Abstract
Introduction
Stroke is the third leading cause of death and a major cause of long-term disability in the adult population. Growing evidence suggests that inflammation may play an important role in the evolution of stroke. Because Rho-associated coiled-coil containing kinases (ROCKs) are important mediators of inflammation, they may contribute to stroke and stroke recovery.
Areas covered
The pathophysiological role of ROCKs in mediating inflammation at different phases of stroke, and the therapeutic opportunities for stroke prevention and stroke treatment with ROCK inhibitors will be discussed.
Expert opinion
Inflammation is a double-edged sword during the evolution of stroke. Immunomodulation might provide a novel therapeutic approach for stroke prevention and stroke treatment. ROCK plays an important role in mediating the inflammatory response following vascular injury as well as platelet activation and thrombus formation. ROCK inhibitors have been shown to be beneficial in stroke prevention, acute neuroprotection and chronic stroke recovery by affecting inflammatory-mediated platelet and endothelial function, smooth muscle contraction and neuronal regeneration. Thus, ROCK-mediated inflammation could be a potential therapeutic target for stroke prevention and stroke treatment. However, the mechanism by which ROCKs regulate the inflammatory response is unclear, and the role of the two ROCK isoforms in stroke and stroke recovery remains to be determined.
Keywords: fasudil, inflammation, Rho kinase, stroke
1. Introduction
Stroke is the third most common cause of death and is a leading cause of long-term disability [1]. On average, stroke occurs every 40 s in the USA, and the mortality rate is about 30%. The total cost of stroke to the USA is estimated at US$68 billion per year, and the mean lifetime cost of ischemic stroke per patient is estimated to be US $140,048. Approximately 30 – 50% of stroke survivors are functionally disabled. More than 65% of the total cost for stroke is due to long-term care and lost productivity. Despite these staggering costs, there are few effective therapies, which can prevent stroke and improve the functional outcome of patients with ischemic stroke. Consequently, there is a pressing need to identify biological targets for potential pharmacological interventions for stroke prevention and treatment. There is increasing evidence showing that the neuro-inflammatory response is an important process that contributes to the risk of stroke as well as the acute and chronic phase, and generates both beneficial and detrimental effects [2]. For example, depending on the timing, severity, type of immune response and microenvironment, the immune system can generate both positive and negative effects following stroke. Therefore, it is important to fine-tune the immune response in order to harness the beneficial while avoiding detrimental effects. In this review, the authors will discuss the diverse role of ROCKs in inflammation during stroke evolution. A better understanding of the ROCK pathway in inflammation may help foster development of innovative therapeutic strategies for stroke prevention and treatment.
2. Function of ROCKs
Rho-GTPases function as signal transducers between cell surface receptors and intracellular signaling pathways. There are three main subclasses of Rho-GTPases, including Rho, Rac and Cdc42, whose activities are regulated by the binding of GTP [3]. These molecules play a pivotal role in many cellular processes such as chemotaxis, contraction, proliferation and apoptosis. One of the first downstream effectors of Rho are ROCKs, which mediate actin cytoskeletal changes through their effects on myosin light-chain (MLC) phosphorylation [4,5]. Consequently, ROCKs affect multiple actin cytoskeletal functions related to cell shape and motility. However, ROCKs could also regulate other important non-cytoskeletal signaling path ways. For example, ROCKs mediates upregulation of NAD (P)H oxidase after treatment with angiotensin II in myocardial hypertrophy [6]. ROCK decreases endothelial nitric oxide (NO) synthase (eNOS) mRNA stability via post-transcriptional regulation [7]. Furthermore, inhibition of ROCK activity leads to rapid phosphorylation and activation of eNOS and cardiovascular protection [8]. Indeed, a potential pleotropic effect of statin may be mediated via the inhibition of ROCKs. For example, statin treatment inhibits ROCK activity, leading to the stabilization of eNOS mRNA and increased level of endothelial-derived NO, which is neuroprotective [7,9].
ROCKs have been found to play important roles in broad range of physiological functions, including vascular smooth muscle contraction, vascular tone, blood flow, vascular inflammation, cytoskeleton organization, cell adhesion and cell mobility [10]. Abnormal ROCK activity has been observed in number of cardiovascular diseases, including hypertension, pulmonary hypertension, atherosclerosis, cerebral and coronary vasospasm, myocardial hypertrophy, myocardial ischemia-reperfusion injury and acute cerebral ischemia [10]. Treatments with ROCK inhibitors such as fasudil has been found to be beneficial for hypertension [11], pulmonary hypertension [12], ischemia stroke [13], cerebral vasospasm after subarachnoid hemorrhage [14] and vasospastic angia [15].
The available ROCK inhibitors are non-selective ROCK inhibitors. There are two isoforms of ROCKs, ROCK1 and ROCK2. The two isoforms share a highly conserved amino-terminal region with 92% homology in the kinase domain. The carboxyl-terminal region is substantially differ ent between the two isoforms with 65% homology [16]. Both isoforms can phosphorylate a variety of substrates such as MLC phosphatase, LIM kinase and ezrin–radixin–moesin proteins. However, the specific functions of the two isoforms are largely unknown. Increasing evidence suggests that ROCK1 and ROCK2 have different roles in cellular and physiological function. ROCK1 is mainly expressed in the lung, liver, spleen, kidney and testis, while ROCK2 is distributed mostly in the brain and heart [16]. Knockout of ROCK1 in mice generates an omphalocele phenotype [17], whereas knockout of ROCK2 leads to intrauterine growth retardation and fetal death [18]. In haploinsufficient ROCK1+/- mice, neointima formation, the levels of pro-inflammatory adhesion molecule expression and leukocyte infiltration were reduced following vascular injury [19]. Knockdown of ROCK2 but not ROCK1 by small interfering RNA (siRNA) inhibits natural killer-kappaB (NK-κB) activation induced by lysophosphatidic acid (LPA), resulting in decreased expression of adhesion molecules [20]. Therefore, it is important to determine the specific function of ROCK1 and ROCK2 in order to develop effective targeted treatment strategies.
3. ROCK-mediated inflammation as a risk factor for stroke
Recent evidence suggests a strong link between inflammation and the risk of stroke. Chronic inflammatory disorders such as inflammatory bowel disease, vasculitis, rheumatoid arthritis [21,22] and systemic lupus erythematosus (SLE) [23,24] are associated with an increased risk of ischemic stroke. Furthermore, the link between inflammation and stroke also comes from an increased incidence of stroke associated with systemic infection [25]. About 18 – 40% of patients with ischemic stroke had recent bacterial or viral infections [26]. Indeed, the incidence of stroke is increased in influenza pandemics and in patients with chronic infections such as periodontitis and chronic bronchitis. This is because inflammation could affect stroke formation through multiple processes, including thrombosis formation, vasculopathy and atherosclerosis. This involves several different cell types such as platelets, endothelial cells, monocytes, macrophages and T lymphocytes. Thus, there is recently emerging concept of ‘thrombo-inflammatory’ cascade in acute ischemic stroke process [27,28]. Because ROCKs mediate inflammation and thrombosis formation via affecting the function of a wide variety of cell types, including vascular and inflammatory cells (as summarized in Table 1), abnormal activation of ROCK could increase the risk of stroke. For example, ROCK activity in peripheral leukocytes is elevated in humans within 48 h of an acute ischemic stroke as compared with healthy controls [29]. In addition, it has been shown that inhibition of ROCK may mediate the non-cholesterol or ‘pleiotropic’ effects of statins in preventing ischemic stroke [30-32] and in inhibiting venous thromboembolic events [33]. Statins inhibit the formation of isoprenoid intermediates such as geranylgeranyl pyrophosphate [34]. These isoprenoid intermediates are required for post-translational modification and trafficking of Rho-GTPases [35]. Therefore, statins could inhibit the activation of the Rho-ROCK pathway.
