Abstract
Acute mesenteric ischemia, a frequently lethal disease, requires prompt diagnosis and intervention for favorable clinical outcomes. This goal remains elusive due, in part, to lack of a noninvasive and accurate imaging study. Traditional angiography is the diagnostic gold standard but is invasive and costly. Computed tomography (CT) is readily available and noninvasive but has shown variable success in diagnosing this disease. The faster scanning time of multidetector row CT (M.D.CT) greatly facilitates the use of CT angiography (CTA) in the clinical setting. We sought to determine whether M.D.CT-CTA could accurately demonstrate vascular anatomy and capture the earliest stages of mesenteric ischemia in a porcine model. Pigs underwent embolization of branches of the superior mesenteric artery, then imaging by M.D.CT-CTA with three-dimensional reconstruction protocols. After scanning, diseased bowel segments were surgically resected and pathologically examined. Multidetector row CT and CT angiography reliably defined normal and occluded mesenteric vessels in the pig. It detected early changes of ischemia including poor arterial enhancement and venous dilatation, which were seen in all ischemic animals. The radiographic findings—compared with pathologic diagnoses—predicted ischemia, with a positive predictive value of 92%. These results indicate that M.D.CT-CTA holds great promise for the early detection necessary for successful treatment of acute mesenteric ischemia.
Keywords: Mesenteric ischemia, computed tomography, angiography, animal model
Acute mesenteric ischemia, the process of insufficient blood supply to the bowel, carries consequences ranging from a transient, totally reversible insult to a lethal event. The prevalence of mesenteric ischemia is increasing in the United States as the population ages. It is estimated that nearly 1% of patients presenting with acute abdominal pain have ischemic intestinal disease,1–3 and it may be responsible for 0.1% of all hospital admissions.1 The lethality of the disease is well documented, with some series reporting mortality rates exceeding 60%.4–7
A critical factor for survival of acute mesenteric ischemia is early diagnosis and intervention. In cases of superior mesenteric artery (SMA) embolism in which surgery is performed once the bowel is infarcted, the mortality rate nearly doubles from 35% to 68%.8 The mean duration of ischemia preceding surgery for noninfarcted bowel is 13 hours, compared with 21 hours for those with infarction.9 Thus, the window of opportunity for intervention is narrow and prompt diagnosis is crucial for patient survival. Nevertheless, this goal remains elusive, due in part to the lack of an accurate diagnostic imaging tool.
Although angiography is considered the standard of reference for the diagnosis of mesenteric ischemia, it is not widely used because it is invasive, time-consuming, and costly. Computed tomography has been historically used with variable success for evaluating small bowel ischemia. It can help detect ischemic changes in the affected small bowel loops and mesentery. Changes that have been reported include bowel wall thickening and edema, submucosal hemorrhage, increased or decreased enhancement of the bowel wall, mesenteric stranding or fluid, and pneumatosis intestinalis.10–13 However, these morphologic descriptions are not adequate for the early detection of reversible bowel ischemia. A recent study reported that spiral CT demonstrated a sensitivity and specificity of 64% and 92%, respectively, for diagnosing mesenteric ischemia.14
Multidetector CT, combining multiple rows of detectors and faster gantry rotation with narrow collimation, increases scanning speed and virtually eliminates motion and respiratory artifact. These factors allow for improved visualization of the small-bowel wall and distal branches of the mesenteric vessels. Faster scanning also allows for more accurate timing of intravenous contrast bolus administration so that the data can be obtained during both the arterial and venous phases. The resulting improved quality of the three-dimensional reformatted images has allowed for significant advances in CT angiography in the cerebral vessels,15 pulmonary arteries,16 and hepatic,17 renal,18 and pancreatic vessels19 of the abdomen. Application of this method to the mesenteric vessels and evaluation of small bowel ischemia has also been reported.20 Although these studies elegantly demonstrate how CT angiography can image acute mesenteric ischemia, its utility as an accurate diagnostic tool must still be demonstrated.
Here we describe the use of multidetector CT angiography (M.D.CT-CTA) in a porcine model of mesenteric ischemia. Branches of the SMA were selectively identified under fluoroscopic guidance. SMA branch occlusion was performed by injection of microspheres and gel foam and confirmed by angiogram. Standard laboratory analyses were sent preischemic insult and prior to surgical excision. Pigs were imaged using 64-slice helical M.D.CT at 1, 3, or 6 hours postembolization to assess qualitative and quantitative markers of ischemia. These radiologic findings were compared to the findings at surgical exploration and to the gross and microscopic pathological diagnoses.
We report that: (1) M.D.CT-CTA was able to accurately define the porcine arterial and venous mesenteric anatomy. (2) M.D.CT-CTA with three-dimensional reconstruction protocols was able to accurately identify and define the occluded arteries when compared to standard angiography. (3) M.D.CT-CTA was able to detect the early changes of mesenteric ischemia, including poor arterial enhancement and venous dilatation. These two findings were seen in all ischemic animals. (4) The radiographic findings, when compared to microscopic pathologic diagnoses, were used to predict ischemia with a positive predictive value of 92% and a negative predictive value of 80%.
MATERIALS AND METHODS
Animal Preparation, Embolization, and Angiography
Approval was obtained from our institution's subcommittee on research animal care, and animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals.21 Each pig, weighing between 35 and 40 kg, was placed under general anesthesia with inhaled isoflurane. An endotracheal tube was placed and intravenous access was obtained in an auricular vein.
Angiography of one of the branches of the mesenteric artery was performed using a modified Seldinger technique. A mesenteric arteriogram was obtained by injecting 20 ml of nonionic iodinated contrast material (Ultravist 300, Berlex Laboratories, Montville, NJ). The vessel was embolized using 700–900 micron polyvinyl alcohol particles and gel foam (Contour SE Microspheres, Boston Scientific, Natick, MA) until stasis was achieved in the branch vessel. The catheter was then pulled back to the ostium of the mesenteric artery and an arteriogram was obtained to confirm vessel occlusion.
CT Imaging
Following predetermined time points of 1, 3, or 6 hours after embolization, each pig underwent a dual-phase scanning of the upper abdomen, in the craniocaudal direction, using a 64-slice M.D.CT scanner (SOMATOM Sensation 64, Siemens Medical Systems, Erlangen, Germany). An 18–20-gauge cannula was placed in an auricular vein, and a noncontrast-enhanced CT of the abdomen was performed from the dome of the diaphragm to the level of pubic symphysis, using a detector collimation of 0.6 mm, slice thickness of 3 mm, and a pitch of 0.75. For initiating the arterial phase scanning, the time to peak aortic enhancement was first estimated by a minitest bolus of 15 ml of iodinated contrast injected at 4 ml/second. Finally, about 2ml/kg of 300 mg I/ml of iodinated contrast (Isovue 300, Bracco Diagnostics, Princeton, NJ) was power-injected at a rate of 4 ml/second, followed by 30 ml of saline flush, also injected at 4 ml/second, and arterial phase scanning was performed. Venous phase scanning followed after a delay of 50 seconds. No oral contrast was administered at any time. Four additional, nonembolized pigs underwent the exact same scanning protocol as the control subjects.
