Abstract
Purpose
Second malignant neoplasms (SMNs) after diagnosis of childhood acute lymphoblastic leukemia (ALL) are rare events.
Patients and Methods
We analyzed data on risk factors and outcomes of 642 children with SMNs occurring after treatment for ALL from 18 collaborative study groups between 1980 and 2007.
Results
Acute myeloid leukemia (AML; n = 186), myelodysplastic syndrome (MDS; n = 69), and nonmeningioma brain tumor (n = 116) were the most common types of SMNs and had the poorest outcome (5-year survival rate, 18.1% ± 2.9%, 31.1% ± 6.2%, and 18.3% ± 3.8%, respectively). Five-year survival estimates for AML were 11.2% ± 2.9% for 125 patients diagnosed before 2000 and 34.1% ± 6.3% for 61 patients diagnosed after 2000 (P < .001); 5-year survival estimates for MDS were 17.1% ± 6.4% (n = 36) and 48.2% ± 10.6% (n = 33; P = .005). Allogeneic stem-cell transplantation failed to improve outcome of secondary myeloid malignancies after adjusting for waiting time to transplantation. Five-year survival rates were above 90% for patients with meningioma, Hodgkin lymphoma, thyroid carcinoma, basal cell carcinoma, and parotid gland tumor, and 68.5% ± 6.4% for those with non-Hodgkin lymphoma. Eighty-nine percent of patients with brain tumors had received cranial irradiation. Solid tumors were associated with cyclophosphamide exposure, and myeloid malignancy was associated with topoisomerase II inhibitors and starting doses of methotrexate of at least 25 mg/m2 per week and mercaptopurine of at least 75 mg/m2 per day. Myeloid malignancies with monosomy 7/5q− were associated with high hyperdiploid ALL karyotypes, whereas 11q23/MLL-rearranged AML or MDS was associated with ALL harboring translocations of t(9;22), t(4;11), t(1;19), and t(12;21) (P = .03).
Conclusion
SMNs, except for brain tumors, AML, and MDS, have outcomes similar to their primary counterparts.
INTRODUCTION
As many as one third of all deaths in childhood acute lymphoblastic leukemia (ALL) are caused by toxicities or second malignant neoplasms (SMNs).1–4 Previously reported cumulative incidences of SMNs have varied from less than 1% to 10% or more because of differences in antileukemic therapy and in duration, accuracy, and completeness of follow-up.1,2,5–18 Partly because of their rarity, little is known about the etiology of SMNs or about the treatment options that offer the best chances of cure.1
With the goal of improving overall survival in childhood ALL and providing guidelines for treatment, the international Ponte di Legno consortium of ALL study groups has studied uncommon subgroups of childhood ALL.19–23 This is the largest study of SMNs after therapy for childhood ALL reported to date, and it presents new potential risk factors and provides survival rates for distinct subsets.
PATIENTS AND METHODS
Review of Patient Data
In the February 2010 issue of Leukemia, 16 cooperative study groups from Europe, North America, and Asia reported clinical outcomes, including the occurrence of SMNs, of 54,068 children and adolescents up to 21 years of age with newly diagnosed ALL enrolled onto controlled clinical trials between 1980 and 2007.5–17,24–26 From these 16 groups as well as from FRALLE (French Acute Lymphoblastic Leukaemia Study Group) and the childhood leukemia branch of the European Organisation for Research and Treatment of Cancer (EORTC), we collected data on individuals with SMNs to form a common database with predefined variables comprising clinical and biologic data (including cytogenetic characteristics for myeloid neoplasias) as well as outcomes (Appendix Table A1, online only). Furthermore, we recorded clinical and biologic characteristics of their primary ALL as well as treatment given and status at latest follow-up. The data available for this study were retrieved from the groups' central ALL databases. If patient data on drug doses were unavailable, the patients were assigned the drugs and doses listed in the ALL protocols onto which they were enrolled. Accrual of data for patients with ALL who did not develop SMNs was not part of the study. The study was approved according to regional institutional review board requirements. All data were compiled at Rigshospitalet (Copenhagen, Denmark), and the database was approved by the Danish Data Protection Authorities.
Statistical Analysis
Differences in distribution of individual parameters among subsets were analyzed by using nonparametric tests.27 Since accrual of data for patients with ALL who did not develop SMNs was not part of this study, odds ratios for SMNs in relation to specific exposures are not included. Instead, we analyzed patterns of ALL characteristics and therapy by subsets of SMNs to determine whether certain ALL subtypes or drug exposures were more prevalent within specific subsets of SMNs. Survival after an SMN was defined as time from diagnosis of the SMN to death as a result of any cause or to last follow-up. The Kaplan-Meier method was used to estimate survival rates with SEs calculated according to Greenwood.28 Differences in survival rates were compared with the log-rank test.29 The Cox proportional hazard model was used for selected analysis of survival after SMNs.30 Two-sided P values below .05 were regarded as significant.
RESULTS
In all, 659 patients diagnosed with ALL between 1980 and 2007 were registered with a malignant neoplasm or a CNS tumor as the first event after diagnosis of ALL. Seventeen SMNs reported as ALL (n = 12), acute undifferentiated leukemia (n = 2), or myeloid malignancies with monosomy 7 (n = 1) or t(9;22)(q34;q11.2) (n = 2) at diagnosis of both ALL and the subsequent SMNs were excluded because the clonal relationship to the original leukemia could not be confidently verified, leaving a total of 642 study patients.
