Abstract
Neospora caninum is an apicomplexan parasite that causes abortion in cattle; hence, accurate diagnosis of this pathogen is important to the cattle farming industry. Our previous proteomics and immunoscreening analyses revealed that the N. caninum subtilisin-like serine protease 1 (NcSUB1) has potential as a serodiagnostic tool for Neospora. Consequently, we expressed two fragments containing five NcSUB1 tandem repeat copies covering amino acids (aa) 524 to 843 (NcSUB1t) and 555 to 679 (NcSUB1tr) to identify the antigenic regions. The serodiagnostic performances of NcSUB1t and NcSUB1tr were compared with that of N54, which contains a single copy of the repeats (aa 649 to 784), and with the truncated NcSAG1 (NcSAG1t), which lacks a signal peptide and C-terminal hydrophobic regions, as a positive reference. Serum samples from N. caninum experimentally infected cattle and mice and cattle from a farm with confirmed cases of Neospora abortion were tested by enzyme-linked immunosorbent assay (ELISA) with the four antigens. In the N. caninum experimentally infected cattle, the highest IgG1 antibody titers were detected against NcSUB1t, while specific IgG1 antibodies were detectable from 16 days postinfection (dpi), with levels peaking at 36 dpi for all of the antigens. On the other hand, the levels of anti-NcSUB1 IgG2 antibodies were lower than those of anti-SAG1t IgG2 antibodies. The ELISA with NcSUB1t and NcSUB1tr had good sensitivity (94.59 to 95.95%) and specificity (80 to 100%) with bovine serum field samples compared to NcSAG1t and showed no cross-reactions with sera from Toxoplasma gondii experimentally infected mice. Moreover, IgG antibodies against NcSUB1t were detected during parturition in the NcSAG1t antibody-positive cattle, and NcSUB1t-specific antibody transfer was observed from a mother to her calf. Our results show that the NcSUB1 tandem repeat is potentially useful for serodiagnosis of N. caninum.
INTRODUCTION
Neospora caninum, the causative agent of neosporosis in cattle, is an obligate intracellular apicomplexan parasite that causes abortion and stillbirth (1, 2). The morphological similarity of this parasite to Toxoplasma gondii previously resulted in them being misclassified as one and the same (3, 4). The cattle with antibodies to N. caninum (seropositive) are more likely to abort than seronegative cows, and most of the live-born calves from seropositive dams will be congenitally infected but clinically normal (5). Neosporosis-associated abortion problems in cattle may have an epidemic or endemic pattern. Some epidemiological studies show that epidemic N. caninum-associated abortions are caused by postnatal infection of naive cattle via the exposure of feed or water containing oocysts shed by the definitive host, dogs. In cattle herd with endemic abortion due to neosporosis, the major route of transmission is vertical between seropositive dams and calves (5).
The economic impact of N. caninum to the worldwide bovine industry underlies the importance of obtaining an accurate diagnosis of the disease (6). Consequently, numerous serological methods have been developed, including the immunofluorescent antibody test (IFAT) and the direct agglutination test, which use whole tachyzoite or whole parasite antigens, and the enzyme-linked immunosorbent assay (ELISA) and competitive inhibition ELISA (iELISA), which use antigenic proteins (7, 8). One of the major problems with serological diagnoses for Neospora infection is that there is no appropriate “gold standard” to detect the infected cattle (5). The detection of antibodies by the immunofluorescent antibody test (IFAT) can give false-positive results. In addition, since IFAT titers are largely dependent on the quality of the equipment used for fluorescence microscopy, it is often impossible to standardize the IFAT results among different laboratories. Most of the ELISA systems used to detect N. caninum-specific antibodies are based on total tachyzoite lysate antigens and recombinant antigens. ELISA with N. caninum lysate antigens showed a possibility of cross-reactivity between sera from animals infected with N. caninum, T. gondii, or Sarcocystis sp. (9). The ELISAs with recombinant antigens would become more important because they can be produced easier in large quantities and better standardized for the production of serological assays. A number of recombinant N. caninum antigens, such as NcGRA6, NcGRA7, NcSRS2, and NcSAG1, of potential diagnostic value have been published (5). NcSAG1 is one of the good antigens to detect antibodies against N. caninum in cattle (10). However, false-positive reactions from insufficiently purified recombinant proteins (11) and low specificity between infected and negative sera (12) have been reported. Hence, there is a strong drive for the development of reliable, sensitive, and specific diagnostic assays that use novel N. caninum-specific recombinant antigens.