For the arterial and venous phase imaging, a detector collimation of 0.6 mm, slice thickness of 1 mm, pitch of 0.75, and tube rotation of 0.5 second were selected. Other parameters were kept constant for each phase of scanning, such as kVp of 120 and effective mAs of 200–240. Source images were reconstructed at 50% overlap. The reconstructed images were processed to create two-dimensional and three-dimensional maps for the mesenteric arterial and venous system using multiplanar reformation (MPR) and maximum intensity projection (MIP) techniques.
Surgery and Pathological Analysis
After the final CT scan, pigs underwent surgical exploration. During laparotomy, the location and severity of bowel ischemia was determined by bowel wall color, the degree of dilation present, inflamma-tory changes, and the degree of vascular cyanosis present. The diseased bowel segment was then photographed and resected prior to euthanasia. The bowel segments were fixed in formalin and subsequently analyzed for both macroscopic and microscopic pathological changes in three regions: proximal jejunum; the segment comprising distal jejunum and proximal ileum; and distal ileum. A pathologist used a 5-point scale (1 = normal; 2 = mucosal congestion; 3 = loss of superficial glandular architecture; 4 = loss of all glandular architecture, including deep crypts, with necrosis of the muscularis mucosae; 5 = transmural necrosis, including muscularis propria) to rank the level of ischemia in each segment; when heterogeneity was present, the most ischemic value was recorded.
CT Data Analysis
All images were transferred to a postprocessing workstation (Advantage Windows; GE Healthcare Information Technologies, Milwaukee, WI) where they were analyzed in a consensus mode by two radiologists who remained blinded to the type of embolization procedure used on each animal. A subjective assessment was performed focusing on the presence of the following signs: reduced small-bowel wall enhancement, mural thickening, mesenteric stranding or fluid, congestion of small mesenteric veins, ascites, and pneumatosis intestinalis.
For the quantitative assessment of small-bowel perfusion, enhancement measurements were performed in multiple small (mean diameter, 1.5 mm) elliptical regions of interest in the small-bowel wall within three separate, predefined regions: the proximal jejunum, the segment comprising the distal jejunum and proximal ileum, and the distal ileum. Based on the measurements taken for these three areas of small-bowel wall, the mean enhancement was calculated for each data set in Hounsfield units (HU). A bowel segment was graded as one of the following: normal (25–45 HU), ischemic insult (<25 HU), or ischemic insult with bowel hyperemia (>45 HU).
The regions of presumed bowel injury recorded by the radiologists and those resulting from the pathological analysis were compared, and radiologic predictions were rated as true-positive, false-positive, false-negative, or true-negative for statistical purposes.
RESULTS
M.D.CT-CTA Identifies Normal Porcine Arterial and Venous Anatomy
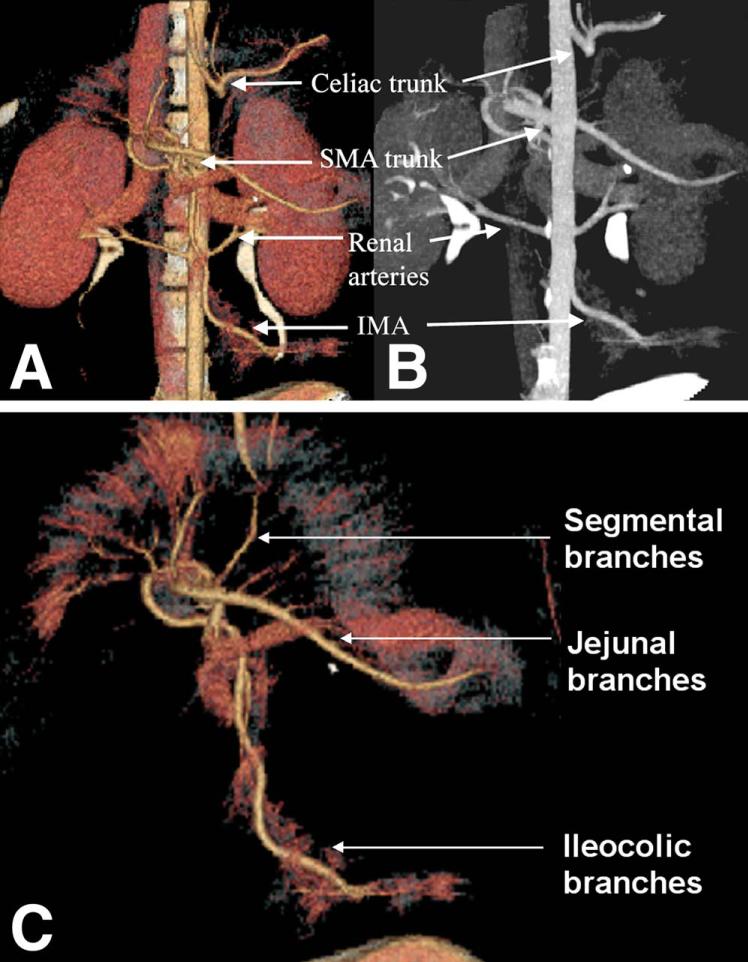
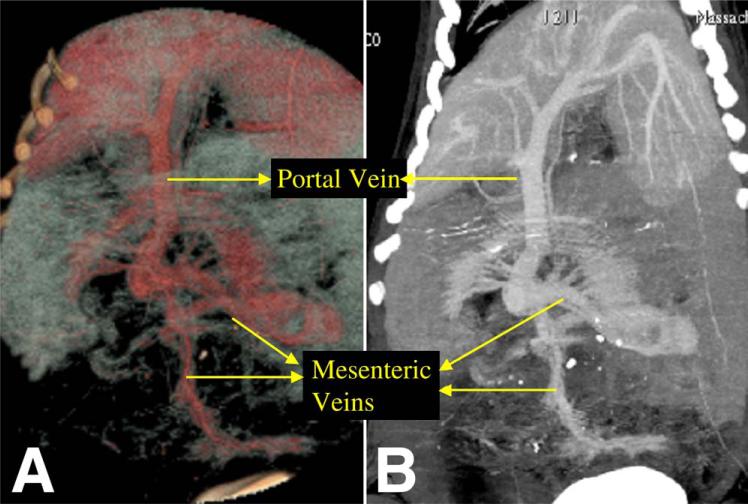
In four control pigs that were tested, M.D.CT-CTA was able to demonstrate normal mesenteric vascular anatomy—both arterial supply (Fig. 1) and venous drainage (Fig. 2) of the bowel were readily identified. MPR two-dimensional reconstruction and MIP three-dimensional reconstruction both reliably identified normal abdominal vascular anatomy. The major branches of the aorta, including the celiac trunk, the SMA, the IMA, and the renal arteries, were all visualized using these reconstruction techniques. Using MIP reconstruction, all major named branches of the SMA, including the jejunal and ileocolic branches, could easily be identified. Furthermore, second-order segmental branches could also be identified readily (Fig. 1, C). Normal portal and mesenteric venous anatomy could also be clearly delineated using the MIP and MPR reconstruction techniques; in this manner, the superior mesenteric, inferior mesenteric, and segmental drainage were easily identified (Fig. 2).