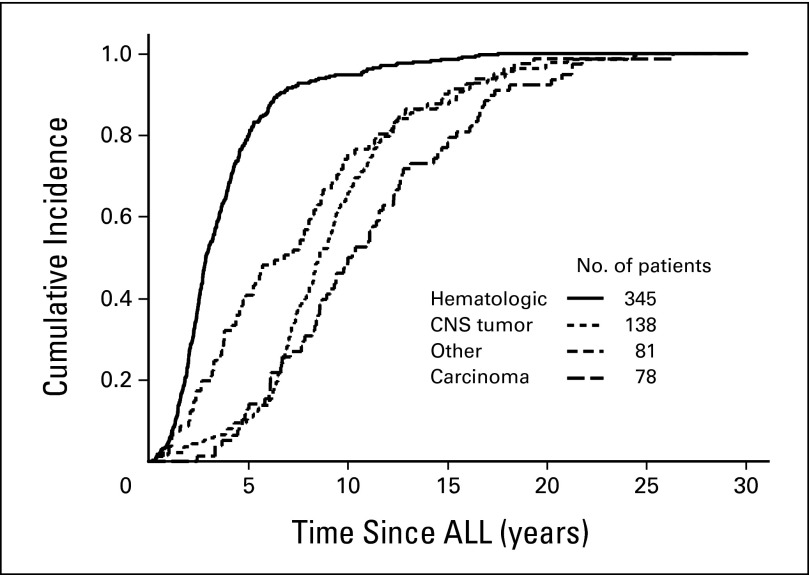
Table 1 reports clinical information on the 642 SMNs by subtype. The interval between diagnosis of ALL and occurrence of SMNs was significantly associated with the subtype of SMN, being shortest for hematologic malignancies and longest for carcinomas and meningiomas (P < .001; Fig 1 and Table 1). Thus, among the 48 SMNs diagnosed more than 15 years from the diagnosis of ALL, 35% were meningiomas (n = 15) or other CNS tumors (n = 2); 31% were non–skin carcinomas (n = 15), including six thyroid cancers; 15% were melanomas (n = 4) or other skin cancers (n = 3); and 17% were hematologic malignancies (n = 5); sarcomas (n = 2); or testicular cancer (n = 2). Eight patients with cancer-predisposing diseases are described in Appendix Table A2 (online only).
Table 1.
Clinical Characteristics and 5-Year Overall Survival of 642 Patients With SMNs by Major Categories and Subtype
| Type of SMN | Total |
Males |
ALL Immunophenotype* (n = 555) |
Age at ALL (years) |
WBC at ALL (×109/L) |
Interval to SMN (years) |
Age at SMN (years) |
5-Year Survival Rate After SMN (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | BCP | % | Median | 50% Range | Median | 50% Range | Median | 50% Range | Median | 50% Range | ||
| Total | 642 | 346 | 53.9 | 434 | 78.2 | 5.2 | 3.2-10.3 | 11.4 | 4.7-45.0 | 4.8 | 2.6-8.9 | 12.6 | 7.8-17.5 | 40.4 ± 2.1† | |
| Hematologic | 345 | 53.7 | 198 | 57.4 | 234 | 79.6 | 5.2 | 3.2-11.2 | 9.0 | 4.2-37.0 | 2.9 | 2.0-4.5 | 9.4 | 6.5-15.2 | 35.2 ± 2.7 |
| Acute myeloid leukemia | 186 | 106 | 57.0 | 116 | 73.4 | 5.6 | 3.3-11.2 | 11.6 | 4.2-45.0 | 2.7 | 1.8-4.5 | 9.5 | 6.4-15.0 | 18.1 ± 2.9 | |
| Myelodysplastic syndrome | 69 | 32 | 46.4 | 54 | 91.5 | 5.2 | 3.1-12.2 | 6.0 | 3.8-12.7 | 3.3 | 2.6-4.6 | 9.7 | 6.9-15.9 | 31.1 ± 6.2 | |
| Chronic myeloid leukemia | 9 | 4 | 44.4 | 7 | 100.0 | 12.5 | 4.2-15.1 | 9 | 4.0-28.5 | 4.1 | 3.5-7.2 | 18.0 | 17.4-19.3 | 62.2 ± 17.8 | |
| Non–Hodgkin lymphomas | 56 | 39 | 69.6 | 39 | 83.0 | 4.7 | 3.0-8.6 | 11.2 | 4.3-31.8 | 2.3 | 1.5-4.0 | 7.8 | 5.5-12.1 | 68.5 ± 6.4 | |
| Hodgkin disease | 25 | 17 | 68.0 | 18 | 78.3 | 4.2 | 3.0-9.2 | 7.4 | 5.0-45.0 | 4.1 | 2.6-5.3 | 10.2 | 6.9-14.9 | 91.1 ± 6.0 | |
| CNS tumor | 138 | 21.5 | 67 | 48.6 | 94 | 78.3 | 4.2 | 2.6-8.7 | 15.7 | 6.1-59.0 | 8.6 | 6.8-11.2 | 14.7 | 11.0-19.2 | 25.9 ± 4.2 |
| Nonmeningioma CNS tumor | 116 | 53 | 45.7 | 79 | 77.5 | 4.4 | 2.7-8.7 | 18.7 | 6.9-82.8 | 8.1 | 6.5-9.8 | 13.9 | 10.5-16.5 | 18.3 ± 3.8 | |
| Meningioma | 22 | 14 | 63.6 | 15 | 83.3 | 3.5 | 2.3-8.5 | 9 | 5.1-30.0 | 16.2 | 12.3-18.3 | 21.7 | 17.8-25.4 | 90.9 ± 8.7 | |
| Carcinoma | 78 | 12.1 | 34 | 43.6 | 62 | 84.9 | 5.8 | 3.3-10.6 | 12.3 | 4.0-45.6 | 10.1 | 6.7-14.5 | 17.5 | 12.4-22.2 | 82.2 ± 4.9 |
| Nonthyroid carcinoma | 46 | 19 | 41.3 | 35 | 81.4 | 8.4 | 3.9-13.0 | 12.9 | 3.6-38.5 | 10.2 | 6.1-15.0 | 18.0 | 12.4-25.8 | 67.3 ± 8.2 | |
| Thyroid carcinoma | 32 | 15 | 46.9 | 27 | 90.0 | 5.0 | 3.1-6.5 | 12.1 | 4.3-58.5 | 10.1 | 7.8-13.5 | 15.5 | 12.1-18.3 | 100 | |
| Other | 81 | 12.6 | 47 | 58.0 | 44 | 64.7 | 5.7 | 4.0-10.4 | 14.0 | 4.9-79.9 | 6.8 | 3.4-10.0 | 14.1 | 8.2-17.9 | 55.3 ± 6.1 |
| Soft tissue sarcoma | 29 | 14 | 48.3 | 14 | 60.9 | 6.0 | 4.1-10.4 | 19.8 | 7.3-66.0 | 5.4 | 3.3-9.6 | 13.3 | 8.0-17.2 | 43.9 ± 9.7 | |
| Bone tumor | 22 | 13 | 59.1 | 14 | 77.8 | 5.3 | 2.9-8.1 | 7.0 | 3.1-30.9 | 7.8 | 5.2-11.4 | 14.4 | 11.9-17.9 | 61.9 ± 11.6 | |
| Melanoma | 11 | 6 | 54.6 | 9 | 90.0 | 10.0 | 5.7-13.9 | 10.0 | 4.7-30.9 | 10.0 | 6.3-17.8 | 19.2 | 16.7-24.3 | 85.7 ± 13.2 | |
| Germ cell tumor | 4 | 4 | 100.0 | 3 | 100.0 | 12.7 | 8.1-15.2 | 7.8 | 2.6-13.2 | 12.3 | 8.4-19.8 | 22.9 | 20.2-31.4 | 100 | |
| Histiocytosis | 12 | 9 | 75.0 | 2 | 16.7 | 4.2 | 2.5-5.5 | 141.0 | 40.4-248.5 | 2.3 | 1.4-3.9 | 6.9 | 6.0-8.2 | 48.6 ± 14.8 | |
| Other | 3 | 1 | 33.3 | 2 | 100.0 | 9.9 | 4.1-12.3 | 4.0 | 2.2-148.0 | 7.6 | 3.3-9.8 | 15.5 | 13.9-17.5 | 33.3 ± 27.2 | |
Abbreviations: ALL, acute lymphoblastic leukemia; BCP, B-cell precursor; SMN, second malignant neoplasm.