The quest for antigens that can be used to diagnose N. caninum infection has focused on identification of immunodominant antigens from the parasite. Proteins containing tandem repeats can elicit strong humoral immune responses in the hosts of other apicomplexan parasites (13, 14). Tandem repeats are regions in a protein that are highly antigenic (15) and are considered major B-cell epitopes that are highly immunogenic (14). A previous study showed that the subtilisin-like serine protease 1 of N. caninum (NcSUB1) contains an internal region of 25 conserved amino acid (aa) repeats that are copied five consecutive times (16). Furthermore, another study demonstrated the antigenicity of a partial fragment of NcSUB1 called N54, which contains a single repeat element (17). These findings suggest that NcSUB1 could have potential for serodiagnosis of N. caninum; therefore, further analyses to determine the most antigenic regions of NcSUB1 are required. Thus, in this study, we constructed two fragments containing five copies of the tandem repeats of the antigen, namely, NcSUB1t (aa 524 to 843) and NcSUB1tr (aa 555 to 679), and expressed them as glutathione S-transferase (GST) fusion proteins in Escherichia coli. We evaluated their diagnostic potential against N. caninum infection by comparing the results obtained from ELISAs using these recombinant proteins with those obtained from established ELISAs based on NcSAG1t and N54.
MATERIALS AND METHODS
Parasite preparation.
N. caninum tachyzoites of the Nc-1 strain and T. gondii tachyzoites of the PLK strain were propagated using monolayers of African green monkey kidney (Vero) cells in Eagle's minimum essential medium (Sigma-Aldrich, St. Louis, MO) supplemented with 8% heat-inactivated fetal bovine serum. Tachyzoite purification was done by washing the parasites and host cell debris with cold phosphate-buffered saline (PBS). The final pellet was resuspended in cold PBS and passed through a 27-gauge needle and an MF-Millipore 5.0-μm-pore membrane filter (Millipore, Bedford, MA).
Construction and expression of recombinant NcSUB1 fragments.
Total RNA from pelleted parasites was isolated using TRIzol reagent (Gibco-BRL, Life Technologies, CA), while cDNA was synthesized utilizing an Invitrogen Superscript first-strand synthesis system for reverse transcription (RT)-PCR (Invitrogen, Carlsbad, CA). Procedures were performed according to the respective manufacturer's instructions. cDNA was used as a template to amplify the coding region of NcSUB1 (GenBank accession no. AF139114). Two NcSUB1 fragments, NcSUB1t and NcSUB1tr, were amplified using a set of oligonucleotide primers that included an EcoRI restriction enzyme site in the forward primer (NcSUB1t, 5′-AAG AAT TCT CAA CCC GAG TGA CAT GGG GC-3′; NcSUB1tr, 5′-AAG AAT TCC CGC CGT CTC ATC CTC CCC CC-3′) and an XhoI site in the reverse primer (NcSUB1t, 5′-GGC TCG AGT TAA GCA TGA CCT TCC GAT GAG TG-3′; NcSUB1tr, 5′-GGC TCG AGT TAC TGA GAA AAA GGA CGT TG-3′). A partial fragment of the NcSUB1 gene designated N54 (GenBank accession no. U76556) was cloned using a set of oligonucleotide primers that included an EcoRI restriction enzyme site in the forward primer (5′-AAG AAT TCC AAC ATC CTC ATC CTC ATC CT-3′) and an XhoI site in the reverse primer (5′-GGC TCG AGT TAT GGT GGA GAT GGC TCG ACA GC-3′). (In the sequences, restriction enzyme sites are in boldface.) PCR products were digested with EcoRI and XhoI before in-frame ligation into the GST fusion protein coding sequence of the Escherichia coli expression vector pGEX-4T1 (GE Healthcare, Buckinghamshire, United Kingdom), which had been digested with the same set of restriction enzymes. Plasmid nucleotide sequences were determined using an ABI 3100 DNA sequencer (Applied Biosystems, Foster City, CA). NcSAG1t was prepared as described previously (18). The recombinant proteins of NcSAG1t, NcSUB1t, NcSUB1tr, and N54 were expressed in E. coli as glutathione S-transferase (GST) fusion proteins and then purified using glutathione Sepharose 4B (Amersham Pharmacia Biotech, Sweden), as described previously (18). The molecular masses of the GST-fused proteins were confirmed through sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie brilliant blue staining (MP Biomedicals, Inc., France). After dialysis in cold PBS, the protein concentrations were measured using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, IL).