Fig. 1.
Normal arterial anatomy in control animal, as visualized by M.D.CT-CTA. Major aortic branches can be clearly seen. (A) MIP three-dimensional reconstruction with volume rendered technique. (B) MPR two-dimensional reconstruction. (C) MIP three-dimensional reconstruction with volume rendered technique reconstruction of SMA anatomy.
Fig. 2.
Normal portal and mesenteric venous anatomy as visualized in control pig by M.D.CT-CTA with (A) three-dimensional volume rendered technique and (B) MPR two-dimensional reconstructions.
Perfusion of healthy bowel was determined by the degree of enhanced signal following contrast injection. Pre-IV contrast values were determined from multiple points in the three bowel regions of interest—the proximal jejunum, the distal ileum, and the segment comprising distal jejunum and proximal ileum. The average signal of nonenhanced bowel was found to be 40 HU (range, 36–44 HU) in all areas of the small bowel, with no variation detected between any of the three regions of interest (Fig. 3, A).
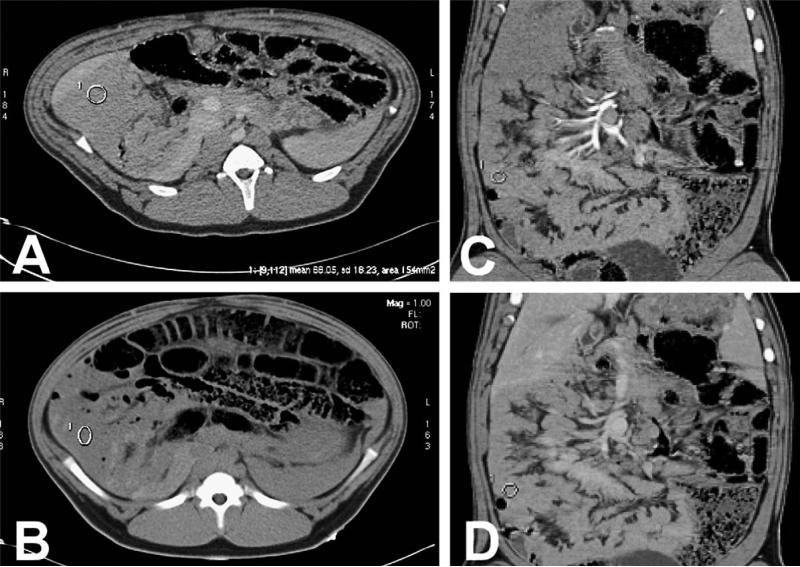
Fig. 3.
Axial and coronal CT images of control pigs demonstrating baseline enhancement of the small bowel. (A) Postcontrast axial CT image shows jejunal enhancement of 67 HU following iodinated contrast administration. (B) Precontrast axial image shows enhancement in the jejunum loops of 41.9 HU. (C) Arterial phase coronal reformatted image shows 67 HU enhancement in the small bowel. (D) Venous phase coronal reformatted image shows 70 HU enhancement in the small bowel.
The maximum bowel wall enhancement was identified at 60 seconds following injection of intravenous iodinated contrast material (Fig. 3, B). At this time point, control animals showed an average contrast enhancement of 70 HU (range, 65–85 HU). Thus, healthy, perfused bowel yielded a mean change in enhancement of 30 HU (range, 25–45). This was consistently found throughout all bowel segments and in all control pigs. These values of healthy, perfused bowel were later used to set objective benchmarks for radiologically diagnosed ischemia.
M.D.CT-CTA Reliably Defines and Identifies Occluded Arterial Anatomy
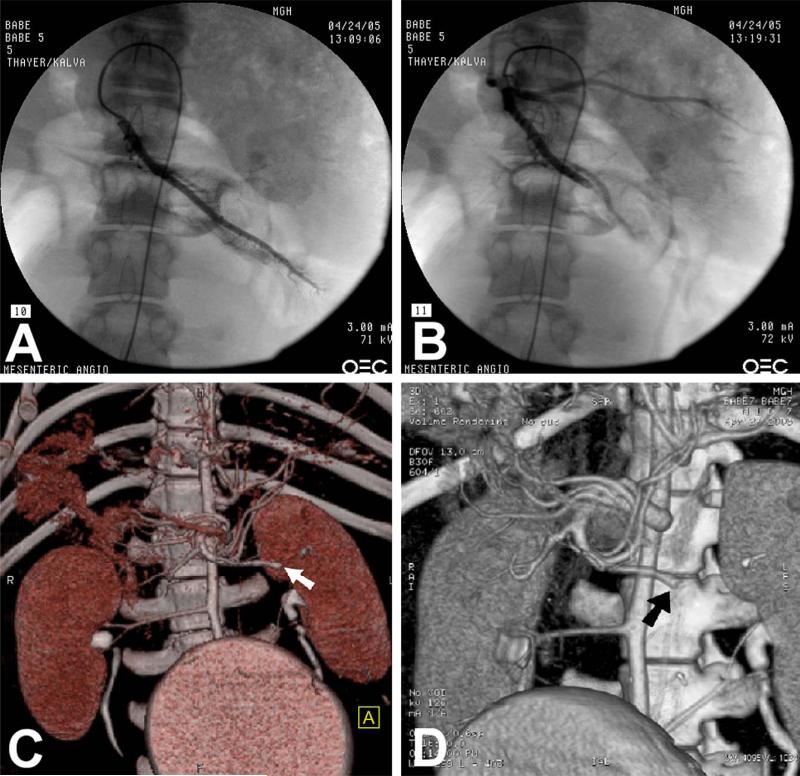
Six pigs underwent catheter-assisted embolization of SMA branches and were assessed by M.D.CT-CTA to determine if the location of arterial occlusion correlated with standard angiography. In each pig, the branch vessel was embolized, and then the same catheter was used to perform an arteriogram and to confirm vessel occlusion (Fig. 4, A, B). The vascular anatomy was then studied at designated time points—1, 3, and 6 hours—by M.D.CT-CTA with MIP reconstruction. In all cases, this technique reliably identified the same occluded vessel visualized with traditional angiography (Fig. 4, C, D).