In all, 87 patients were excluded because immunophenotype was not reported (n = 75) or was not specified as either BCP or T-cell ALL (n = 12).
Ten-year survival rate was 38.7% ± 2.2%.
Fig 1.
Kaplan-Meier estimates of the interval between diagnosis of acute lymphoblastic leukemia (ALL) and development of the four major categories of second malignant neoplasms.
Patterns of SMNs by ALL-Presenting Features
Although distribution of sex, age, and WBC count at diagnosis of ALL varied significantly among the major categories of SMNs for the entire cohort (Table 1), this was not the case for the subset of 201 patients who were not irradiated and did not undergo hematopoietic stem-cell transplantation during first-line ALL treatment (P > .45 for all analyses; Appendix Table A3, online only).
Immunophenotype
Of the 186 patients with AML and 69 patients with myelodysplastic syndrome (MDS), the ALL lineage (B-cell precursor or T-cell lineage) was available for 217 patients. When analyzing only the 192 patients who did not receive irradiation and did not receive transplantation but who did have ALL immunophenotype available, the prevalence of T-cell ALL did not differ significantly among the categories of hematologic malignancies, CNS tumors, carcinomas, and other tumors (7.8%, 10.0%, and 16.7%, respectively; P = .38), but 26.6% of all patients with AML (42 of 158) and 8.5% of all patients with MDS (five of 59) initially had T-cell ALL. Patients with AML were overall more likely than those with other hematologic malignancies (n = 136) to have had T-cell ALL (26.6% v 13.2%; P = .005) with the same trend (10.0% v 5.6%; P = .33) in the subsets of patients who did not receive irradiation and did not receive transplantation. The interval between diagnosis of ALL and SMN was significantly shorter for the 11 patients who did not receive irradiation and did not receive transplantation but who had T-cell ALL than for the 130 patients with B-cell precursor ALL who had developed hematologic malignancies (median, 1.6 v 3.0 years; P = .001). Finally, 91% (10 of 11) of the patients who developed Langerhans cell histiocytosis had T-cell ALL compared with 20.4% among the other SMNs (P < .001).
Karyotype and Therapy-Related Myeloid Neoplasias
The time to develop AML was shorter than the time to develop MDS (median, 2.7 v 3.3 years; P = .01), reflecting a higher proportion of 11q23/MLL rearrangements with short latency (median, 2.5 years) in patients with AML (58% v 5% of patients with MDS with an aberrant karyotype; P < .001). By contrast, treatment-related myeloid neoplasia (t-MN; ie, AML or MDS) with monosomy 7 (median interval, 3.7 years) occurred in 22% of patients with AML and in 50% of patients with MDS with an aberrant karyotype (P = .002).
Among the 44 patients with t-MN with monosomy 7, 5q−, or 11q23/MLL rearrangements (one t-MN with both monosomy 7 and 11q23/MLL rearrangements was excluded) and an available karyotype for the ALL clone, the cytogenetic aberrations of their ALL and t-MN were highly correlated. Thus, among the 25 patients who developed 11q23/MLL-rearranged t-MN, 13 had ALL with classical recurrent translocations—t(9;22)(q34;q11.2) (n = 1), t(1;19)(q23;p13.3) (n = 2), t(12;21)(p13;q22) (n = 8), or 11q23/MLL rearrangements (n = 2 [different 11q23/MLL rearrangement in the two clones]—and six had a high hyperdiploid ALL karyotype (modal chromosome number above 50), and six had other structural and/or numeric aberrations. In contrast, among the 19 patients who developed t-MN with 5q– or monosomy 7, 10 had a high hyperdiploid ALL karyotype, three had ALL clones with one of the above-listed classical translocations, and six had other aberrations (P = .03 by likelihood-ratio χ2 test).
Patterns of SMNs by ALL Therapy
The pattern of SMNs was significantly influenced by the preceding ALL therapy (Table 2). The 12 patients with CNS tumors who had not received CNS irradiation were diagnosed at significantly shorter intervals after ALL than the 97 patients with CNS tumors that occurred after CNS irradiation (median, 6.6 v 9.1 years; P = .01).
Table 2.
Pattern of SMNs in Relation to Their First-Line ALL Treatment in Patients Who Did Not Receive Hematopoietic Stem-Cell Transplantation
| Type of Second Cancer | CNS Irradiation* (n = 432) |
Epipodophyllotoxin* (n = 446) |
Cyclophosphamide* |
6-Mercaptopurine† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNS Irradiation (n = 228) |
No CNS Irradiation (n = 199) |
CNS Irradiation (n = 230) |
No CNS Irradiation (n = 192) |
|||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Total | 230 | 202 | 185 | 261 | 186 | 42 | 126 | 73 | 53 | 177 | 94 | 98 |
| Hematologic SMN | 79 | 145 | 105 | 127 | 67 | 11 | 82 | 61 | 25 | 50 | 76 | 61 |
| t-MN was AML or MDS | 64 | 109 | 84 | 96 | 54 | 9 | 60 | 47 | 22 | 38 | 61 | 43 |
| CNS tumors | 97 | 12 | 48 | 63 | 76 | 20 | 7 | 5 | 24 | 68 | 5 | 7 |
| Non-CNS solid tumors | 54 | 45 | 32 | 79 | 43 | 11 | 37 | 7 | 4 | 49 | 13 | 30 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; SMN, second malignant neoplasm; t-MN, therapy-related myeloid neoplasia.
Only patients who did not receive transplantation who had available information on their therapy are included.