Serum samples.
Seven-week-old female BALB/c mice were purchased from CLEA Japan (Tokyo, Japan). All of the mice used in the present study were treated under the guiding principles for the care and use of research animals promulgated by Obihiro University of Agriculture and Veterinary Medicine, Japan. They were intraperitoneally inoculated with 1 × 105 tachyzoites of the N. caninum Nc-1 strain or 1 × 103 tachyzoites of the T. gondii PLK strain. The sera were separated from the clot 30 min after the blood collection from mice via the tail vein at 30 days after infection. The blood was centrifuged at 1,000 × g for 10 min, and the serum (20 μl) was collected and stored at −20°C until use to measure the levels of N. caninum-specific antibodies by ELISA. To confirm the lack of an antibody response in uninfected mice, control sera were taken from all of the animals on day 0 before they were infected with the parasites. The optical density (OD) values against NcSUB1 antigens and NcSAG1t in day 0 samples were similar to those in day 30 samples of uninfected animals. Bovine serum samples were previously obtained from four calves experimentally infected with 1 × 107 tachyzoites of the N. caninum Nc-1 strain and were serially collected on different days before and after infection (19). The serum samples from cows (n = 79) were obtained from one Holstein dairy herd of Tochigi, Japan, with a history of Neospora-associated abortions. The samples were collected during the period from November to December 2010. In this herd, 18 cases of abortion were observed during the period from June 2009 to November 2010. In November 2010, two fetal samples (cerebrum, cerebellum, and skeletal muscle) from this herd were examined using an immunohistochemical test, and N. caninum tachyzoites were detected in the lesions obtained from both animals. From the same herd, serum samples from one pregnant dam and from her calf, both of which had antibodies against NcSAG1t, were also obtained for analysis.
ELISA.
The ELISA was performed as described previously (20, 21). The GST-fused NcSUB1t, NcSUB1tr, N54, and NcSAG1t recombinant proteins and the GST control were diluted in coating buffer (50 mM carbonate-bicarbonate buffer [pH 9.6]) at a final concentration of 0.1 μM. Each well of a flat-bottomed 96-well microtiter plate (Nunc, Denmark) was coated with 50 μl of the corresponding protein and incubated overnight at 4°C. The subsequent steps were performed as previously described (21), except that the secondary antibodies used were horseradish peroxidase (HRP)-conjugated sheep anti-bovine (total IgG, IgG1, or IgG2) or anti-mouse total IgG antibody (Bethyl Laboratories) diluted at 1:10,000 (for anti-bovine) or 1:4,000 (for anti-mouse). Absorbance values were calculated as the difference in the mean optical densities at 415 nm (OD415) between the recombinant antigens (NcSUB1t, NcSUB1tr, N54, and NcSAG1t) and the GST protein. To define cutoff values, sera from a total of 25 cattle that were experimentally infected with N. caninum were used. Before the infection, the sera were confirmed negative for the parasite by IFAT. The 25 sera from uninfected cattle and 25 sera from the infected animals of three independent experiments were used for optimized cutoff selection by receiver operating characteristic (ROC) analysis: experiment 1, 42 days after intravenous infection with 1 × 107 tachyzoites (n = 9); experiment 2, 56 days after intravenous infection with 1 × 107 tachyzoites (n = 2) or 5 × 107 tachyzoites (n = 2); and experiment 3, 57 days after intravenous infection with 1 × 107 tachyzoites (n = 12). The cutoff values were 0.085 for anti-NcSUB1t IgG, 0.040 for anti-NcSUB1tr IgG, 0.043 for anti-N54 IgG, and 0.034 for anti-NcSAG1t IgG. Based on the cutoff values, the antibody titers of each antigen against 2× serially diluted sera from experimentally infected cattle (1:250 to 1:16,000) were calculated.