Fig. 4.
Accurate identification and detection of occluded arterial anatomy by M.D.CT-CTA. (A) Fluoroscopic angiography demonstrates normal filling pattern of jejunal branch of superior mesenteric artery. (B) Angiography postembolization reveals occluded jejunal branch. (C) M.D.CT-CTA using volume rendered technique reconstruction demonstrates the occlusion of the jejunal branch of the SMA (white arrow). (D) M.D.CT-CTA using MIP three-dimensional reconstruction demonstrates the occlusion of the jejunal branch of SMA (black arrow).
Catheter-Assisted Arterial Embolization Induces Mesenteric Ischemia
During laparotomy, the location and severity of bowel ischemia was determined by bowel wall color, the degree of dilation present, inflammatory changes, and the degree of vascular cyanosis present. Ischemic segments were photographed (Fig. 5) and saved for gross and microscopic pathologic analysis. For each pig, the bowel was divided into three anatomic regions—proximal jejunum; the segment comprising distal jejunum and proximal ileum; and distal ileum—and the grade of ischemia was determined for these regions. A pathologist (M.M.-K.) was asked to histologically evaluate the three regions of interest and grade the injury on a 5-point scale, ranging from normal to transmural necrosis. All occlusion time points resulted in ischemic damage to the small intestine (Fig. 6; Table 1).
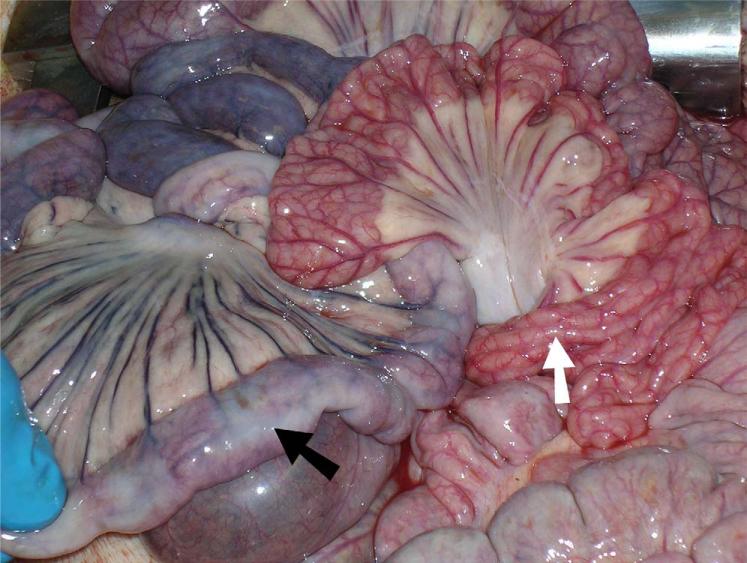
Fig. 5.
Six-hour ischemic bowel segment as visualized at laparotomy. Ischemic bowel (black arrow) demonstrates cyanosis, devascularization, and darkening of the mesenteric vessels. Surrounding healthy bowel (white arrow) is, by contrast, well-vascularized and pink.
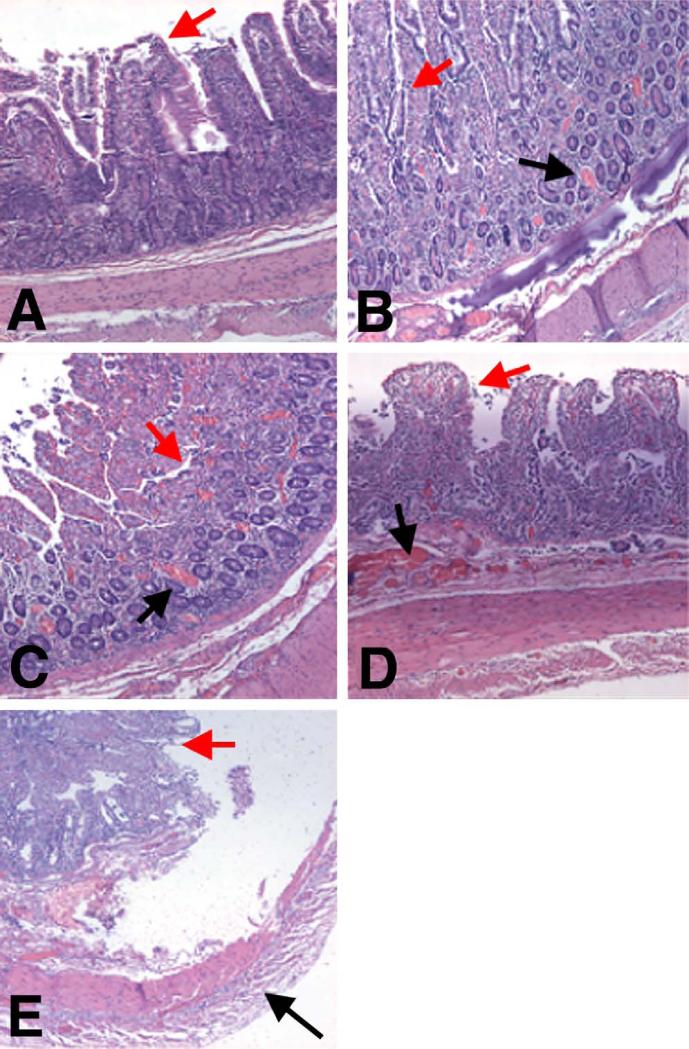
Fig. 6.
Histopathology of ischemic bowel, with five representative ischemic grades. Villous structures represented by red arrows. (A) Grade 1. Normal. No vascular congestion is present, and both the villous architecture and muscular layer are preserved. (B) Grade 2. Villous architecture is preserved, with some mucosal congestion and dilated capillaries (black arrow). (C) Grade 3. Mucosa is congested (black arrow) with loss of superficial glandular architecture, but deep villous architecture is preserved. (D) Grade 4. The mucosa is completely involved, with loss of all superficial and deep glandular architecture, but the muscular layer is preserved. (E) Grade 5. There is total loss of glandular architecture, and the muscularis propria shows degeneration, fragmentation, and myocyte death, all indicative of transmural infarction (black arrow).