Dose ≥ 75 mg/m2.
Thirty-eight (76.0%) of 50 patients with t-MN with an aberrant karyotype and previous exposure to epipodophyllotoxins had 11q23/MLL rearrangements, whereas only four (8.0%) had monosomy 7 and none had 5q–. In contrast, among the 46 patients with t-MN (52.2%) who had not been exposed to epipodophyllotoxins, 24 developed monosomy 7 (n = 20) or 5q– (n = 4) t-MN, and only 13 (28.3%) had 11q23/MLL rearrangements (P < .001).
Among patients who did not receive irradiation, 44 (79%) of 56 patients with solid tumors had previously received cyclophosphamide compared with 82 (57%) of 143 patients with hematologic malignancies or CNS tumors (P = .005).
Among the patients who did not receive transplantation for whom data on maintenance therapy methotrexate (n = 431) and mercaptopurine dosage (n = 422) were available, the patients who developed t-MN received higher starting doses of methotrexate and mercaptopurine than did patients who developed other SMNs (P < .001 for both drugs), and this was the case for both CNS patients who received irradiation (P < .001 and P = .001, respectively) and those who did not (P = .007 and P = .02, respectively). Thus, compared with patients with other SMNs, the patients who developed t-MNs were more likely to have received methotrexate starting doses of at least 25 mg/m2 per week (45% v 28%; P < .001) and mercaptopurine starting doses of at least 75 mg/m2 per day (52% v 29%; P < .001).
Neither the distribution of the four major categories of SMNs (P = .37) nor the time interval to SMN (P = .84) differed significantly between patients with low (n = 13; 10 by genotype and three by phenotype) versus normal (n = 114) thiopurine methyltransferase activity. Among the 413 patients who did not undergo transplantation but who did have data on the total duration of therapy, 65 (31.3%) of the 208 patients with t-MN and 36 (17.6%) of the 205 patients with solid tumors had received ALL therapy for 2.5 years or longer (P = .001).
Transplantation during first remission of ALL had been performed in 29 (5.7%) of the 510 ALL patients with available information. One (1.4%) of 74 patients with CNS tumors and seven (3.6%) of 193 patients with t-MN had received transplantation compared with nine (28.1%) of 32 patients with carcinomas and eight (15.4%) of 52 with other SMNs (P < .001).
Survival After SMNs
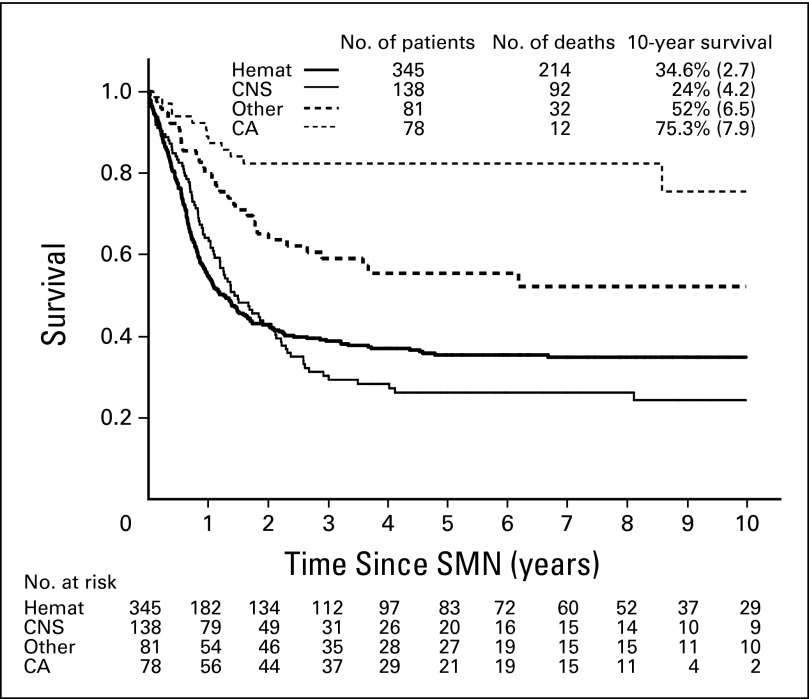
The median follow-up after diagnosis of an SMN was 4.9 years for the 292 patients who were alive at their latest follow-up. In all, 350 patients died within 20.6 years from diagnosis of an SMN (median, 0.75 years; 25th to 75th percentile: 0.4 to 1.4). The overall cumulative probability of death as a result of any cause was 59.6% ± 2.1% at 5 years and 61.3% ± 2.2% at 10 years after an SMN (Table 1 and Fig 2). The 10-year cumulative incidence of death as a result of the second (n = 236) or third (n = 1) cancer was 41.1% ± 2.1%; it was 5.6% ± 1.0% for relapsed ALL (n = 31), 10.4% ± 1.3% for treatment-related toxicities among patients who received a transplantation (n = 39) and those who did not (n = 20), and 4.2% ± 0.9% for unknown causes (n = 23; Fig 3). The 10-year probability of survival was 18.9% ± 6.9% (n = 33) for patients whose SMN occurred before 1990 (n = 54), 34.8% ± 2.8% (n = 296) for patients with SMNs diagnosed between 1990 and 1999, and 40.9% ± 6.3% (n = 313) for patients diagnosed from 2000 onward (P < .001).
Fig 2.
Survival curves according to the four major categories of second malignant neoplasms (SMNs). Hemat, hematologic; CA, carcinoma.
Fig 3.
Cause-specific cumulative incidences (CIs) of death after development of a second malignant neoplasm (SMN).
Hematologic Malignancies
Survival remained consistently lower for patients with AML compared with those who had MDS (P < .001). The 5-year survival estimate for AML was 11.2% ± 2.9% for 125 patients diagnosed before 2000 and 34.1% ± 6.3% for 61 patients diagnosed after 2000 (P < .001). For MDS, the 5-year survival was 17.1% ± 6.4% for 36 patients diagnosed before 2000 and 48.2% ± 10.6% for 33 patients diagnosed after 2000 (P = .005). In a Cox regression model, adjusting for sex and age at diagnosis of SMNs and the use of CNS irradiation for ALL treatment, the improved outcome after 2000 was confirmed for both AML (estimated hazard ratio [HR], 0.62; 95% CI, 0.42 to 0.90; P = .01) and MDS (HR, 0.30; 95% CI, 0.15 to 0.60; P < .001). The hazard of death after t-MN decreased by approximately 10% for every additional year of interval between ALL and AML (HR, 0.88; 95% CI, 0.80 to 0.96; P = .004) with a similar trend for MDS (HR, 0.92; 95% CI, 0.80 to 1.06; P = .23).