Statistical analyses.
ELISA was performed on the recombinant proteins to estimate their sensitivity and specificity. The percentage agreement was determined using kappa values with 95% confidence intervals (http:/vassarstats.net/) between the different antigens. The kappa values were used to assess the strength of agreement and were ranked as fair (0.21 to 0.40), moderate (0.41 to 0.60), or substantial (0.61 to 0.80) (22). Statistical differences were calculated by analysis of variance (ANOVA) followed by Tukey's multiple comparison with a 95% confidence interval (CI).
RESULTS
Expression of NcSUB1 fragments in E. coli.
The two newly designed fragments, NcSUB1t and NcSUB1tr, comprised 320 amino acids (aa), including those residues encoding the complete 5 copies of the tandem repeats (here called “125 aa”), and the 125-aa region containing the tandem repeats alone, respectively (Fig. 1A). The N54 protein comprised 135 aa and contained only 1 copy of the tandem repeat (Fig. 1A). As shown in Fig. 1B, the results of the SDS-PAGE analysis of the recombinant NcSUB1t, NcSUB1tr, and N54 GST fusion proteins were consistent with their estimated molecular masses (68, 42, and 42 kDa, respectively). To confirm whether the protein bands on lanes 1 to 3, slightly above or below the 30-kDa marker are cleavage fragments of the recombinant proteins, Western blotting using serum against N. caninum was performed. The result showed that the antiserum detected these minor bands, indicating the cleavage fragments of the recombinant NcSUB1 (data not shown).
Fig 1.
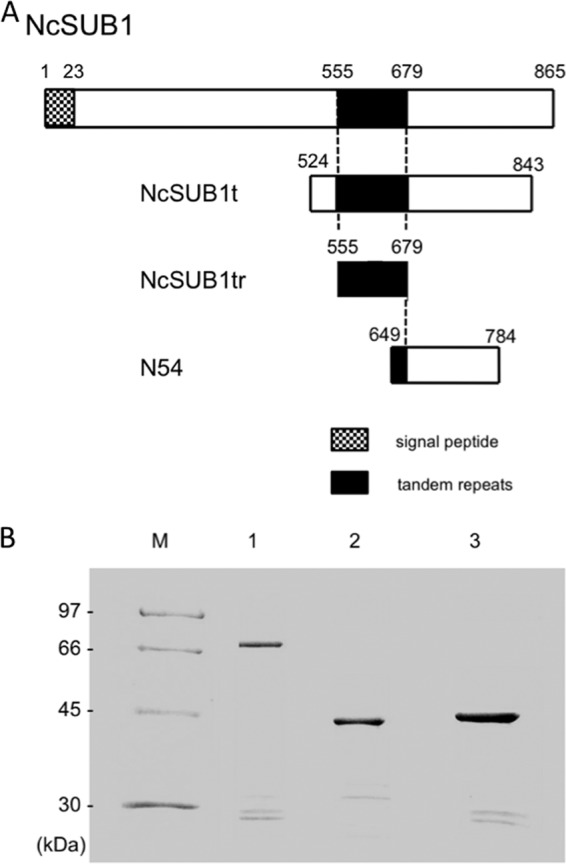
(A) Schematic depiction of the NcSUB1 fragments used in this study. The amino acid fragment lengths were 320, 125, and 136 for NcSUB1t, NcSUB1tr, and N54, respectively. The conserved 25-aa repeats are consecutively copied five times from aa 555 to 679. (B) SDS-PAGE analysis of purified recombinant NcSUB1t (lane 1), NcSUB1tr (lane 2), and N54 (lane 3). All of the recombinant proteins were GST fusions. M, molecular mass markers.