Table 1.
Potential role of ROCK in mediating the inflammatory response in stroke.
| Cell type | Cellular functions of ROCK | Role in stroke | Ref. |
|---|---|---|---|
| Endothelium | Increase endothelial permeability, decrease tight junctions, increase cell adhesion between endothelial cell and extracellular matrix, increase inflammatory cell invasion, mediate cerebral blood flow via eNOS-dependent mechanisms | Increase risk of stroke and increase infarct volume | [58-61,68,139,140] |
| Smooth muscle | Promote smooth muscle cell differentiation, proliferation, and migration via MLCK, increase intimal thickness | Increase risk of stroke | [62-64] |
| Platelets | Platelet contractile function via PIP5K, assembly of platelet actin cytoskeleton, thrombus contraction, aggregation of platelet | Increase risk of stroke | [45-47] |
| Neutrophils | Increase neutrophil recruitment and adhesion to endothelium | Increase infarct volume | [68] |
| T lymphocytes | T-cell proliferation via actomyosin polymerization, T-cell receptor activation via activation of JNK and NF-κB, increased IL-17 production via IRF4 activation | Unclear | [117-119,123] |
| Macrophages | Macrophage migration via induction of MCP-1 | Unclear | [134] |
| Microglia | Pro-inflammatory cytokine production | Unclear | [67] |
| Astrocyte | Promote astrocyte migration, process formation, inhibit glutamate transport via EAAT 1/2 | Unclear | [136,137] |
EAAT: Excitatory amino acid transporter; eNOS: Endothelial nitric oxide synthase; IRF4: Interferon regulatory factor 4; JNK: Jun N-terminal kinase; MCP-1: Monocyte chemotactic protein-1; MLCK: Myosin light-chain kinase; NF-κB: Nuclear transcription factor-κB; PIP5K: Phosphatidylinositol 4-phosphoate 5-kinase; ROCKs: Rho-associated coiled-coil containing kinases.
3.1 ROCK and platelet function
The adhesion of platelets to damaged blood vessels initiates thrombosis, which is the precipitating event and final common pathway in ischemic strokes [36,37]. The cascade of platelet adhesion, spreading, aggregation and thrombus formation is initiated by interaction between the platelet receptor glycoprotein (GP)Ib and von Willebrand factor (vWF) under high shear stress [38]. vWF has been suggested as a potential target in stroke therapy [39]. Several studies indicate that high plasma level of vWF is associated with risk of ischemic stroke [40,41]. In consistent with the clinical study, mice with deletion of vWF gene (vWF-/-) have reduced infarct volume and improved neurological score after middle cerebral artery occlusion (MCAO) [42].
Several collagen-binding proteins that are expressed on the platelets, including GPIV, GPVI and integrin α2β1 regulates subsequent collagen-induced adhesion and aggregation. Activated platelets also stimulate inflammatory response. Platelets that aggregate at the injured sites secrete cytokines, including platelet-activating factor (PAF), macrophage inflammatory protein (MIP)-1α, TGF-β, platelet-derived growth factor (PDGF) and LPA which promote leukocyte recruitment, smooth muscle cell proliferation and collagen synthesis. In addition, the platelet receptor-GPIbα not only binds to vWF for platelet adhesion, but also binds to macrophage-1 antigen (Mac-1) which mediates neutrophil adhesion, transendothelial migration and phagocytosis [43]. Mac-1-deficient mice have less infarct volume after transient middle cerebral artery occlusion [44]. Furthermore, GPVI activation induces upregulation of pro-inflammatory cytokines including IL-1α and bradykinin. Mice with deficiency of bradykinin receptor B1 (B1R) have reduced infarct volume after MCAO. Therefore, thrombosis and inflammation are closely coupled. Chronic inflammation activates platelet aggregation and thrombosis formation, while activated platelets stimulate further platelet and leukocyte recruitment.
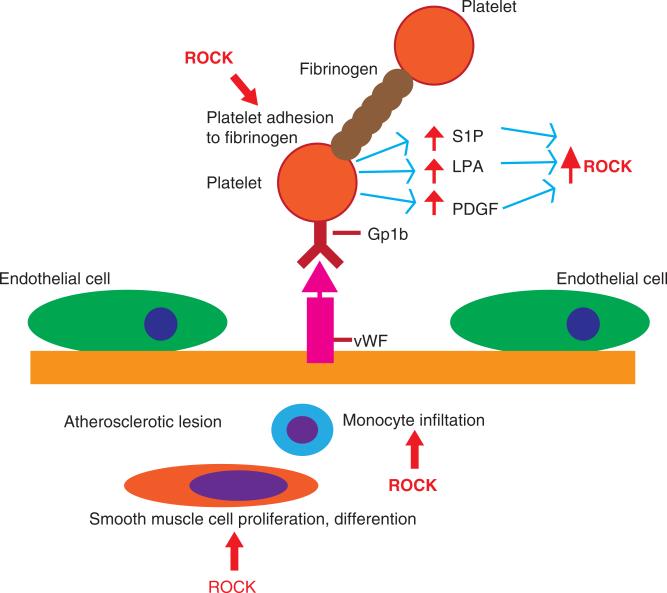
Recent evidence suggests that the Rho/ROCK pathway is involved in platelet activation. ROCK regulates the assembly of the platelet actin cytoskeleton. ROCK activates and indu ces trafficking of platelet phosphatidylinositol 4-phosphoate 5-kinase (PIP5K) [45]. First, PIP5K regulates actin dynamics by stimulating the production of phosphatidylinositol 4,5-bisphosphate (PIP2) [45]. Second, ROCK inhibits myosin light-chain phosphatase (MLCP), which regulates phosphory lation of MLC. Phosphorylation of MLC leads to increased interaction with actin resulting in contraction of the platelet actin cytoskeleton [46]. Third, inhibition of ROCKs with Y-27632 reduces platelet adhesion to fibrinogen by decreasing the phosphorylation of MLC (Figure 1). In addition, ROCK increases actin stress fibers in spreading platelets and increasing focal adhesion [47]. Therefore, inhibition of ROCKs reduces thrombus contraction and aggregation of platelets, which reduces thrombus stability [46]. Finally, stimulation of platelets by thrombin results in the activation of Rho and ROCK. Activated platelets release LPA, PDGF and sphingosine-1-phosphate (S1P), which are known to increase the protein levels of ROCK (Figure 1) [48-50]. The involvement of ROCK in thrombus formation has also been suggested by the induction of atherosclerosis by abnormal ROCK activation [51]. Consequently, deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis [52]. Indeed, accelerated atherosclerosis in apoliprotein E-deficient mice was reduced by the ROCK inhibitor, Y-27632 [53].
Figure 1. The role of ROCKs in thrombus formation and inflammation at neurovascular bed.