For 185 patients with available information on transplantation after t-MN, the 5-year survival was 30.3% ± 4.4% for the 119 patients who received a transplantation and 11.4% ± 4.0% for the 66 who did not (P < .001). However, with a landmark at the median waiting time to transplantation of 4.1 months from SMN diagnosis, the 5-year survival estimates for patients who had received a transplantation and those who had not did not differ (26.7% ± 4.2% and 27.2% ± 7.7%, respectively),28,31 and this was also the case for 78 patients with t-MN diagnosed in 2000 or later (42.0% ± 7.6% v 46.9% ± 11.5%). Among the patients with t-MN who received a transplantation, the 10-year survival for 30 patients with 11q23/MLL rearrangements (24.7% ± 8.3%) did not differ significantly from that of 26 patients with monosomy 7 (28.0% ± 9.0%).
Only two of the 25 patients with Hodgkin lymphoma died, both of whom were diagnosed with Hodgkin lymphoma in the 1980s. Excluding patients who received transplantation as part of their ALL therapy, the 5-year survival was 70.5% ± 7.9% for the 34 patients with non-Hodgkin lymphoma diagnosed in the 1990s and 65.4% ± 10.8% for the 22 patients diagnosed later (P = .64). The 5-year survival was 76.9% ± 8.3% for the 27 patients who had developed mature B-cell non-Hodgkin lymphoma.
CNS Tumors
Although only one of 22 patients with meningioma died, the 5-year survival was very poor for the remaining 116 patients with brain tumors (18.3% ± 3.8%), including eight patients with low-grade tumors (45.0% ± 18.8%), 76 with high-grade tumors including medulloblastomas and supratentorial primitive neuroectodermal tumors (6.5% ± 3.6%), and 13 unspecified glial tumors (8.5% ± 8.2%). Overall survival after nonmeningioma brain tumor did not improve over time, with 5-year estimates of 19.6% ± 5.5% before 2000 and 16.6% ± 5.3% afterward (P = .76).
Nonthyroid Carcinomas
All seven patients with basal cell carcinoma and nine with parotid gland tumors survived, and the 5-year survival for the nine patients with squamous cell carcinoma was 71.4% ± 17.1%. In contrast, the overall survival for the 18 patients with other carcinomas (five, breast; four, gastrointestinal; three, liver; and one each, peritoneal, pancreas, lung, cervix uteri, urinary tract, and nasopharyngeal) was only 40.1% ± 13.7% at 5 years and 0% at 10 years (P < .001).
DISCUSSION
In this study, the largest reported to date, patients with t-MN or nonmeningioma brain tumor had a poor prognosis, whereas patients with secondary meningioma, Hodgkin lymphoma, thyroid carcinoma, basal cell carcinoma, and parotid gland carcinoma had a 5-year survival exceeding 90%.
This study had some limitations since it did not allow calculations of HRs by ALL characteristics or therapy components, and it could not identify exposures that had equal influence on the risk of all major categories of SMNs. In addition, the data must be interpreted cautiously, since the completeness of recording of SMNs was influenced by the individual study groups' frequency and duration of follow-up,1 screening strategies for thyroid carcinomas, meningiomas, or breast cancer in irradiated patients,32–34 and linkage with population-based nationwide cancer registries.18 The impact of such differences will be limited for secondary hematologic malignancies but will be more profound for SMNs that have long latency such as carcinomas and meningiomas. Furthermore, hematologic SMNs can be misinterpreted as relapse of ALL, and some cases of ALL and SMNs may have a common clonal origin.35,36 Thus, an association between T-cell ALL and histiocytosis has previously been reported,35,36 and patients with early T-cell precursor ALL have been shown to have genetic profiles similar to those of patients with myeloid malignancies,37 which could indicate a common ancestral clone for the primary and second malignancies.
The observed association between high-hyperdiploid ALL and the development of t-MN with monosomy 7/5q– has been observed in a much smaller study,2 although the association between ALL with specific chromosomal translocations (ie, t(9;22)(q34;q11.2), t(1;19)(q23;p13.3), t(12;21)(p13;q22)) and t-MN with 11q23/MLL rearrangements has hitherto not been reported. The more frequent use of topoisomerase II inhibitors such as epipodophyllotoxins in high-risk ALL cases with specific chromosomal translocation might have contributed to the development of t-MN with 11q23/MLL rearrangements. However, the unique gene expression profiles of ALL blast from those patients who subsequently developed SMNs, including t-MN, could also reflect inherited genetic variants38 that could influence drug disposition (eg, glutathione S-transferases, cytochrome P-450 enzymes, quinone oxidoreductase, or the folate pathway39,40) or be related to cancer predisposition syndromes. International collaboration with extensive mapping of host genomic variants could be instrumental in identifying subsets of patients with ALL with genetic predispositions for whom modification of first-line ALL therapy or individualized follow-up should be offered.
This study supports previously reported associations of t-MN with higher mercaptopurine dosages during maintenance therapy and longer duration of therapy. Some study groups that offer a maintenance therapy mercaptopurine starting dose of 75 mg/m2 have found an association between an increased risk of SMN and low-activity thiopurine methyltransferase genotypes or phenotypes.2,41 Notably, others who used a mercaptopurine starting dose of only 50 mg/m2 failed to find such an association.42 The linkage between thiopurine therapy and risk of SMN may reflect that these anticancer agents, when given at high dosage or for an extended period, may interfere with DNA repair rather than directly induce mutations.41,43 Accordingly, the omission or interruption of maintenance therapy for patients who received a transplantation as part of their ALL therapy may explain why very few patients with brain tumor or t-MN in this cohort had received transplantation. Overall, the risk of relapse if mercaptopurine/methotrexate-based maintenance therapy is truncated44 is far higher than the risk of t-MN indicated by this and previous studies. The goal for future research is thus to identify patients with a clearly excessive risk of t-MN and consider treatment modification only for such a limited patient subset.
Patients with t-MN have had significant improvements in survival over the last few decades, but the cure rates are still below those obtained by the best treatment protocols for primary AML.45 Although the survival of patients with t-MN who did not receive transplantation was only 11.4% ± 4.0%, the study did not support that hematopoietic stem-cell transplantation would be beneficial for these patients when the data were adjusted for the waiting time to transplantation. Thus, future studies of this important issue, including the impact of t-MN cytogenetics, are needed.