Evaluation of recombinant proteins for serodiagnosis of N. caninum.
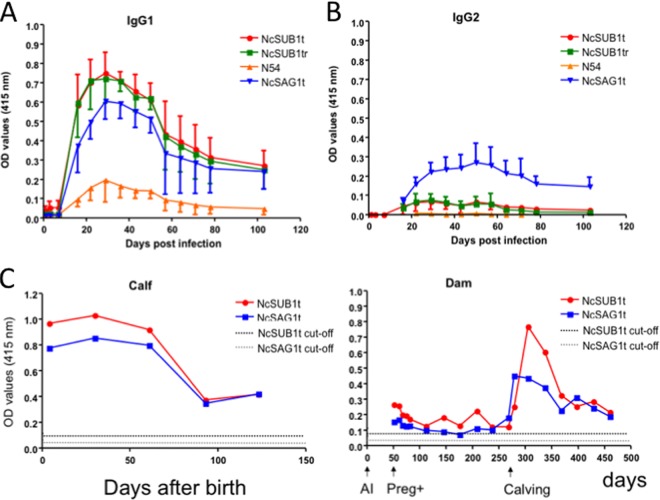
ELISAs were used to evaluate the diagnostic potential of the NcSUB1 fragments for N. caninum serodiagnosis and to compare their antigenicity against established parasite antigen NcSAG1t. The ELISAs measured the IgG1 and IgG2 antibody levels against the antigens in the sera of four experimentally infected calves. The results showed that all four proteins (NcSUB1t, NcSUB1tr, N54, and NcSAG1t) were able to bind the IgG1 antibodies that were present in the samples from 16 days postinfection (dpi), with peak levels being detectable at 36 dpi (Fig. 2A). NcSUB1t and NcSUB1tr had higher OD values than NcSAG1t although the differences in the OD values were not statistically significant. In contrast, the NcSAG1t ELISAs generated significantly higher IgG2 antibody OD values than the other three antigens (Fig. 2B). To confirm the difference in IgG1 antibody levels among the antigens, the sera from 16 dpi were tested at higher dilutions (Table 1). The results showed that at 1:8,000, NcSUB1t was still detectable, while NcSUB1tr and NcSAG1t were only detectable at dilutions of up to 1:2,000 and 1:1,000, respectively. In addition, N54 had a lower antibody titer than the other antigens. Next, the total IgG antibody levels against NcSUB1t and NcSAG1t in a Neospora-positive dam were monitored during pregnancy. Figure 2C shows the antibody production dynamics of a dam that successfully gave birth to a calf. Antibodies against each antigen were detectable from the start of the experiment, the levels of which peaked within a month after calving. The graph of the calf shows high antibody levels against both antigens during the first 2 months after birth. These antibody levels steadily decreased to reach an OD value of 0.4 3 months after birth. For specificity testing, the recombinant antigens were used to test the sera from 10 mice infected with N. caninum, 10 mice infected with T. gondii, and 10 uninfected mice (Fig. 3). All of the serum samples from the N. caninum-infected mice showed reactivity against the four truncated antigens, while negative results were obtained from the sera of uninfected and T. gondii-infected mice.
Fig 2.
IgG1 (A) and IgG2 (B) antibody production against NcSUB1t, NcSUB1tr, N54, and NcSAG1t in four cattle at 0, 1, 3, 7, 16, 22, 29, 36, 43, 50, 57, 64, 71, 78, and 103 days after infection with N. caninum tachyzoites. OD values are the means ± standard deviations (SD) from four cattle. (C) Antibody production dynamics in an N. caninum-seropositive pregnant dam and her calf. The cutoff values were 0.085 for anti-NcSUB1t IgG and 0.034 for anti-NcSAG1t IgG. AI, artificial insemination; Preg+, confirmed pregnant; calving, calf was alive. The dam was artificially inseminated on day 0, confirmed pregnant on day 51, and had the calf on day 275.