Thrombus formation is initiated by adhesion of platelet to damaged blood vessels through interaction between platelet receptor GP1b to vWF. Fibrinogen activates platelet aggregation. Several collagen-binding proteins regulate subsequent collagen-induced adhesion and aggregation. Thrombus formation is also triggered by atherosclerosis which is marked by monocyte infiltration and smooth muscle differentiation and proliferation. ROCKs affect thrombus formation at multiple points. ROCKs regulate assembly of the platelet actin cytoskeleton and induce platelet adhesion to fibrinogen. ROCKs stimulate monocyte infiltration and smooth muscle cell proliferation at the atheroscleoric lesion. Activated platelets also secrete pro-inflammatory factors such as LPA, PDGF and S1P which activate ROCKs.
3.2 ROCKs and vascular inflammation
Endothelial dysfunction and vascular inflammation also contribute to atherosclerosis formation. Activation of vascular endothelial cells and circulating leukocytes leads to the recruitment and infiltration of inflammatory cells into the vessel wall, which contributes to vascular inflammation and atherosclerosis. The activated endothelial cells initiate cell adhesion cascade by expressing cell adhesion molecules such as P- and E-selectin, and secreting monocyte chemoattractant protein 1 [54,55]. Circulating leukocytes can bind to the endothelial adhesion molecules and migrate into the vascular intima. Mice with targeted deletion of P- and E-selectin genes have a deficiency in leukocyte rolling and monocyte recruitment to the sites of inflammation [56,57].
ROCKs play an important role in endothelial dysfunction, vascular inflammation and vasculopathy. It has been shown that ROCK mediates endothelial cell permeability [58,59] by regulating cell–cell tight junction [58], regulates cell adhesion between endothelial cells and extracellular matrix [60] and increases inflammatory cell infiltration [61], all of which contribute to vascular inflammation. Indeed, abnormal activation of ROCK has been shown to induce vascular inflammation [51]. In addition, vascular inflammation could be reduced by ROCK inhibition, and this effect is correlated with reduced atherosclerotic lesion formation [51]. Furthermore, haploinsufficient ROCK1+/- mice exhibit reduced neointima formation, decreased levels of pro-inflammatory adhesion molecule expression and reduced leukocyte infiltration following vascular injury [19]. ROCKs could also stimulate smooth muscle cell proliferation [62], differentiation [63], contraction [64] and neointimal formation (Figure 1). Alteration of ROCK signaling has been shown to lead to endothelial dysfunction in diabetes and aortic stiffness in aging and smoking subjects [65]. Indeed, treatment with fasudil improves endothelial function in human subjects with coronary artery disease [66].
4. ROCK-mediated post-stroke inflammation
Increasing evidence suggests an important role of ROCK in acute stroke and chronic stroke recovery through the modulation of neuro-inflammation (Table 1). For example, treatment of cultured microglia with ROCK inhibitor reduces pro-inflammatory factors such as IL-6 and IFN-γ, and increases anti-inflammatory factors such as IL-10. ROCK2 expression was increased in microglia after hypoxia/re-oxygenation injury [67]. Acute administration of ROCK inhibitor fasudil decreased leukocyte recruitment and adhesion to the endothelium after ischemia/reperfusion injury [68]. Therefore, ROCK has become a promising target for modulating inflammation following ischemia-reperfusion injury.
4.1 Inflammation following stroke
The neuro-inflammatory response is an important process during both acute and late phases of stroke [69]. Post-ischemic inflammation is a double-edged sword and involves a complex sequence of events. As a natural defense mechanism of the body to injured tissue, inflammation helps to isolate the damaged area and prevent the spread of the damage. On the other hand, excessive inflammation could exacerbate ischemic injury. Despite intense investigation, the roles of various types of immune responses in the pathophysiology of ischemic stroke are still unclear. Minutes to hours after cerebral ischemia, the inflammatory process is triggered by activation of resident cells such as microglia and recruitment of neutrophils. In animal models of transient ischemic stroke, infiltration of neutrophils in the ischemic brain occurs within 30 min to a few hours and peaks within 72 h [70]. Studies from stroke patients confirm the increased accumulation of neutrophils in infarcted tissues. Furthermore, the amount of neutrophil infiltration correlates with the severity of brain infarction and poor neurological outcome [71,72].
Hypoxic brain cells release a panel of inflammatory mediators called damage-associated molecular patterns (DAMPs), including high-mobility group (HMGB1) box 1, hyaluronan and heat shock proteins [73]. Activation of DAMPs, partially mediated through Toll-like receptors (TLRs), triggers the production of cytokines (IL-1, TNF, IL-6), and reactive oxygen species (ROS), which cause cell death and damage to the blood–brain barrier (BBB). The elevated production of cytokines and chemokines leads to increased expression of cellular adhesion molecules on the vascular endothelium, which help recruit circulating leukocytes into the damaged cerebral parenchyma [74]. Deficiency of TLR2- or TLR-4 results in less cerebral infarct volume with reduced inflammation [75,76]. Furthermore, endothelial dysfunction during ischemia-reperfusion injury results in less production of endothelium-derived nitric oxide. This reduction of endothelium-derived NO leads to increased expression of endothelial adhesion molecules (i.e., P-selectin and intercellular adhesion molecule 1 (ICAM-1)), resulting in increased neutrophil infiltration to the ischemic tissue. Indeed, inhibition of leukocyte adhesion molecules (e.g., ICAM-1, P-selectin) has been shown to reduce infarct volume and brain edema in experimental stroke models [77-79].
By contrast, some inflammatory response has a biphasic pattern. For example, HMGB1, which generates a harmful effect at early stages, has been shown to promote neurogenesis and angiogenesis at later phases [80]. At the later phases of stroke recovery, inflammation could also exert both beneficial and detrimental effects. On the positive side, for example, astrocytes, which are brain inflammatory cells, become activated following central nervous system (CNS) insults and produce neurotrophic factors such as nerve growth factor and brain-derived neurotrophic factors (BDNF) [81-83] required for neuroplasticity. Inhibition of astrogliosis results in axonal loss and worsening of motor function. Some studies suggest that macrophages can stimulate neuronal recovery in spinal cord injury. On the other hand, inflammation has been shown to be detrimental for neurogenesis in the adult brain. Lipopolysaccharide-induced inflammation impairs hippocampal neurogenesis in rats [84]. This impaired neurogenesis is rescued by systemic administration of anti-inflammatory drugs such as indomethacin [85] and minocycline [84]. The detrimental effect of inflammation is supported by the finding that long-term treatment with minocycline after stroke leads to increased neurogenesis and functional stroke recovery [86].
Further studies suggest that the positive and negative effects of inflammation depend on multiple factors such as the phenotype and activities of immune cells, the microenvironment and the origins of immune cells (resident or blood-borne). For example, neurogenesis is dependent on the activation state of microglia. The detrimental effect of microglia is mostly mediated through IL-1, IL-6, TNF-α, NO (from neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS)) and ROS. On the other hand, microglial cells treated with IL-4 switch from TNF-α to IGF-1-producing cells. TNF-α has a negative effect on neurogenesis after stroke [87], whereas IGF-1 promotes proliferation and differentiation of neuronal progenitors [88,89]. T cells and other cells in the neurovascular unit are important regulator of the phenotype of microglial cells. Therefore, the regulation of the inflammatory response after stroke needs to be further elucidated in order to generate an effective immunomodulatory therapy that could promote stroke recovery.