Table 1.
Pathological evaluation of bowel segments at time of laparotomy
| Duration of ischemia | No. Pig | Proximal jejunum | Distal jejunum/proximal ileum | Distal ileum | Overall level of ischemia |
|---|---|---|---|---|---|
| 1 h | 2 | 1 | 2 | 2 | 2 |
| 1 h | 6 | 1 | 3 | 3 | 3 |
| 3 h | 1 | 1 | 3 | 3 | 3 |
| 3 h | 5 | 1 | 2 | 4 | 4 |
| 6 h | 3 | 2 | 3 | 5 | 5 |
| 6 h | 4 | 1 | 4 | 4 | 4 |
A 5-point scale of ischemia was used (1 = normal; 2 = mucosal congestion; 3 = loss of superficial glandular architecture; 4 = loss of all glandular architecture, including deep crypts, with necrosis of the muscularis mucosae; 5 = transmural necrosis, including muscularis propria). When heterogeneity was present, the most severe ischemic value was recorded. Ischemic values emphasized in bold face.
The overall level of bowel ischemia in each pig was based on the ischemic grade of the most severely affected segment. Based on this criterion, the overall degree of ischemic insult roughly correlated with the duration of arterial occlusion, as shown in Table 1. Occlusions of 1 hour resulted in bowel injuries ranging from mucosal congestion (Grade 2) to superficial loss of glandular architecture (Grade 3). Occlusions of 3 hours resulted in injuries ranging from Grade 3 to total loss of glandular architecture in the mucosa (Grade 4). Finally, occlusions of 6 hours resulted in injuries ranging from Grade 4 to transmural necrosis with myocyte degeneration and total loss of glandular architecture in the mucosa (Grade 5).
Early Changes in Mesenteric Ischemia are Detectable by M.D.CT-CTA
After embolization of either the jejunal or the ileocolic branches of the superior mesenteric artery, each pig was assigned to remain ischemic for a specific duration of time prior to scanning. Two pigs were selected for each time point: 1 hour, 3 hours, or 6 hours. Following the prespecified ischemic period, each animal underwent analysis by precontrast and postcontrast M.D.CT-CTA.
Initially, a qualitative radiologic assessment was performed, looking for the standard indicators of mesenteric ischemia. Blinded radiologists were asked to comment on the accepted assessments for bowel ischemia: the state of the mesenteric vasculature, bowel wall thickening and edema, submucosal hemorrhage, increased or decreased enhancement of the bowel wall, mesenteric stranding or fluid, ascites, and pneumatosis intestinalis. In all six ischemic animals, even at the latest time points, bowel wall thickening, stranding, and pneumatosis were not identified. Ascites was only detected in one animal. However, all ischemic animals, including those imaged only 1 hour after embolization, showed a significant decrease in arterial phase enhancement following contrast administration (Fig. 7), as well as marked venous dilatation (Fig. 8). Thus, these two signs appear to be early and sensitive indicators of bowel wall ischemia. Because decreased arterial enhancement was a sensitive indicator of perfusion in all six ischemic animals, and because it can be easily identified by physicians with a broad spectrum of clinical training, we chose this sign for quantitative characterization.
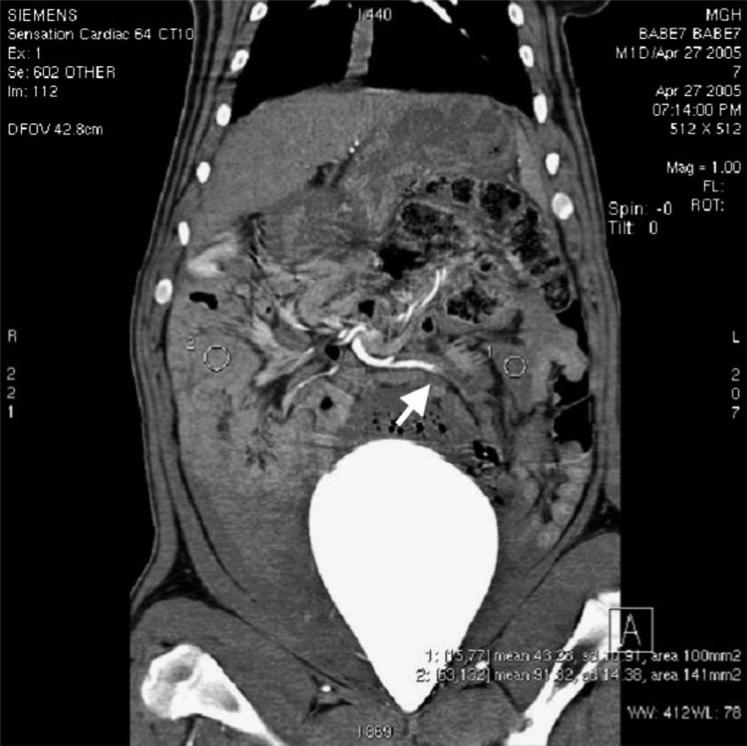
Fig. 7.
Coronal reformatted contrast-enhanced CT image of pig abdomen, 6 hours postembolization, demonstrates poor enhancement of distal jejunum at 43 HU (1) as compared to 91 HU in the distal ileum (2). Occlusion of jejunal branch (white arrow) of SMA supplying the nonviable bowel is visualized.
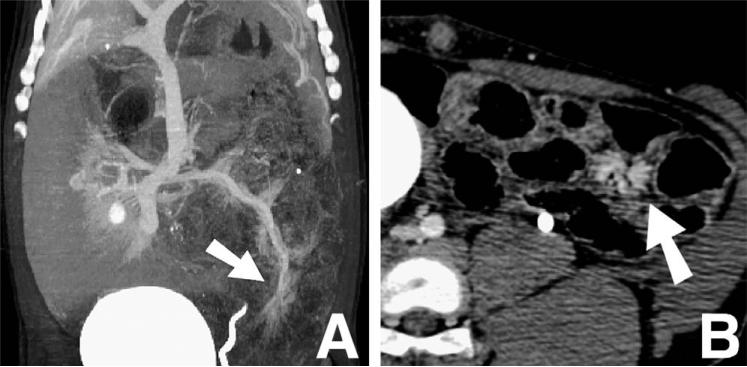
Fig. 8.
Venous dilatation (white arrows) noted in coronal (A) and axial (B) images by M.D.CT-CTA (postcontrast injection) at the site of arterial occlusion, indicating ischemia.
