radiology.193.3.7972808. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa T, Haradome H, Hachiya J, et al. Pancreatic ductal adenocarcinoma: preoperative assessment with helical CT versus dynamic MR imaging. Radiology. 1997;202:655–662. doi: 10.1148/radiology.202.3.9051012. [DOI] [PubMed] [Google Scholar]
- 17.Kauhanen SP, Komar G, Seppanen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–963. doi: 10.1097/SLA.0b013e3181b2fafa. [DOI] [PubMed] [Google Scholar]
- 18.Schima W, Fugger R, Schober E, et al. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. AJR Am J Roentgenol. 2002;179:717–724. doi: 10.2214/ajr.179.3.1790717. [DOI] [PubMed] [Google Scholar]
- 19.Schima W, Ba-Ssalamah A, Goetzinger P, et al. State-of-the-art magnetic resonance imaging of pancreatic cancer. Top Magn Reson Imaging. 2007;18:421–429. doi: 10.1097/rmr.0b013e31816459e0. [DOI] [PubMed] [Google Scholar]
- 20.Olivie D, Lepanto L, Billiard JS, et al. Predicting resectability of pancreatic head cancer with multi-detector CT. Surgical and pathologic correlation. JOP. 2007;8:753–758. [PubMed] [Google Scholar]
- 21.Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging. 2009;20:3–9. doi: 10.1097/RMR.0b013e3181b48392. [DOI] [PubMed] [Google Scholar]
- 22.Walters DM, Lapar DJ, de Lange EE, et al. Pancreas-protocol imaging at a high-volume center leads to improved preoperative staging of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2764–2771. doi: 10.1245/s10434-011-1693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 24.Taieb J, Lecomte T, Aparicio T, et al. FOLFIRI.3, a new regimen combining 5-fluorouracil, folinic acid and irinotecan, for advanced pancreatic cancer: results of an Association des Gastro-Enterologues Oncologues (Gastroenterologist Oncologist Association) multicenter phase II study. Ann Oncol. 2007;18:498–503. doi: 10.1093/annonc/mdl427. [DOI] [PubMed] [Google Scholar]
- 25.Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658–1663. doi: 10.1038/sj.bjc.6605374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Xiong HQ, Varadhachary GR, Blais JC, et al. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008;113:2046–2052. doi: 10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 28.Washington K, Berlin J, Branton P, et al. [Accessed March 20. 2012];Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. Available at: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/PancreasExo_11protocol.pdf.
- 29.Gumbs AA, Rodriguez Rivera AM, Milone L, Hoffman JP. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol. 2011;18:1335–1341. doi: 10.1245/s10434-010-1503-4. [DOI] [PubMed] [Google Scholar]
- 30.Nakeeb A, Lillemoe KD, Grosfeld JL. Surgical techniques for pancreatic cancer. Minerva Chir. 2004;59:151–163. [PubMed] [Google Scholar]
- 31.Yeo TP, Hruban RH, Leach SD, et al. Pancreatic cancer. Curr Probl Cancer. 2002;26:176–275. doi: 10.1067/mcn.2002.129579. [DOI] [PubMed] [Google Scholar]
- 32.Wayne JD, Abdalla EK, Wolff RA, et al. Localized adenocarcinoma of the pancreas: the rationale for preoperative chemoradiation. Oncologist. 2002;7:34–45. doi: 10.1634/theoncologist.7-1-34. [DOI] [PubMed] [Google Scholar]
- 33.Baque P, Iannelli A, Delotte J, et al. Division of the right posterior attachments of the head of the pancreas with a linear stapler during pancreaticoduodenectomy: vascular and oncological considerations based on an anatomical cadaver-based study. Surg Radiol Anat. 2009;31:13–17. doi: 10.1007/s00276-008-0353-2. [DOI] [PubMed] [Google Scholar]
- 34.Evans DB, Pisters PW. Novel applications of endo GIA linear staplers during pancreaticoduodenectomy and total pancreatectomy. Am J Surg. 2003;185:606–607. doi: 10.1016/s0002-9610(02)01400-9. [DOI] [PubMed] [Google Scholar]
- 35.Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma. A contraindication for resection? Ann Surg. 1996;224:342–347. doi: 10.1097/00000658-199609000-00010. discussion 347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riediger H, Makowiec F, Fischer E, et al. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106–1115. doi: 10.1016/j.gassur.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 38.Stitzenberg KB, Watson JC, Roberts A, et al. Survival after pancreatectomy with major arterial resection and reconstruction. Ann Surg Oncol. 2008;15:1399–1406. doi: 10.1245/s10434-008-9844-y. [DOI] [PubMed] [Google Scholar]
- 39.Christein JD, Kendrick ML, Iqbal CW, et al. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J Gastrointest Surg. 2005;9:922–927. doi: 10.1016/j.gassur.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Shoup M, Conlon KC, Klimstra D, Brennan MF. Is extended resection for adenocarcinoma of the body or tail of the pancreas justified? J Gastrointest Surg. 2003;7:946–952. doi: 10.1016/j.gassur.2003.08.004. discussion 952. [DOI] [PubMed] [Google Scholar]
- 41.Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg. 2007;204:244–249. doi: 10.1016/j.jamcollsurg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Verbeke CS. Resection margins and R1 rates in pancreatic cancer—are we there yet? Histopathology. 2008;52:787–796. doi: 10.1111/j.1365-2559.2007.02935.x. [DOI] [PubMed] [Google Scholar]
- 43.Gebhardt C, Meyer W, Reichel M, Wunsch PH. Prognostic factors in the operative treatment of ductal pancreatic carcinoma. Langenbecks Arch Surg. 2000;385:14–20. doi: 10.1007/s004230050004. [DOI] [PubMed] [Google Scholar]
- 44.Mitsunaga S, Hasebe T, Iwasaki M, et al. Important prognostic histological parameters for patients with invasive ductal carcinoma of the pancreas. Cancer Sci. 2005;96:858–865. doi: 10.1111/j.1349-7006.2005.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Japan Pancreatic Society . Classification of Pancreatic Carcinoma. 2nd ed Kanehara; Tokyo, Japan: 2003. [Google Scholar]
- 46.Campbell F, Foulis AK, Verbeke CC. Dataset for the Histopathological Reporting of Carcinomas of the Pancreas, Ampulla of Vater and Common Bile Duct. The Royal College of Pathologists; London, England: 2010. [Google Scholar]
- 47.Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas: AFIP Atlas of Tumor Pathology, 4th Series, Fascicle 6; Washington, DC. Armed Forces Institutes of Pathology; 2007. [Google Scholar]