4.2 ROCK and neutrophils
Overactivation of ROCK during acute cerebral ischemia likely contributes to early worsening of cerebral injury partially through stimulating the inflammatory response. Increased expression of adhesion molecules (i.e., P-selectin and ICMA-1) secondary to reduction of endothelium-derived NO during ischemic injury is mediated by ROCK. ROCK is the upstream negative regulator of eNOS. Inhibition of ROCK increases eNOS mRNA stability and expression [7]. Indeed, ROCK inhibitors reduce neutrophil accumulation in the infarct tissue and reduce infarct volume in animal mod els of ischemic stroke [68,90,91]. ROCK activation also stimulates neutrophil infiltration in vascular inflammation via NADHP oxidase activation and ROS production. In addition, in spinal cord injury, ROCK inhibitor reduces leukocyte infiltration into the spinal cord and promotes neurological recovery [92]. Therefore, ROCK could be a promising target for therapeutic intervention for acute stroke.
4.3 Immunomodulatory role of T cells
T lymphocytes play an important role in mediating post-stroke inflammation [93]. Under normal conditions, T cells are prevented from entering the CNS by the BBB. After experimental ischemia in rodents, T cells infiltrate the ischemic region from day 1 and reach the peak around day 7. There is a twofold increase of T lymphocytes in the ischemic versus non-ischemic hemisphere [94]. The role of T cells in stroke and stroke recovery, however, is also a double-edged sword. For example, T cells promote adhesion of neutrophils and platelets, resulting in further tissue injury after ischemic stroke. T-cell-deficient mice have a decreased neuro-inflammatory response and cerebral infarct size following experimental stroke [95]. The immunosuppressant fingolimod, which is used to suppress T-cell-mediated allograft rejection, reduced infarct volume in rats after MCAO [96]. By contrast, T cells may exert protective effects on brain tissue from secondary injury. Copolymer 1, which is a T-cell-specific stimulator, induces neuroprotection following optic nerve injury [97]. The action of T cells mostly depends on the interaction between T-cell subsets, cytotoxic T cells and T-helper cells. Thus, the role of T cells in stroke recovery remains to be determined.
Cytotoxic T cells (CD8+) induce tissue damage by releasing cytotoxic granules or through TNF receptor-mediated cytotoxicity. The activity of CD8+ T cells is regulated by T-helper cells (CD4+). Naïve CD4+ T cells can differentiate into four different subsets depending on activation signals. These subsets include T-helper 1 (Th1), T-helper 2 (Th2), T-helper 17 (Th17) and induced regulatory T cells (Treg) [98]. CD4+ Th1 cells secrete pro-inflammatory cytokines, such as IFN-γ, IL-2, IL-12 and TNF-α, and Th1 cells are strongly associated with neurodegenerative diseases. CD4+ Th2 cells secrete anti-inflammatory cytokines, such as IL-4, IL-5, IL-10 and IL-13. Th2 cells also downregulate the activity of Th1 cells. The anti-inflammatory response mediated through Th2 cells has been shown to be beneficial in promoting stroke recovery. High levels of acute IL-10 secreted from Th2 cells correlates with better clinical outcomes after ischemic stroke [99]. In addition, TGF-β produced by Th2 cells has been shown to protect neurons from hypoxic- and apoptotic-induced injury [99,100]. TGF-β increases anti-apoptotic factors such as Bcl-2 and Bcl-xL [101]. Furthermore, it was found that in the late phases of post-stroke patients, there is a shift of the immune system toward a Th2 response, which may counter the potential harmful effects of the IFN-γ-mediated post-stroke immune response [102].
Interestingly, Th17 cells produce IL-17 [103], IL-21 [104] and IL-22 [103], play a role in autoimmunity and are involved in the clearance of extracellular pathogens. Th17 cells produce large quantities of IL-17, and most of Th17-regulated effects are mediated through this cytokine. IL-17 induces production of pro-inflammatory cytokines (IL-6, TNF-α and IL-1β), chemokines (CXCL1, GCP-1, CXCL8, CINC, monocyte chemotactic protein-1 (MCP-1)) [105] resulting in the recruitment of neutrophils to tissues.
Induced Treg cells phenotypically resemble natural Treg cells, which makes distinguishing their functions difficult. Treg cells possess anti-inflammatory effects via production of TGF-β, IL-10 and IL-35. Treg cells play an important role in maintaining self-tolerance. Activation of Treg cells prevents both acute and chronic allograft rejection in mice; whereas deletion of Treg cells enhances immunity against tumors and chronic infectious agents.
T-cell lineage differentiation plays an important role in determining the immunomodulatory response and is mediated by cytokines. For example, IFN-γ activates Th1 pathways by upregulating transcription factors such as STAT1. STAT1 activate T-box expressed in T cells (T-bet), which is a master transcription activator for Th1 differentiation. T-bet is exclusively expressed in Th1 and stimulates Th1 to produce IFN-γ and IL-2. T-bet also inhibits production of IL-4 and IL-5 from Th2 cells [106]. By contrast, lineage differentiation to the Th2 phenotype requires binding of IL-4 to its receptor, which triggers activation of STAT6. Activation of STAT6 upregulates GATA-binding protein 3 (GATA-3), which is the potent activator of Th2 to produce Th2 cytokines IL-4 and IL-5, and inhibit Th1 cytokine IFN-γ [107].
Th17 differentiation requires both IL-6 and TGF-β [108,109], which have opposite effects. IL-6 is a pro-inflammatory cytokine that is induced by infection or local inflammation, whereas TGF-β is an anti-inflammatory cytokine. Deficiency in either IL-6 [110] or TGF-β [108,109] results in failed development of Th17 cells. While combination of TGF-β and IL-6 is needed to induce Th17 differentiation, IL-23 mediates maintenance of Th17 phenotype and IL-21 mediates amplification of the Th17 response [111]. In the presence of IL-6, TGF-β upregulates IL-23 receptor (IL-23R) [112] and stimulates production of IL-21. IL-21 generates a positive feedback effect on Th17 proliferation by stimulating expression of IL-23R and retinoic acid-related orphan nuclear hormone receptor (ROR-γt) [113]. Importantly, all three cytokines (IL-6, IL-21 and IL-23) activate STAT3.
TGF-β also induces naïve CD4+ T cells to differentiate into Treg in the absence of pro-inflammatory cytokines. Co-activation of Smad3 by TGF-β and of NFAT by TCR (T-cell antigen receptor) induces the expression of the transcription factor forkhead box P3 (Foxp3), which is the key regulator of Treg differentiation [114]. STAT5 activation induced by IL-2 is also required for Foxp3 expression.
4.4 ROCK and T-cell function
The role of ROCKs in T-cell function is not well known. However, much evidence suggests that ROCKs mediate T-cell activation either by affecting actin cytoskeletal changes or by affecting transcription factors involved in T-cell activation. The T-cell cytoskeletal complex may act to support signaling transduction during T-cell activation. T-cell activation is accompanied by the rearrangement of cytoskeletal complexes triggered by the binding of CD3 to the T-cell receptor [115,116]. In addition, phosphorylation of the actin and myosin cytoskeleton is required for T-cell activation. Since one of the main functions of ROCKs is to induce cytoskeletal rearrangement, it is conceivable that ROCKs play important roles in T-cell activation. Indeed, inhibition of ROCK with Y-27632 in T cells blocked actomyosin polymerization, leading to reduced T-cell proliferation and T-cell receptor activation [117]. ROCK has been shown to regulate the migration of CD4+ T cells. ROCK activity is increased in CD4+ T cells from SLE patients, and inhibition of ROCK activity decreases cytoskeletal abnormalities in T cells from SLE patients.