Table 1.
Comparison of the IgG antibody titers of NcSUB1t, NcSUB1tr, N54, and NcSAG1t against serially diluted sera from experimentally infected cattle
| Antigen | Antibody titera |
|||
|---|---|---|---|---|
| Calf 1 | Calf 2 | Calf 3 | Calf 4 | |
| NcSUB1t | 1:4,000 | 1:8,000 | 1:1,000 | 1:1,000 |
| NcSUB1tr | 1:1,000 | 1:1,000 | 1:1,000 | 1:2,000 |
| N54 | 1:1,000 | 1:250 | 1:250 | BC |
| NcSAG1t | 1:1,000 | 1:1,000 | 1:1,000 | 1:500 |
Serum samples used were at 16 days postinfection. The values shown represent the highest serum serial dilution by which specific antibodies against N. caninum were detected. The cutoff values are 0.085 for anti-NcSUB1t IgG and 0.040 for anti-NcSUB1tr. BC, below cutoff.
Fig 3.
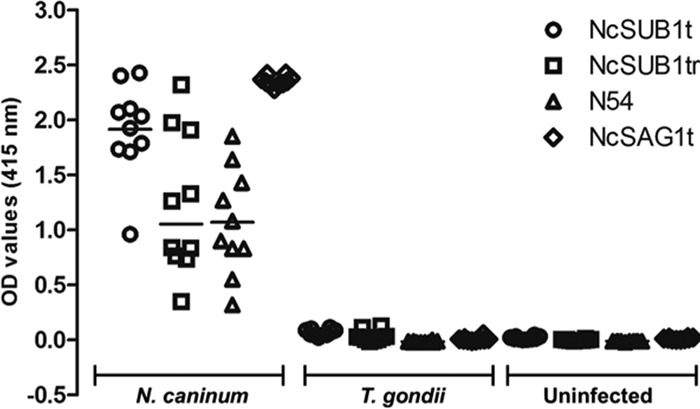
ELISA with recombinant antigens using sera from mice experimentally infected with N. caninum (n = 10) or T. gondii (n = 10) and sera from uninfected mice (n = 10).
Evaluation of NcSUB1 fragments using field samples of bovine sera.
Bovine serum samples were collected from a farm with confirmed Neospora abortion cases (Table 2). The ELISA results from these samples revealed that NcSUB1t and NcSUB1tr detected more N. caninum-positive samples than N54. The sensitivity of the three NcSUB1 antigen fragments was also compared by ELISA using NcSAG1t as the reference, and NcSUB1 antigens had higher sensitivity (Table 3). For specificity, the ELISA with NcSUB1t and NcSUB1tr showed higher values, while N54 assay showed the lowest values (Table 3). The ELISA results using the recombinant NcSUB1tr were consistent with those from NcSAG1t, as evidenced by the kappa values (Table 3). By comparison of seropositive cattle, ELISA using NcSUB1t showed higher antibody levels (Fig. 4).
Table 2.
Comparison of the results for field bovine samples from a farm with confirmed abortion cases examined by NcSUB1 fragments and NcSAG1t
| ELISA (NcSAG1t) result | No. (%) of samples with ELISA resulta: |
||||||
|---|---|---|---|---|---|---|---|
| NcSUB1t |
NcSUB1tr |
N54 |
Total | ||||
| Positive | Negative | Positive | Negative | Positive | Negative | ||
| Positive | 70 | 1 | 71 | 0 | 60 | 11 | 71 (89.9) |
| Negative | 4 | 4 | 3 | 5 | 1 | 7 | 8 (10.1) |
| Total | 74 (93.7) | 5 (6.3) | 74 (93.7) | 5 (6.3) | 61 (77.2) | 18 (22.8) | 79 (100) |
Samples with OD415 values for the specific IgG higher than the cutoff values (NcSUBt, 0.085; NcSUB1tr, 0.040; N54, 0.043; NcSAG1t, 0.034) were judged positive.
Table 3.