We therefore considered the measured enhancement values before and after the administration of intravenous contrast as indicators of poor arterial flow to the small bowel. Using the range of normal bowel wall enhancement obtained from the study of control animals, predetermined cutoff points were set to define the level of enhancement indicative of ischemia. A bowel segment demonstrating 25–45 HU change in small-bowel attenuation following contrast injection was considered to be normal. On the other hand, a change of less than 25 HU in attenuation was considered consistent with poor perfusion and ischemia. At the same time, a change in attenuation greater than 45 HU was considered consistent with an ischemic insult causing vascular congestion and hyperemia.
Every embolized pig was found to be ischemic as defined by these criteria, while control pigs were not (data not shown). Areas of diminished enhancement in the arterial phase were readily identified in the supplied segments of occluded arteries (Fig. 7), and these findings were validated by the gross pathological findings at laparotomy (Fig. 6). These radiographic findings were present as early as 1 hour following embolization, before there was a measurable change in serum ischemic markers. They were also seen at the later time points, when markers such as potassium, alkaline phosphatase, and creatine kinase were elevated (data not shown).
M.D.CT-CTA Reliably Predicts the Presence of Mesenteric Ischemia
We finally sought to quantify how reliably the measured enhancement levels predicted mesenteric ischemia in the 18 bowel segments studied (3 segments/pig × 6 pigs). The radiologic prediction for each bowel segment was compared to the corresponding gold standard pathological diagnosis, and each prediction was rated as true-positive, false-positive, false-negative, or true-negative for statistical purposes.
The histopathologic findings are summarized in Table 1, the radiologic findings in Table 2, and Table 3 shows a comparison of the two data sets. Briefly, these data show that M.D.CT angiography correctly predicted ischemia in 12 of 13 bowel segments with pathologically defined ischemia (PPV = 92%). The one false-negative was seen in a 6-hour pig that developed low-level ischemia (Grade 2) in the proximal jejunum and was radiologically classified as normal (Grade 1). Likewise, 12 of 13 segments that were predicted to be ischemic by M.D.CT-CTA were ultimately found to demonstrate pathologic signs of ischemia (sensitivity = 92%). The one false-positive was seen in a 3-hour pig that was found on pathologic examination to have normal proximal jejunum, but it was radiologically predicted to have early ischemia and hyperemia based on >45 HU enhancement. The negative predictive value and specificity were both calculated as 80% (Table 3).
Table 2.
Qualitative and quantitative assessment of ischemic bowel segments
| Duration of ischemia | No. Pig | Proximal jejunum | Distal jejunum/proximal ileum | Distal ileum | Qualitative assessment by radiologist |
|---|---|---|---|---|---|
| 1 h | 2 | 35 | 22 | 6 | Distal jejunum and proximal ileum are the worst affected. Venous dilatation is clearly present in the distal jejunum and proximal ileum. |
| 1 h | 6 | 29 | 15 | 24 | Proximal jejunum appears normal. Some areas of ileum appear ischemic. |
| 3 h | 1 | 73 | 62 | 4.1 | Very significant injury, involving a long segment of distal jejunum, entire ileum, and cecum. Only a small segment of proximal jejunum looks viable. The rest of the bowel appears nonviable. Venous dilatation primarily in jejunum. |
| 3 h | 5 | 41 | 20 | 0.4 | Distal jejunum looks ischemic, proximal ileum looks ischemic, but not to the point of nonviability. Some venous dilatation present in the proximal jejunum. |
| 6 h | 3 | 31 | 4.6 | 0.5 | A long segment of involvement, from the distal jejunum through distal ileum. Very poor enhancement in some areas, likely ischemic/infarcted with nonviability. |
| 6 h | 4 | 40 | 12 | 7 | Ileum is most significantly affected. Hyperemia and venous dilatation exist in some areas of jejunum. |
Average degree of arterial phase enhancement change in bowel segments over pre contrast imaging by M.D.CT-CTA, listed in Hounsfield units. Boldface signifies segments deemed to be ischemic based on degree of arterial enhancement (>45 HU or <25 HU).
Table 3.
Radiographic findings compared with pathologic diagnoses
| Ischemia (+) | Ischemia (–) | Total | |
|---|---|---|---|
| Scan (+) | 12 | 1 | 13 |
| Scan (–) | 1 | 4 | 5 |
| Total | 13 | 5 | 18 |
DISCUSSION
Acute mesenteric ischemia is an often devastating disease that is currently the leading cause of the acute abdomen in medical intensive care unit patients22 and in hemodialysis patients.23 As the incidences of cardiac and thromboembolic disease increase worldwide, we may expect the same for mesenteric ischemia. Despite the importance of early detection in preventing the high mortality associated with bowel infarction, it continues to pose a diagnostic challenge to clinicians. Angiography remains the diagnostic gold standard, but its implementation is costly and it is not available in all clinical settings. While traditional CT imaging has improved the diagnosis of many abdominal diseases, it has been shown to have a sensitivity of only 64% for the diagnosis of mesenteric ischemia.14 Because of the ability of M.D.CT-CTA to visually represent arterial anatomy and perfusion, it may represent a great improvement over single-detector CT in imaging this disease.
We therefore aimed to study the M.D.CT imaging of acute mesenteric ischemia of the small bowel in a porcine model. First, we demonstrated M.D.CT-CTA readily identifies both normal and occluded vascular anatomy, and the two radiologic signs that appeared to correlate best with the presence of ischemia were venous dilatation and abnormal postcontrast arterial enhancement. These findings were present before the onset of serum chemistry abnormalities that are often characteristic of bowel ischemia.
Using the predetermined criteria of hypo-enhancement (<25 HU) and hyper-enhancement (>45 HU) yields both a sensitivity and positive predictive value of 92%, suggesting that these may ultimately be useful parameters for clinical practice. Whereas areas of severe, irreversible ischemia should logically demonstrate significantly lower enhancement than areas of reversible, less severe ischemia, we were unable to statistically distinguish ischemic grades 3, 4, and 5 based on radiology alone. Bowel that took on a hyperemic character, that is, a change in signal intensity of >45 HU, was not identified in any late ischemic lesions (Grades 3–5). Thus, it appears that M.D.CT-CTA is a highly sensitive indicator of bowel perfusion and ischemia, and it can be used to detect mesenteric ischemia at its earliest stages as well as throughout its progression from early hypo-perfusion to severe, nonviable bowel ischemia. However, our results do not support the use of M.D.CT-CTA to definitively exclude the diagnosis of mesenteric ischemia in the case of a negative radiographic finding, as the negative predictive value was found to be only 80%.