It is uncertain whether the extremely poor survival rate for CNS tumors, the vast majority of which developed after CNS irradiation, reflects a more aggressive biology, difficulties in performing complete tumor resection in previously irradiated regions, limitations in irradiating previously irradiated regions, or a pessimistic attitude toward curative therapy for such patients. Because this subset is the second most common SMN among survivors of childhood ALL and is overall one of the most common SMNs after a childhood cancer,18 a review of patients' records of these tumors is needed to explore these issues in depth.
Although the cure rates for some SMNs were as favorable as those obtained for their primary cancer counterparts, future strategies should continue to focus on prevention of SMNs. Thus, the frequency of secondary brain tumor is expected to fall dramatically during the coming decades with the reduced use of CNS irradiation in first-line ALL therapy,46 and given the few patients on contemporary protocols who are exposed to epipodophyllotoxins, the risk of 11q23/MLL-rearranged t-MN is likely to be lower in future childhood ALL cohorts.
Acknowledgment
We thank all participating centers, data managers, and local physicians as well as patients and parents. We also thank Hester de Groot, data manager of the Dutch Childhood Oncology Group; Jane O'Brien, Leukemia Program Manager, and Kristen Stevenson, statistician (Dana-Farber Cancer Institute); Yoshifumi Kawano, MD, and Yasuto Shimomura, MD, for data collection (Japanese Pediatric Leukemia/Lymphoma Study Group); and Mats Heyman, Nordic Society of Paediatric Haematology and Oncology leukemia registry manager.
Appendix
Table A1.
SMNs Reported by the Seventeen Participating Collaborative Groups
| Trial Group Name | Trial Group Acronym | Trial Group Location | No. of Patients | Date of Diagnosis of First SMN | Date of Diagnosis of Last SMN | Trial Registration Numbers |
|---|---|---|---|---|---|---|
| Associazione Italiana Ematologia Oncologia Pediatrica | AIEOP | Italy | 22 | January 4, 1985 | December 11, 2007 | ALL-BFM 90, ALL-BFM 95, ALL-BFM 2000 (NCT00430118) |
| Berlin-Frankfurt-Münster | BFM | Austria | 14 | September 1, 1992 | June 26, 2009 | ALL-BFM 86, ALL-BFM 90, ALL-BFM 95, ALL-BFM 2000 (NCT00430118) |
| Berlin-Frankfurt-Münster | BFM | Germany | 107 | December 12, 1984 | February 1, 2009 | ALL-BFM 2000 (NCT00430118), NCI Protocol ID 68529 |
| Cooperative Study Group for Childhood Acute Lymphoblastic Leukaemia | COALL | Germany | 36 | May 10, 1984 | July 19, 2007 | COALL 07-03, EU-205104, NCT00343369 |
| Children's Oncology Group (includes both the US Children's Cancer Group and the Pediatric Oncology Group) | COG | USA | 136 | April 4, 1990 | February 12, 2008 | Separate list of POG and CCG protocols |
| Dutch Childhood Oncology Group | DCOG | Holland | 18 | February 26, 1991 | May 30, 2008 | |
| Dana-Farber Cancer Institute | DFCI | USA | 13 | August 14, 1986 | March 17, 2008 | DFCI ALL Consortium Protocols 85-001, 87-001, 91-001, 96-001 |
| European Organisation for Research and Treatment of Cancer | EORTC | Belgium and France | 16 | June 30, 1991 | June 15, 2002 | EORTC 58881 study |
| French Acute Lymphoblastic Leukaemia Study Group | FRALLE | France | 52 | March 12, 1991 | June 15, 2010 | FRALLE protocols 83, 87-89, 93, 2000 |
| Israel National ALL Studies | INS | Israel | 11 | June 16, 1993 | December 15, 2008 | ALL INS 89 (mod BFM 86), ALL INS 93 (mod BFM 90), ALL INS 98 (mod BFM 95) |
| Tokyo Children's Cancer Study Group | TCCSG | Japan | 49 | June 23, 1987 | May 6, 2010 | TCCSG L84-11, L89-12, L92-13, L95-14 |
| Japan Association of Childhood Leukemia Study | JACLS | Japan | Tokai-POG 9104, OCLSG 94, JACLS ALL-96, JACLS ALL-97 | |||
| Japanese Children's Cancer and Leukemia Study Group | JCCLSG | Japan | CCLSG ALL841, ALL851, ALL874, ALL911, ALL941 | |||
| Kyushu-Yamaguchi Children's Cancer Study Group | KYCCSG | Japan | KYCCSG AL841, HR88, ALL90, ALL96 | |||
| Nordic Society for Paediatric Haematology and Oncology | NOPHO | Denmark, Finland, Iceland, Norway, Sweden | 53 | January 15, 1986 | May 15, 2010 | ALL-86, ALL-92, ALL-2000 |
| St Jude Children's Research Hospital | SJCRH | USA | 69 | February 9, 1982 | November 18, 2002 | Total Therapies 4, 5, 6, 7, 8, 9, 10, 11, 12, 13A, and 13B |
| Taiwan Pediatric Oncology Group | TPOG | Taiwan | 19 | August 5, 1987 | January 13, 2007 | TCALL 84; TPOG-ALL 88, 93, 97, 2002 |
| National Cancer Research Institute Children's Leukaemia Clinical Studies Group | NCRI | United Kingdom | 27 | January 15, 1994 | September 15, 2007 | UKALLXI ISRCTN 16757172, ALL97 ISRCTN 26727615 |
| Total | 642 | February 9, 1982 | June 15, 2010 |
Table A2.
Clinical Characteristics of Patients With Cancer-Predisposing Syndromes
| Predisposing Syndrome | Type of Second Cancer | Sex | Age at ALL (years) | WBC at ALL (×109/L) | BCP or T-Cell ALL | Interval to SMN (years) | Age at SMN (years) | Status | Survival (years) |
|---|---|---|---|---|---|---|---|---|---|
| Down syndrome | AML | Male | 3.2 | 16.8 | B | 4.0 | 7.2 | Dead | 0.8 |
| Down syndrome | AML | Female | 2.0 | 7.8 | B | 5.9 | 7.9 | Dead | 1.1 |
| Down syndrome | Mature B-cell NHL | Male | 6.2 | 38.1 | B | 2.6 | 8.8 | Alive | 7.0 |
| Down syndrome | Ewing sarcoma | Female | 6.6 | 2.1 | B | 8.3 | 14.9 | Alive | 5.4 |
| Li Fraumeni syndrome | AML | Male | 12.4 | 6.6 | B | 2.5 | 15.0 | Dead | 0.6 |
| Ataxia telangiectasia | T-cell NHL | Male | 9.5 | 86.0 | T | 12.5 | 22.0 | Dead | 0.6 |
| Noonan syndrome | MDS | Female | 16.0 | 2.0 | B | 2.7 | 18.7 | N/A | |
| AIDS | Mature B-cell NHL | Male | 13.7 | 1.8 | B | 4.0 | 17.7 | Alive | 10.2 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCP, B-cell precursor; MDS, myelodysplastic syndrome; N/A, not available; NHL, non-Hodgkin lymphoma; SMN, second malignant neoplasm.