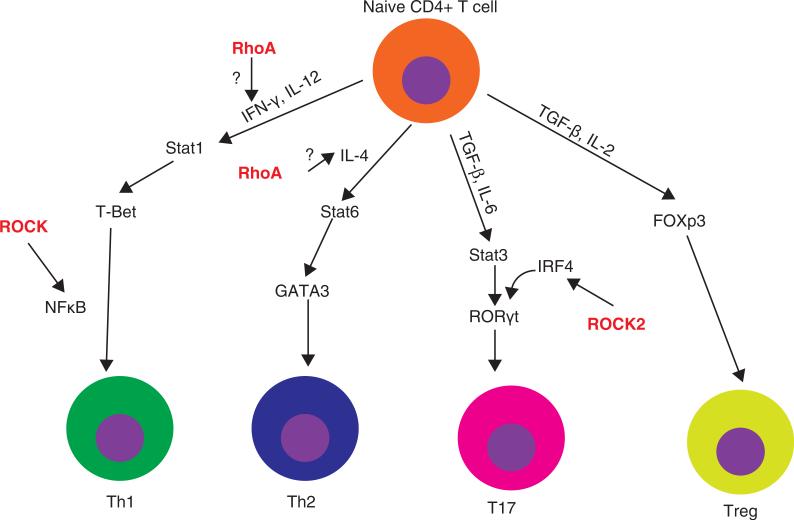
The ROCK pathway may also regulate several transcription factors required for T-cell activation (Figure 2). ROCK induces activation of Jun N-terminal kinase (JNK) in T cells [118]. The activated JNK, in turn, phosphorylates downstream transcription factors. ROCK has also been found to activate a nuclear transcription factor-kB (NF-κB), which is a potent activator of T-cell function [119]. In addition, stimulation of TCR activates RhoA [120], which leads to activation of p38 mitogen-activated protein kinase (p38MARK), which induces upregulation of IL-4 and IL-10, and IFN-γ production [121,122]. Furthermore, ROCK2 phosphorylates interferon regulatory factor 4 (IRF4), which is required for induction of ROR-γt and increases production of IL-17 and IL-21 [123] in Th17 cell differentiation.
Figure 2. Role of ROCK in T-cell lineage differentiation.
On stimulation, T cells differentiate into different subsets including Th1, Th2, Th17 and Treg. ROCK has been shown to regulate T-cell lineage differentiation at multiple levels. Stimulation of T-cell receptor activates RhoA, which leads to activation of p38 mitogen-activated protein kinase (p38MARK). p38MARK upregulates production of IFN-γ and IL-4. In addition, ROCK has been shown to activate NK-κB which plays important role differentiation into Th1 cells. Furthermore, ROCK2 phosphorylates interferon regulatory factor 4 (IRF4), which is required for the induction of retinoic acid-related orphan nuclear hormone receptor (ROR-γt) in Th17-cell differentiation.
4.5 ROCK and other inflammatory cells
Microglial cells are the resident macrophages of the brain and are activated within minutes of onset of cerebral ischemia. Microglial proliferation peaks at 48 – 72 h after onset and may last for several weeks after cerebral ischemia [124]. They produce pro-inflammatory cytokines including IL-1β and TNF-α. IL-1β has been well shown to generate detrimental effect in cerebral ischemia [125,126], whereas TNF-α may increase tissue damage [127,128] or protect the brain against excitotoxic injury [129]. Activation of ROCK stimulates cytokines production by microglial cells [67], and thus, could regulate the inflammatory response post-ischemic stroke.
It is difficult, however, to distinguish reactive microglial cells from the blood-derived macrophages because they are similar in morphology and function [130]. Blood-derived macrophages infiltrate ischemic brain tissue at days 3 – 7 after stroke but rarely before 2 days after stroke [131-133]. Recent data support the hypothesis that the majority of macrophage-like cells in the ischemic brain tissue are activated resident microglial. ROCK stimulates blood-derived macrophage adhesion and migration during atherosclerosis. Bone marrow-derived ROCK1-deficient macrophages have decreased chemotaxis to MCP-1 and have a reduced ability to develop into cells [52]. Inhibition of ROCK by Y-27632 attenuates TNF-α-mediated monocyte migration and induction of MCP-1 via the p38MAPK pathway [134]. However, the role of ROCK in macrophage following stroke is still unclear.
Astrocytes are important in a wide variety of functions within the CNS, including homeostasis at the synapse, regulation of the BBB and cerebral blood flow, promotion of glial scar formation, regulation of neuronal signaling, determination of the fate of endogenous neural precursors and protection of neurons from oxidant toxicity [135]. Astrocyte process formation and astrocytic morphology can be altered by actin dynamics. ROCK inhibitors have been shown to produce rapid stellation of astrocytes and promote astroctye migration in vitro [136]. ROCK inhibitors also increase expression of excitatory amino acid transporters (EAAT 1/2) on the astrocyte cell surface, leading to elevated glutamate transport, thereby preventing cell death secondary to excitotoxicty [137].
5. Adverse effects of ROCK inhibitors
There is concern that treatment with ROCK inhibitor could potentially increase the risk of cerebral hemorrhage because ROCK inhibitors could also inhibit platelet function. However, clinical trials with ROCK inhibitors do not show increased incidence in bleeding or cerebral hemorrhage. Indeed, ROCK inhibitor has been used to treat vasospasms after hemorrhagic stroke [14]. Several other adverse effects have been reported such as hepatic toxicity and hypotension. Clinical trial of fasudil for treatment of subarachnoid hemorrhage and acute stroke did not report severe adverse effects. It should also be noted that ROCK inhibitors are teratogenic [138].
6. Expert opinion
Based on accumulating evidence, overactivation of inflammation appears to be detrimental for stroke prevention, evolution of stroke and stroke recovery. However, there are some reports that suggest that inflammation plays beneficial roles in isolating and repairing ischemic injury. Therefore, the timing and extent of inflammation during the stroke evolution need to be further elucidated. In addition, the degree and type of immune response may differ at different phases of stroke development. Consequently, immunomodulation as a therapy for stroke and stroke recovery needs to be further studied in terms of timing, type of immune response, microenvironment and intensity.
6.1 ROCK inhibitors as immunomodulator for stroke prevention
ROCK inhibitors may be beneficial in stroke prevention through anti-inflammatory effect. Abnormal ROCK activity contributes to stroke occurrence through numbers of different processes, including atherosclerosis, platelet activation, endothelial dysfunction and vascular dysfunction. Inflammation is involved in all the above processes. Much of the evidence of efficacy of ROCK inhibitors in stroke prevention is drawn from statins, which indirectly inhibits ROCK. Because statins could also improve endothelial function and vascular disease, it is unclear how much of the neuroprotective effects of statins are due to their anti-inflammatory properties. It is likely that the broad effects of statins contribute importantly to stroke prevention. However, the efficacy of the drugs might be improved by specifically targeting ROCKs in inflammatory cells instead of inhibiting ROCK activity in all tissues.