The small number of animals studied, and therefore the small number of bowel segments examined, limited the overall statistical significance. Another difficulty was the ability to distinguish various segments of the pig bowel radiographically. While porcine intestinal anatomy closely resembles that of humans, it does differ radically in a few ways; namely, the jejunum and ileum are often transposed, and ileal loops can be difficult to identify on CT. As a result, differentiating much of the pig jejunum and ileum is often not possible; we thus limited our study to only those segments that could reliably be distinguished from one another: the proximal jejunum, the distal ileum, and the intervening segment of distal jejunum and proximal ileum. Future studies may better elucidate the anatomic definition of ischemic segments.
Despite these weaknesses, M.D.CT-CTA appears to represent a great improvement over single-detector CT scanning in its ability to diagnose acute mesenteric ischemia. One of the drawbacks of traditional imaging modalities is that they only identify the end-organ damage resulting from low-perfusion states, such as pneumatosis, mural wall thickening, mesenteric stranding or fluid, and ascites. In contrast, M.D.CT-CTA appears to detect the initial perfusion defects, as opposed to end-organ tissue damage, that are characteristic of this process. It thus may represent a sensitive, early indicator of mesenteric ischemia, which is critical in the prevention of irreversible tissue changes and in improving surgical and patient outcomes.
M.D.CT-CTA also has several advantages over traditional angiography. First, it requires less time to mobilize all members of the care team, and acquire data by M.D.CT-CTA, than angiography requires. Second, this technique may be more cost-effective; it requires fewer personnel and less technical expertise, and the M.D.CT equipment may be used for other CT-based imaging studies. Third, because of its lower degree of invasiveness and associated morbidity, it may be performed with a lower index of clinical suspicion, and it thus may represent an ideal screening modality.
This imaging technique may have broad applications in any given clinical situation. Because diminished perfusion is characteristic of all forms of acute mesenteric ischemia, M.D.CT-CTA may be useful in detecting not only the embolic ischemia demonstrated here, but also ischemia from other etiologies such as venous thrombosis, low-flow states (nonocclusive mesenteric ischemia), or acute-on-chronic SMA ischemia. Although there are many facets of M.D.CT-CTA analysis, such as the MIP and MPR reconstructions that can readily identify compromised vascular anatomy, this diagnostic tool is not limited to complex and time-consuming image processing algorithms. Rather, the simple quantification of decreased bowel enhancement has a 92% positive predictive value in this disease, it is easily attainable in most emergency rooms, and it can be easily identified by or communicated to a surgeon.
CONCLUSION
In summary, we have shown that M.D.CT-CTA can be reliably used to diagnose mesenteric ischemia in a porcine model. Venous dilatation and abnormal arterial enhancement were found to be consistent signs of ischemia, as early as 1 hour following arterial occlusion. Based on our quantitative data, arterial phase enhancement of less than 25 HU was generally associated with severe ischemia, and enhancement of greater than 45 HU, which is believed to be a sign of vascular congestion, was seen in less severe ischemia. These parameters taken together predict ischemia with 92% sensitivity and positive predictive value. Any results extrapolated from this animal study must first be validated in clinical trials, but the relatively high sensitivity and positive predictive value of these tests suggest an encouraging avenue for future diagnosis of this disease in human patients.
Acknowledgments
We thank Arthur Foubert for his technical assistance in handling and operating on the pigs.
Supported by the Karin Grunebaum Research Fellowship, Harvard Medical School (D.E.R.), the German Research Fellowship, German Research Foundation STR 690/1-1 (O.S.), and the Phillip H. Meyers Grant from the Society of Gastrointestinal Radiologists (S.P.T.).
Discussion
Dr. Stanley Ashley (Boston, MA): This is a very nice animal study suggesting that CT might be used for diagnosis in an area where it has not been used previously, mesenteric ischemia. The MGH has proven the utility of CT in the diagnosis of appendicitis, and this study suggests that, in another difficult area, it is going to be useful as well. I have a couple of questions.
First, what is the pathophysiology of the venous dilatation that you saw in the ischemic segments? That is not something that I think about occurring with mesenteric ischemia, and I wonder what that means.
The second relates to the model. A large number of these patients have nonocclusive disease. Microspheres were good for reproducing the pathophysiology of thrombosis or embolus but do you have any data about this technique's utility in low-flow states?
Finally, the data would suggest that you could actually make a distinction between viable and nonviable bowel, preoperatively. One of the big dilemmas in these patients is whether to revascularize, with the inherent morbidity, versus just going ahead and resecting the segment. Do you think this technique might allow us to make that decision a little better?
Dr. Rosow: The first question regards the pathophysiology of venous dilatation. In the earliest stages of ischemia, as seen on CT, we were able to see congestion of the small veins as an indication of early tissue death. From a radiologic standpoint, venous dilatation seems to represent this early ischemic state.
The second question regards the nonocclusive variety. We didn't create a nonocclusive state to yield ischemia, as the end products are relatively similar. We do speculate, because our findings are based on the Houndsfield enhancement unit alone, that even in nonocclusive states, one would be able to see a similar reduction in the amount of arterial phase enhancement.
Our study was not designed to determine whether or not someone can preoperatively assess viability versus nonviability. The results do suggest that there is an objective set of criteria that can be used to determine this. However, our data were used to determine ischemia versus nonischemia, as opposed to early ischemia (viable) versus severe ischemia (nonviable). A greater degree of statistical power is required to make that assessment.
Dr. John Christein (Rochester, MN): I think you are doing important work here in an attempt identify this early group of ischemic bowel patients who are, in fact, salvageable. However, when we see these patients in the emergency department with vague abdominal pain, usually they are just run through the scanner by the ED physician, usually before a surgical consult and typically with standard IV contrast and cuts. Is that completely different than the M.D.CT you are using, and if it is, is there a price difference or technology difference to the M.D.CT scanner?
Dr Rosow. It is actually a fairly similar protocol. It requires simple injection of intravenous contrast, as well as no oral contrast administration. The price difference, I think, would only come in the purchasing of the equipment. Once the multidetector CT scanner is in place, we use a very similar protocol in terms of the computing power and technical help that is required.
Dr. Thomas Howard (Indianapolis, IN): This may be a follow-up to the first question, but with regard to motion artifact, to get those very, very good images that you showed with three-dimensional reconstructions in small peripheral vessels, what was required technically? Is the pig anesthetized and immobilized, how fast are the images obtained, and is technique able to be translated to a clinical scenario where a patient may or may not be able to cooperate with that type of study?