Table A3.
Clinical Characteristics and Overall Survival of the Four Major Categories of SMNs in the Subset of 201 Patients Who Were Not Irradiated and Did Not Undergo Hematopoietic Stem-Cell Transplantation as Part of Their First-Line Treatment for ALL
| Type of Second Cancer | Total |
Males |
ALL Immunophenotype* (n = 192) |
Age at ALL (years) |
WBC at ALL (×109/L) |
Interval to SMN (years) |
Age at SMN (years) |
5-Year Survival Rate After SMN (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | BCP | % | Median | 50% Range | Median | 50% Range | Median | 50% Range | Median | 50% Range | ||
| Total | 201 | 107 | 53.2 | 173 | 90.1 | 3.6 | 2.3-6.6 | 9.0 | 6.5-15.1 | 44.1 ± 3.7 | |||||
| Hematologic† | 145 | 72.1 | 79 | 54.5 | 130 | 92.2 | 4.3 | 3.0-6.5 | 6.1 | 4.0-15.3 | 2.9 | 2.1-4.3 | 8.2 | 6.0-12.7 | 41.1 ± 4.2 |
| CNS tumor† | 12 | 6.0 | 6 | 50.0 | 9 | 90.0 | 5.0 | 3.5-8.9 | 7.4 | 3.7-34.4 | 6.8 | 2.7-7.4 | 13.1 | 8.7-17.2 | 32.1 ± 15.0 |
| Carcinoma† | 19 | 9.5 | 7 | 36.8 | 15 | 83.3 | 4.7 | 3.0-8.7 | 6.6 | 3.3-38.5 | 11.8 | 6.1-16.1 | 16.2 | 10.7-23.4 | 77.4 ± 10.0 |
| Other† | 25 | 12.4 | 15 | 60.0 | 19 | 82.6 | 5.7 | 3.4-8.1 | 4.9 | 2.5-26.2 | 7.8 | 4.4-9.8 | 14.0 | 10.4-17.9 | 44.9 ± 11.3 |
Abbreviations: ALL, acute lymphoblastic leukemia; BCP, B-cell precursor; SMN, second malignant neoplasm.
Nine patients were excluded because immunophenotype was not reported (n = 8) or was not specified as either BCP or T-cell ALL (n = 1).
Seventy-one acute myeloid leukemia, 38 myelodysplastic syndrome, three chronic myeloid leukemia, 23 non-Hodgkin lymphoma, 10 Hodgkin disease, 10 nonmeningioma CNS tumors, two meningioma, 10 nonthyroid carcinoma, nine thyroid carcinoma, seven soft tissue sarcoma, 12 bone tumors, one germ cell tumor, four Langerhans cell histiocytosis, one other tumor.
Footnotes
Supported by Grant No. IG 5017 from the Associazione Italiana per la Ricerca sul Cancro (M.G.V.); St Anna Kinderkrebsforschung; Deutsche Krebshilfe; Fördergemeinschaft Kinderkrebszentrum Hamburg; Grants No. CA098543 and U10 CA98413 from the Children's Oncology Group; Grant No. 5 P01CA068484 from the National Cancer Institute; The European Organisation for Research and Treatment of Cancer Charitable Trust and the Schröder Foundation; Direction Recherche Clinique-Assistance Publique-Hôpitaux de Paris; Centre de Recherche en Oncologie, Hematologie et Pediatrie Association; Israel Cancer Association; Hayim Association for Children with Cancer in Israel; Ministry of Health, Labour and Welfare of Japan; Children's Cancer Association of Japan; Grant No. R40-A2154 from the Danish Cancer Society; Danish Childhood Cancer Foundation; Swedish Childhood Cancer Foundation; Grant No. CA-21765 from the National Institutes of Health; American Lebanese Syrian Associated Charities; Childhood Cancer Foundation Taiwan; and the Medical Research Council (UK).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kjeld Schmiegelow, Maria Grazia Valsecchi
Collection and assembly of data: Kjeld Schmiegelow, Mette Frandsen Levinsen, Andishe Attarbaschi, Andre Baruchel, Mini Devidas, Gabriele Escherich, Brenda Gibson, Christiane Heydrich, Keizo Horibe, Yasushi Ishida, Der-Cherng Liang, Franco Locatelli, Gérard Michel, Rob Pieters, Caroline Piette, Ching-Hon Pui, Susana Raimondi, Lewis Silverman, Martin Stanulla, Batia Stark, Naomi Winick, Maria Grazia Valsecchi
Data analysis and interpretation: Kjeld Schmiegelow, Maria Grazia Valsecchi
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 2.Schmiegelow K, Al-Modhwahi I, Andersen MK, et al. Methotrexate/6-mercaptopurine maintenance therapy influences the risk of a second malignant neoplasm after childhood acute lymphoblastic leukemia: Results from the NOPHO ALL-92 study. Blood. 2009;113:6077–6084. doi: 10.1182/blood-2008-11-187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prucker C, Attarbaschi A, Peters C, et al. Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: A population-based analysis of the Austrian Berlin-Frankfurt-Münster study group. Leukemia. 2009;23:1264–1269. doi: 10.1038/leu.2009.12. [DOI] [PubMed] [Google Scholar]
- 4.Lund B, #x00C5;sberg A, Heyman M, et al. Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2011;56:551–559. doi: 10.1002/pbc.22719. [DOI] [PubMed] [Google Scholar]
- 5.Schmiegelow K, Forestier E, Hellebostad M, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345–354. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 6.Kamps WA, van der Pal-de Bruin KM, Veerman AJ, et al. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309–319. doi: 10.1038/leu.2009.258. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchida M, Ohara A, Manabe A, et al. Long-term results of Tokyo Children's Cancer Study Group trials for childhood acute lymphoblastic leukemia, 1984-1999. Leukemia. 2010;24:383–396. doi: 10.1038/leu.2009.260. [DOI] [PubMed] [Google Scholar]
- 9.Tsurusawa M, Shimomura Y, Asami K, et al. Long-term results of the Japanese Childhood Cancer and Leukemia Study Group studies 811, 841, 874 and 911 on childhood acute lymphoblastic leukemia. Leukemia. 2010;24:335–344. doi: 10.1038/leu.2009.259. [DOI] [PubMed] [Google Scholar]
- 10.Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000) Leukemia. 2010;24:320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conter V, Aricò M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–264. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 12.Stary J, Jabali Y, Trka J, et al. Long-term results of treatment of childhood acute lymphoblastic leukemia in the Czech Republic. Leukemia. 2010;24:425–428. doi: 10.1038/leu.2009.255. [DOI] [PubMed] [Google Scholar]