6.2 ROCK inhibitors as immunomodulator for acute stroke treatment
For acute stroke treatment, evidence shows that the effect of ROCK on endothelial function and inflammation might be the main contributor of neuroprotection by ROCK inhibi tors. In rodent stroke models, ROCK inhibitor improves endothelial function and cerebral blood flow via eNOS-dependent mechanisms [139,140]. In addition, ROCK inhibitor also reduced neutrophil infiltration into brain tissue during the acute ischemic phase [68,90,91]. A multi-center, double-blinded, placebo-controlled study in 160 patients showed that treatment with ROCK inhibitor fasudil within 48 h of acute ischemic stroke onset significantly improved neurological functions at 1 month after the onset of symptoms [13]. Larger clinical trials are currently ongoing in Japan. However, it is not clear which of the mechanisms is predominately beneficial.
6.3 ROCK inhibitors as immunomodulator for chronic stroke recovery
For chronic stroke recovery, the anti-inflammatory response such as the Th2-mediated response appears to be beneficial, whereas the Th1-mediated response appears to be detrimental. It is important to determine the mediators and mechanisms for T-cell lineage differentiation in order to develop effective drug therapies that could modulate the T-cell phenotype. Currently, the available ROCK inhibitors are non-specific inhibitors for ROCK1 and ROCK2 isoforms. There is some evidence that ROCK1 and ROCK2 isoforms might play different roles in inflammation and T-cell lineage differentiation. By taking advantage of ROCK1- and ROCK2-specific knockout mice and cell-specific knockout of ROCK1 and ROCK2, one could determine which ROCK isoforms and cells (endothelial, inflammatory cells, neuronal cells) are important mediators of stroke.
In summary, evidence strongly suggests that ROCKs are potential targets for ischemic stroke prevention and stroke treatment. The role of inflammation mediated by ROCKs in stroke pathogenesis and evolution starts to be recognized. It is important to fine-tune the immune response before or after stroke in order to harness the benefits while avoiding detrimental effects. A greater understanding of neuro-inflammation after cerebral ischemia will provide a novel pathway, which could be modulated to maximize the beneficial effects of immunomodulation after stroke.
Acknowledgments
Declaration of interest
The authors were supported by grants from the National Institutes of Health (HL052233 and NS070001) and the Rehabilitation Medicine Scientist Training Program.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10(5):471–80. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 3•.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290(3):C661–8. doi: 10.1152/ajpcell.00459.2005. [Excellent review on the structure and physiological role of ROCK in the cardiovascular system.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15(9):2208–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16(10):5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higashi M, Shimokawa H, Hattori T, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93(8):767–75. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 7.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273(37):24266–71. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 8.Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24(10):1842–7. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endres M, Laufs U, Huang Z, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95(15):8880–5. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50(1):17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masumoto A, Hirooka Y, Shimokawa H, et al. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38(6):1307–10. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto Y, Matoba T, Ito A, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91(3):391–2. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Shibuya M, Hirai S, Seto M, et al. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238(1-2):31–9. doi: 10.1016/j.jns.2005.06.003. [This Phase III clinical trial shows that treatment with fasudil within 48 h of acute stroke onset improves clinical outcome.] [DOI] [PubMed] [Google Scholar]
- 14.Shibuya M, Suzuki Y, Sugita K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76(4):571–7. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- 15.Masumoto A, Mohri M, Shimokawa H, et al. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105(13):1545–7. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa O, Fujisawa K, Ishizaki T, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392(2):189–93. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 17•.Shimizu Y, Thumkeo D, Keel J, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168(6):941–53. doi: 10.1083/jcb.200411179. [This paper describes the phenotype of the ROCK1 knockout mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Thumkeo D, Keel J, Ishizaki T, et al. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23(14):5043–55. doi: 10.1128/MCB.23.14.5043-5055.2003. [This paper describes the phenotype of the ROCK2 knockout mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Noma K, Rikitake Y, Oyama N, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118(5):1632–44. doi: 10.1172/JCI29226. [An important work showing that ROCK1 but not ROCK2 mediates leukocyte recruitment and neointima formation following vascular injury.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285(17):12536–42. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sodergren A, Stegmayr B, Ohman ML, Wallberg-Jonsson S. Increased incidence of stroke and impaired prognosis after stroke among patients with seropositive rheumatoid arthritis. Clin Exp Rheumatol. 2009;27(4):641–4. [PubMed] [Google Scholar]
- 22.Lindhardsen J, Ahlehoff O, Gislason GH, et al. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ. 2012;344:e1257. doi: 10.1136/bmj.e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Futrell N, Millikan C. Frequency, etiology, and prevention of stroke in patients with systemic lupus erythematosus. Stroke. 1989;20(5):583–91. doi: 10.1161/01.str.20.5.583. [DOI] [PubMed] [Google Scholar]
- 24.Bessant R, Hingorani A, Patel L, et al. Risk of coronary heart disease and stroke in a large British cohort of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2004;43(7):924–9. doi: 10.1093/rheumatology/keh213. [DOI] [PubMed] [Google Scholar]
- 25.Elkind MS. Infectious burden: a new risk factor and treatment target for atherosclerosis. Infect Disord Drug Targets. 2010;10(2):84–90. doi: 10.2174/187152610790963519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7(4):341–53. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 27••.Stoll G, Kleinschnitz C, Nieswandt B. Combating innate inflammation: a new paradigm for acute treatment of stroke? Ann NY Acad Sci. 2010;1207:149–54. doi: 10.1111/j.1749-6632.2010.05730.x. [This is an important review article that defines acute ischemic stroke as a ‘thrombo-inflammatory disorder’.] [DOI] [PubMed] [Google Scholar]
- 28.Nieswandt B, Kleinschnitz C, Stoll G. Ischaemic stroke: a thrombo-inflammatory disease? J Physiol. 2011;589(Pt 17):4115–23. doi: 10.1113/jphysiol.2011.212886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feske SK, Sorond FA, Henderson GV, et al. Increased leukocyte ROCK activity in patients after acute ischemic stroke. Brain Res. 2009;1257:89–93. doi: 10.1016/j.brainres.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amarenco P, Bogousslavsky J, Callahan A, III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 31.Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27(5):283–9. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Sacco RL, Liao JK. Drug insight: statins and stroke. Nat Clin Pract Cardiovasc Med. 2005;2(11):576–84. doi: 10.1038/ncpcardio0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360(18):1851–61. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 35.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295–322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 36.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91(11):2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 37.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008;112(9):3555–62. doi: 10.1182/blood-2008-04-144758. [DOI] [PubMed] [Google Scholar]