Dr. Rosow: Yes, the pigs are anesthetized and intubated; therefore, perhaps motion artifacts aren't represented as significantly as they could be. However, respiratory artifacts were taken into consideration using this method, so the amount of precision required to get these angiographic findings using the multidetector protocol is significantly enhanced. However, you are able to do the same protocol on a 16-detector CT as well. It does not require just a 64-detector CT.
Dr. Michael Sarr (Rochester, MN): Great study. Even I could see those occluded vessels. What about using smaller vessels? I am sure you are going to go on and occlude progressively smaller vessels. And are you blinding your radiologists to looking at the small occluded vessels?
Dr. Rosow: In answer to the first question, we have considered using occlusion of the smaller vessels. It is more of a limitation of the technique at this point; really, I think in terms of the availability of the size of the microspheres that can be used for embolization. The radiologists were completely blinded to whether or not the pig was ischemic versus not ischemic, and as to the branch of the artery that was selected for embolization as well. So, they had no knowledge prior to reading of these scans which artery had been embolized.
Footnotes
Presented at the Forty-Sixth Annual Meeting of The Society for Surgery of the Alimentary Tract, Chicago, Illinois, May 14–18, 2005 (oral presentation).
REFERENCES
- 1.Kaleya RN, Sammartano RJ, Boley SJ. Aggressive approach to acute mesenteric ischemia. Surg Clin North Am. 1992;72:157–182. doi: 10.1016/s0039-6109(16)45633-1. [DOI] [PubMed] [Google Scholar]
- 2.Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg. 1978;15:1–85. doi: 10.1016/s0011-3840(78)80018-5. [DOI] [PubMed] [Google Scholar]
- 3.Moore WM, Jr, Hollier LH. Mesenteric artery occlusive disease. Cardiol Clin. 1991;9:535–541. [PubMed] [Google Scholar]
- 4.Klempnauer J, Grothues F, Bektas H, et al. Long-term results after surgery for acute mesenteric ischemia. Surgery. 1997;121:239–243. doi: 10.1016/s0039-6060(97)90351-2. [DOI] [PubMed] [Google Scholar]
- 5.Sachs SM, Morton JH, Schwartz SI. Acute mesenteric ischemia. Surgery. 1982;92:646–653. [PubMed] [Google Scholar]
- 6.Rogers DM, Thompson JE, Garrett WV, et al. Mesenteric vascular problems. A 26-year experience. Ann Surg. 1982;195:554–565. doi: 10.1097/00000658-198205000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krausz MM, Manny J. Acute superior mesenteric arterial occlusion: A plea for early diagnosis. Surgery. 1978;83:482–485. [PubMed] [Google Scholar]
- 8.Batellier J, Kieny R. Superior mesenteric artery embolism: eighty-two cases. Ann Vasc Surg. 1990;4:112–116. doi: 10.1007/BF02001363. [DOI] [PubMed] [Google Scholar]
- 9.Horgan PG, Gorey TF. Operative assessment of intestinal viability. Surg Clin North Am. 1992;72:143–155. doi: 10.1016/s0039-6109(16)45632-x. [DOI] [PubMed] [Google Scholar]
- 10.James S, Balfe DM, Lee JK, et al. Small-bowel disease: Categorization by CT examination. Am J Roentgenol. 1987;148:863–868. doi: 10.2214/ajr.148.5.863. [DOI] [PubMed] [Google Scholar]
- 11.Alpern MB, Glazer GM, Francis IR. Ischemic or infarcted bowel: CT findings. Radiology. 1988;166:149–152. doi: 10.1148/radiology.166.1.3336673. [DOI] [PubMed] [Google Scholar]
- 12.Rha SE, Ha HK, Lee SH, et al. CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics. 2000;20:29–42. doi: 10.1148/radiographics.20.1.g00ja0629. [DOI] [PubMed] [Google Scholar]
- 13.Chou CK. CT manifestations of bowel ischemia. Am J Roentgenol. 2002;178:87–91. doi: 10.2214/ajr.178.1.1780087. [DOI] [PubMed] [Google Scholar]
- 14.Taourel PG, Deneuville M, Pradel JA, et al. Acute mesenteric ischemia: diagnosis with contrast-enhanced CT. Radiology. 1996;199:632–636. doi: 10.1148/radiology.199.3.8637978. [DOI] [PubMed] [Google Scholar]
- 15.Korogi Y, Takahashi M, Katada K, et al. Intracranial aneurysms: Detection with three-dimensional CT angiography with volume rendering–comparison with conventional angiographic and surgical findings. Radiology. 1999;211:497–506. doi: 10.1148/radiology.211.2.r99ma02497. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PT, Fishman EK, Duckwall JR, et al. Interactive three-dimensional volume rendering of spiral CT data: Current applications in the thorax. Radiographics. 1998;18:165–187. doi: 10.1148/radiographics.18.1.9460115. [DOI] [PubMed] [Google Scholar]
- 17.Kamel IR, Kruskal JB, Pomfret EA, et al. Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. Am J Roentgenol. 2001;176:193–200. doi: 10.2214/ajr.176.1.1760193. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PT, Halpern EJ, Kuszyk BS, et al. Renal artery stenosis: CT angiographydcomparison of real-time volume-rendering and maximum intensity projection algorithms. Radiology. 1999;211:337–343. doi: 10.1148/radiology.211.2.r99ap17337. [DOI] [PubMed] [Google Scholar]
- 19.Hong KC, Freeny PC. Pancreaticoduodenal arcades and dorsal pancreatic artery: Comparison of CT angiography with three-dimensional volume rendering, maximum intensity projection, and shaded-surface display. Am J Roentgenol. 1999;172:925–931. doi: 10.2214/ajr.172.4.10587122. [DOI] [PubMed] [Google Scholar]
- 20.Horton KM, Fishman EK. Multi-detector row CT of mesenteric ischemia: Can it be done? Radiographics. 2001;21:1463–1473. doi: 10.1148/radiographics.21.6.g01nv091463. [DOI] [PubMed] [Google Scholar]
- 21.Guide for the care and use of laboratory animals. National Academy of Sciences; Washington, D.C.: 1996. [Google Scholar]
- 22.Gajic O, Urrutia LE, Sewani H, et al. Acute abdomen in the medical intensive care unit. Crit Care Med. 2002;30:1187–1190. doi: 10.1097/00003246-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Bender JS, Ratner LE, Magnuson TH, et al. Acute abdomen in the hemodialysis patient population. Surgery. 1995;117:494–497. doi: 10.1016/s0039-6060(05)80247-8. [DOI] [PubMed] [Google Scholar]