- 13.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children's Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: A report from the Children's Oncology Group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 16.Escherich G, Horstmann MA, Zimmermann M, et al. Cooperative study group for childhood acute lymphoblastic leukaemia (COALL): Long-term results of trials 82, 85, 89, 92 and 97. Leukemia. 2010;24:298–308. doi: 10.1038/leu.2009.249. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell C, Richards S, Harrison CJ, et al. Long-term follow-up of the United Kingdom Medical Research Council protocols for childhood acute lymphoblastic leukaemia, 1980-2001. Leukemia. 2010;24:406–418. doi: 10.1038/leu.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen JH, Möller T, Anderson H, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst. 2009;101:806–813. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 19.Aricò M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- 20.Pui CH, Gaynon PS, Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–1915. doi: 10.1016/S0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 21.Nachman JB, Heerema NA, Sather H, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110:1112–1115. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28:4755–4761. doi: 10.1200/JCO.2010.30.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrappe M, Hunger SP, Pui CH, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang DC, Yang CP, Lin DT, et al. Long-term results of Taiwan Pediatric Oncology Group studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:397–405. doi: 10.1038/leu.2009.248. [DOI] [PubMed] [Google Scholar]
- 25.Stark B, Nirel R, Avrahami G, et al. Long-term results of the Israeli National Studies in childhood acute lymphoblastic leukemia: INS 84, 89 and 98. Leukemia. 2010;24:419–424. doi: 10.1038/leu.2009.254. [DOI] [PubMed] [Google Scholar]
- 26.Schrappe M, Nachman J, Hunger S, et al. ‘Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985-2000)'. Leukemia. 2010;24:253–254. doi: 10.1038/leu.2009.276. [DOI] [PubMed] [Google Scholar]
- 27.Glantz SA. Primer of Biostatistics (ed 6) New York, NY: McGraw-Hill Medical Publications; 2005. [Google Scholar]
- 28.Marubini E, Valsecchi MG. Analysing Survival Data From Clinical Trials and Observational Studies. West Sussex, United Kingdom: John Wiley and Sons; 2004. [Google Scholar]
- 29.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 30.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 31.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus non-responder bias. Stat Med. 1984;3:35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 32.Giovanella L, Toffalori E, Tozzoli R, et al. Multiplexed immunoassay of thyroglobulin autoantibodies in patients with differentiated thyroid carcinoma. Head Neck. 2012;34:1369–1371. doi: 10.1002/hed.21933. [DOI] [PubMed] [Google Scholar]
- 33.Goshen Y, Stark B, Kornreich L, et al. High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:294–297. doi: 10.1002/pbc.21153. [DOI] [PubMed] [Google Scholar]
- 34.Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: A report from the childhood cancer survivor study. Ann Intern Med. 2010;153:442–451. doi: 10.1059/0003-4819-153-7-201010050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trebo MM, Attarbaschi A, Mann G, et al. Histiocytosis following T-acute lymphoblastic leukemia: A BFM study. Leuk Lymphoma. 2005;46:1735–1741. doi: 10.1080/10428190500160017. [DOI] [PubMed] [Google Scholar]
- 36.Szczepanski T, van der Velden VH, Waanders E, et al. Late recurrence of childhood T-cell acute lymphoblastic leukemia frequently represents a second leukemia rather than a relapse: First evidence for genetic predisposition. J Clin Oncol. 2011;29:1643–1649. doi: 10.1200/JCO.2010.30.2877. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartford C, Yang W, Cheng C, et al. Genome scan implicates adhesion biological pathways in secondary leukemia. Leukemia. 2007;21:2128–2136. doi: 10.1038/sj.leu.2404885. [DOI] [PubMed] [Google Scholar]
- 39.Bolufer P, Collado M, Barragan E, et al. Profile of polymorphisms of drug-metabolising enzymes and the risk of therapy-related leukaemia. Br J Haematol. 2007;136:590–596. doi: 10.1111/j.1365-2141.2006.06469.x. [DOI] [PubMed] [Google Scholar]
- 40.Stanulla M, Dynybil C, Bartels DB, et al. The NQO1 C609T polymorphism is associated with risk of secondary malignant neoplasms after treatment for childhood acute lymphoblastic leukemia: A matched-pair analysis from the ALL-BFM study group. Haematologica. 2007;92:1581–1582. doi: 10.3324/haematol.10260. [DOI] [PubMed] [Google Scholar]
- 41.Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–39. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- 42.Stanulla M, Schaeffeler E, Möricke A, et al. Thiopurine methyltransferase genetics is not a major risk factor for secondary malignant neoplasms after treatment of childhood acute lymphoblastic leukemia on Berlin-Frankfurt-Münster protocols. Blood. 2009;114:1314–1318. doi: 10.1182/blood-2008-12-193250. [DOI] [PubMed] [Google Scholar]
- 43.Karran P, Attard N. Thiopurines in current medical practice: Molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8:24–36. doi: 10.1038/nrc2292. [DOI] [PubMed] [Google Scholar]
- 44.Toyoda Y, Manabe A, Tsuchida M, et al. Six months of maintenance chemotherapy after intensified treatment for acute lymphoblastic leukemia of childhood. J Clin Oncol. 2000;18:1508–1516. doi: 10.1200/JCO.2000.18.7.1508. [DOI] [PubMed] [Google Scholar]
- 45.Kaspers GJ, Creutzig U. Pediatric acute myeloid leukemia: International progress and future directions. Leukemia. 2005;19:2025–2029. doi: 10.1038/sj.leu.2403958. [DOI] [PubMed] [Google Scholar]
- 46.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]