- 38.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94(5):657–66. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 39•.De Meyer SF, Stoll G, Wagner DD, Kleinschnitz C. von Willebrand factor: an emerging target in stroke therapy. Stroke. 2012;43(2):599–606. doi: 10.1161/STROKEAHA.111.628867. [This is an excellent review on vWF as a target in stroke therapy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folsom AR, Rosamond WD, Shahar E, et al. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100(7):736–42. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 41.Tzoulaki I, Murray GD, Lee AJ, et al. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115(16):2119–27. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 42.Kleinschnitz C, De Meyer SF, Schwarz T, et al. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. 2009;113(15):3600–3. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 43.Berndt MC, Shen Y, Dopheide SM, et al. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb Haemost. 2001;86(1):178–88. [PubMed] [Google Scholar]
- 44.Soriano SG, Coxon A, Wang YF, et al. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30(1):134–9. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 45.Yang SA, Carpenter CL, Abrams CS. Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4-phosphate 5-kinase trafficking in platelets. J Biol Chem. 2004;279(40):42331–6. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
- 46.Ono A, Westein E, Hsiao S, et al. Identification of a fibrin-independent platelet contractile mechanism regulating primary hemostasis and thrombus growth. Blood. 2008;112(1):90–9. doi: 10.1182/blood-2007-12-127001. [DOI] [PubMed] [Google Scholar]
- 47.Leng L, Kashiwagi H, Ren XD, Shattil SJ. RhoA and the function of platelet integrin alphaIIbbeta3. Blood. 1998;91(11):4206–15. [PubMed] [Google Scholar]
- 48.Shimada H, Rajagopalan LE. Rho-kinase mediates lysophosphatidic acid-induced IL-8 and MCP-1 production via p38 and JNK pathways in human endothelial cells. FEBS Lett. 2010;584(13):2827–32. doi: 10.1016/j.febslet.2010.04.064. [DOI] [PubMed] [Google Scholar]
- 49.Akiyama N, Naruse K, Kobayashi Y, et al. High glucose-induced upregulation of Rho/Rho-kinase via platelet-derived growth factor receptor-beta increases migration of aortic smooth muscle cells. J Mol Cell Cardiol. 2008;45(2):326–32. doi: 10.1016/j.yjmcc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Hemmings DG, Hudson NK, Halliday D, et al. Sphingosine-1-phosphate acts via rho-associated kinase and nitric oxide to regulate human placental vascular tone. Biol Reprod. 2006;74(1):88–94. doi: 10.1095/biolreprod.105.043034. [DOI] [PubMed] [Google Scholar]
- 51.Mallat Z, Gojova A, Sauzeau V, et al. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93(9):884–8. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 52•.Wang HW, Liu PY, Oyama N, et al. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR-/- mice. FASEB J. 2008;22(10):3561–70. doi: 10.1096/fj.08-108829. [This paper demonstrates that ROCK1 in bone marrow-derived macrophages plays important role in atherosclerosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rekhter M, Chandrasekhar K, Gifford-Moore D, et al. Immunohistochemical analysis of target proteins of Rho-kinase in a mouse model of accelerated atherosclerosis. Exp Clin Cardiol. 2007;12(4):169–74. [PMC free article] [PubMed] [Google Scholar]
- 54.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67(6):1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 55.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 56.Mayadas TN, Johnson RC, Rayburn H, et al. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74(3):541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 57.Frenette PS, Mayadas TN, Rayburn H, et al. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84(4):563–74. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 58.Gavard J, Gutkind JS. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J Biol Chem. 2008;283(44):29888–96. doi: 10.1074/jbc.M803880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun H, Breslin JW, Zhu J, et al. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13(3):237–47. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 60.Breyer J, Samarin J, Rehm M, et al. Inhibition of Rho kinases increases directional motility of microvascular endothelial cells. Biochem Pharmacol. 2012;83(5):616–26. doi: 10.1016/j.bcp.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Greenwood J, Walters CE, Pryce G, et al. Lovastatin inhibits brain endothelial cell Rho-mediated lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J. 2003;17(8):905–7. doi: 10.1096/fj.02-1014fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sauzeau V, Le Mellionnec E, Bertoglio J, et al. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ Res. 2001;88(11):1102–4. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- 63.Mack CP, Somlyo AV, Hautmann M, et al. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276(1):341–7. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 64.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 65.Noma K, Goto C, Nishioka K, et al. Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49(6):698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99(12):1426–32. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding J, Li QY, Wang X, et al. Fasudil protects hippocampal neurons against hypoxia-reoxygenation injury by suppressing microglial inflammatory responses in mice. J Neurochem. 2010;114(6):1619–29. doi: 10.1111/j.1471-4159.2010.06876.x. [DOI] [PubMed] [Google Scholar]
- 68•.Satoh S, Kobayashi T, Hitomi A, et al. Inhibition of neutrophil migration by a protein kinase inhibitor for the treatment of ischemic brain infarction. Jpn J Pharmacol. 1999;80(1):41–8. doi: 10.1254/jjp.80.41. [This paper demonstrates that inhibition of ROCK attenuates neutrophil infiltration to ischemic brain.] [DOI] [PubMed] [Google Scholar]
- 69.Magnus T, Wiendl H, Kleinschnitz C. Immune mechanisms of stroke. Curr Opin Neurol. 2012;25(3):334–40. doi: 10.1097/WCO.0b013e328352ede6. [DOI] [PubMed] [Google Scholar]
- 70.Weston RM, Jones NM, Jarrott B, Callaway JK. Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J Cereb Blood Flow Metab. 2007;27(1):100–14. doi: 10.1038/sj.jcbfm.9600324. [DOI] [PubMed] [Google Scholar]
- 71.Price CJ, Menon DK, Peters AM, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. 2004;35(7):1659–64. doi: 10.1161/01.STR.0000130592.71028.92. [DOI] [PubMed] [Google Scholar]
- 72.Buck BH, Liebeskind DS, Saver JL, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39(2):355–60. doi: 10.1161/STROKEAHA.107.490128. [DOI] [PubMed] [Google Scholar]
- 73.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2011;10(12):826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amantea D, Nappi G, Bernardi G, et al. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276(1):13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- 75.Caso JR, Pradillo JM, Hurtado O, et al. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115(12):1599–608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 76.Lehnardt S, Lehmann S, Kaul D, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190(1-2):28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 77.Zhang RL, Chopp M, Jiang N, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26(8):1438–42. doi: 10.1161/01.str.26.8.1438. discussion 43. [DOI] [PubMed] [Google Scholar]
- 78.Kitagawa K, Matsumoto M, Mabuchi T, et al. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18(12):1336–45. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Ishikawa M, Cooper D, Arumugam TV, et al. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004;24(8):907–15. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- 80.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–7. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Batchelor PE, Liberatore GT, Wong JY, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19(5):1708–16. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miwa T, Furukawa S, Nakajima K, et al. Lipopolysaccharide enhances synthesis of brain-derived neurotrophic factor in cultured rat microglia. J Neurosci Res. 1997;50(6):1023–9. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1023::AID-JNR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 83.Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(1):65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- 84•.Ekdahl CT, Claasen JH, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100(23):13632–7. doi: 10.1073/pnas.2234031100. [This paper shows that LPA-induced inflammation activates microglia and suppresses neurogenesis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85•.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. doi: 10.1126/science.1088417. [This paper indicates that chronic inflammation inhibits neurogenesis.] [DOI] [PubMed] [Google Scholar]
- 86.Liu Z, Fan Y, Won SJ, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38(1):146–52. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 87.Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci. 2005;29(3):381–93. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Kalluri HS, Vemuganti R, Dempsey RJ. Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. Eur J Neurosci. 2007;25(4):1041–8. doi: 10.1111/j.1460-9568.2007.05336.x. [DOI] [PubMed] [Google Scholar]
- 89.Otaegi G, Yusta-Boyo MJ, Vergano-Vera E, et al. Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci. 2006;119(Pt 13):2739–48. doi: 10.1242/jcs.03012. [DOI] [PubMed] [Google Scholar]
- 90.Satoh S, Toshima Y, Hitomi A, et al. Wide therapeutic time window for Rho-kinase inhibition therapy in ischemic brain damage in a rat cerebral thrombosis model. Brain Res. 2008;1193:102–8. doi: 10.1016/j.brainres.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 91.Satoh S, Utsunomiya T, Tsurui K, et al. Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci. 2001;69(12):1441–53. doi: 10.1016/s0024-3205(01)01229-2. [DOI] [PubMed] [Google Scholar]
- 92.Hara M, Takayasu M, Watanabe K, et al. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J Neurosurg. 2000;93(1 Suppl):94–101. doi: 10.3171/spi.2000.93.1.0094. [DOI] [PubMed] [Google Scholar]
- 93.Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2005;7(3):229–42. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- 94.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33(2):586–92. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 95.Hurn PD, Subramanian S, Parker SM, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27(11):1798–805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei Y, Yemisci M, Kim HH, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011 doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kipnis J, Yoles E, Porat Z, et al. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proc Natl Acad Sci USA. 2000;97(13):7446–51. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vila N, Castillo J, Davalos A, et al. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34(3):671–5. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 100.Prehn JH, Bindokas VP, Marcuccilli CJ, et al. Regulation of neuronal Bcl2 protein expression and calcium homeostasis by transforming growth factor type beta confers wide-ranging protection on rat hippocampal neurons. Proc Natl Acad Sci USA. 1994;91(26):12599–603. doi: 10.1073/pnas.91.26.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim ES, Kim RS, Ren RF, et al. Transforming growth factor-beta inhibits apoptosis induced by beta-amyloid peptide fragment 25-35 in cultured neuronal cells. Brain Res Mol Brain Res. 1998;62(2):122–30. doi: 10.1016/s0169-328x(98)00217-4. [DOI] [PubMed] [Google Scholar]
- 102.Theodorou GL, Marousi S, Ellul J, et al. T helper 1 (Th1)/Th2 cytokine expression shift of peripheral blood CD4+ and CD8+ T cells in patients at the post-acute phase of stroke. Clin Exp Immunol. 2008;152(3):456–63. doi: 10.1111/j.1365-2249.2008.03650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98(26):15137–42. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21(10):479–83. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 108.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 109.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 110.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 111.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453(7198):1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morishima N, Mizoguchi I, Takeda K, et al. TGF-beta is necessary for induction of IL-23R and Th17 differentiation by IL-6 and IL-23. Biochem Biophys Res Commun. 2009;386(1):105–10. doi: 10.1016/j.bbrc.2009.05.140. [DOI] [PubMed] [Google Scholar]
- 113.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 114.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 115.Caplan S, Zeliger S, Wang L, Baniyash M. Cell-surface-expressed T-cell antigen-receptor zeta chain is associated with the cytoskeleton. Proc Natl Acad Sci USA. 1995;92(11):4768–72. doi: 10.1073/pnas.92.11.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rozdzial MM, Pleiman CM, Cambier JC, Finkel TH. pp56Lck mediates TCR zeta-chain binding to the microfilament cytoskeleton. J Immunol. 1998;161(10):5491–9. [PubMed] [Google Scholar]
- 117•.Tharaux PL, Bukoski RC, Rocha PN, et al. Rho kinase promotes alloimmune responses by regulating the proliferation and structure of T cells. J Immunol. 2003;171(1):96–105. doi: 10.4049/jimmunol.171.1.96. [This paper demonstrates that Rho kinase plays important role in T-cell activation.] [DOI] [PubMed] [Google Scholar]
- 118.Teramoto H, Salem P, Robbins KC, et al. Tyrosine phosphorylation of the vav proto-oncogene product links FcepsilonRI to the Rac1-JNK pathway. J Biol Chem. 1997;272(16):10751–5. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- 119.Perona R, Montaner S, Saniger L, et al. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11(4):463–75. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 120.Corre I, Gomez M, Vielkind S, Cantrell DA. Analysis of thymocyte development reveals that the GTPase RhoA is a positive regulator of T cell receptor responses in vivo. J Exp Med. 2001;194(7):903–14. doi: 10.1084/jem.194.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schafer PH, Wadsworth SA, Wang L, Siekierka JJ. p38 alpha mitogen-activated protein kinase is activated by CD28-mediated signaling and is required for IL-4 production by human CD4+CD45RO+ T cells and Th2 effector cells. J Immunol. 1999;162(12):7110–19. [PubMed] [Google Scholar]
- 122.Koprak S, Staruch MJ, Dumont FJ. A specific inhibitor of the p38 mitogen activated protein kinase affects differentially the production of various cytokines by activated human T cells: dependence on CD28 signaling and preferential inhibition of IL-10 production. Cell Immunol. 1999;192(2):87–95. doi: 10.1006/cimm.1998.1448. [DOI] [PubMed] [Google Scholar]
- 123•.Biswas PS, Gupta S, Chang E, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120(9):3280–95. doi: 10.1172/JCI42856. [This study demonstrates that ROCK2 mediates Th17-cell differentiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–89. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16(5):932–40. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 126.Stroemer RP, Rothwell NJ. Cortical protection by localized striatal injection of IL-1ra following cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1997;17(6):597–604. doi: 10.1097/00004647-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 127.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 128.Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem. 2001;130(2):169–75. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- 129.Lambertsen KL, Clausen BH, Babcock AA, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29(5):1319–30. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanaka R, Komine-Kobayashi M, Mochizuki H, et al. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117(3):531–9. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 131.Schilling M, Strecker JK, Schabitz WR, et al. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience. 2009;161(3):806–12. doi: 10.1016/j.neuroscience.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 132.Petry KG, Boiziau C, Dousset V, Brochet B. Magnetic resonance imaging of human brain macrophage infiltration. Neurotherapeutics. 2007;4(3):434–42. doi: 10.1016/j.nurt.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kleinschnitz C, Bendszus M, Frank M, et al. In vivo monitoring of macrophage infiltration in experimental ischemic brain lesions by magnetic resonance imaging. J Cereb Blood Flow Metab. 2003;23(11):1356–61. doi: 10.1097/01.WCB.0000090505.76664.DB. [DOI] [PubMed] [Google Scholar]
- 134.Matoba K, Kawanami D, Ishizawa S, et al. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun. 2010;402(4):725–30. doi: 10.1016/j.bbrc.2010.10.093. [DOI] [PubMed] [Google Scholar]
- 135.Blackburn D, Sargsyan S, Monk PN, Shaw PJ. Astrocyte function and role in motor neuron disease: a future therapeutic target? Glia. 2009;57(12):1251–64. doi: 10.1002/glia.20848. [DOI] [PubMed] [Google Scholar]
- 136.Lau CL, Perreau VM, Chen MJ, et al. Transcriptomic profiling of astrocytes treated with the Rho kinase inhibitor fasudil reveals cytoskeletal and pro-survival responses. J Cell Physiol. 2012;227(3):1199–211. doi: 10.1002/jcp.22838. [DOI] [PubMed] [Google Scholar]
- 137.Lau CL, O'Shea RD, Broberg BV, et al. The Rho kinase inhibitor Fasudil up-regulates astrocytic glutamate transport subsequent to actin remodelling in murine cultured astrocytes. Br J Pharmacol. 2011;163(3):533–45. doi: 10.1111/j.1476-5381.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wei L, Roberts W, Wang L, et al. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128(15):2953–62. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- 139.Shin HK, Salomone S, Potts EM, et al. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab. 2007;27(5):998–1009. doi: 10.1038/sj.jcbfm.9600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36(10):2251–7. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]