Specificity and sensitivity of the ELISAs using recombinant NcSUB1 fragments compared with the ELISA results with the reference antigen, NcSAG1ta
| Antigen | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Kappa value (95% CI) |
|---|---|---|---|
| NcSUB1t | 94.59 (86.02–98.25) | 80.00 (29.88–98.95) | 0.58 (0.23–0.94) |
| NcSUB1tr | 95.95 (87.82–98.95) | 100 (46.29–100) | 0.75 (0.47–1) |
| N54 | 98.36 (90.02–99.90) | 38.89 (18.26–63.86) | 0.46 (0.18–0.74) |
Shown are the specificity and sensitivity of the ELISAs using recombinant NcSUB1 fragments for detection of the IgG antibodies to infection in field bovine sera compared with the ELISA results with the reference antigen, NcSAG1t (n = 79). Kappa values with 95% confidence intervals (CI) were computed using http:/vassarstats.net/. The strength of agreement between antigens was graded with kappa values of fair (0.21 to 0.40), moderate (0.41 to 0.60), and substantial (0.61 to 0.80).
Fig 4.
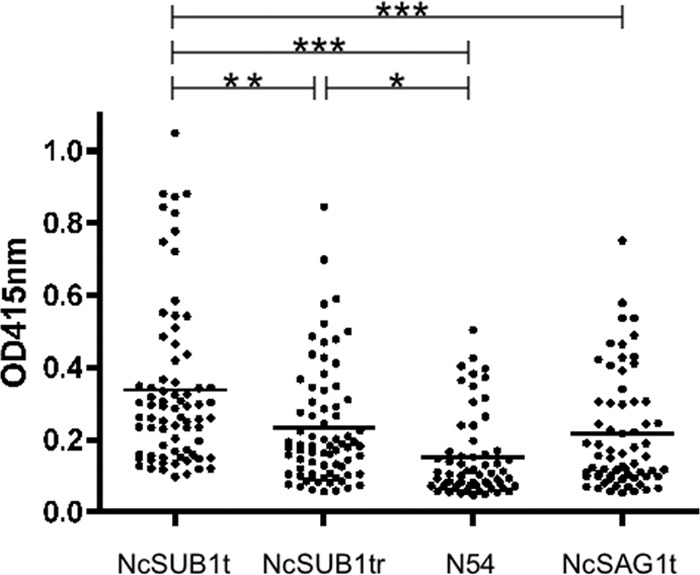
ELISA with recombinant antigens using sera from a farm with confirmed Neospora-associated abortion. Horizontal bars represent the averages for seropositive samples from each experimental group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
N. caninum has a similar morphology to T. gondii (3, 4); nevertheless, specificity testing and BLAST homology analyses of NcSUB1 indicate that this protein is highly conserved in N. caninum and exhibits only 63% sequence identity to the T. gondii orthologue. In addition, the tandem repeats in NcSUB1 are not found in T. gondii SUB1. This implies that the NcSUB1 tandem repeats are specific to N. caninum, and cross-reactions with the closely related parasite T. gondii are less likely to occur if they are used as diagnostic antigens. In fact, specificity testing using sera from mice experimentally infected with T. gondii indicates that the NcSUB1t and NcSUB1tr recombinant proteins did not cross-react with T. gondii, a result that is similar to those obtained for N54 (17) and NcSAG1t (18). The newly developed fragments (NcSUB1t and NcSUB1tr) were designed to include all copies of the NcSUB1 tandem repeats, unlike N54, which contains only 1 copy of the conserved repeats. The use of N54 as an antigen for ELISA using a panel of bovine sera as the gold standard revealed its high sensitivity (95%) and specificity (96%) (17). Our proteomic study that tested sera from Neospora abortion and nonabortion cases showed that NcSUB1 was consistently expressed in all of the cases along with other established serodiagnostic antigens, NcSAG1, NcSRS2, and NcGRA7 (data not shown). This suggested that recombinant NcSUB1 containing all 5 copies of the tandem repeats would have good antigenicity. We surmised that the higher density/number of epitopes in the tandem repeats may facilitate B-cell activation, leading to higher antibody production and greater antigenicity. In the present study, the antigenicity of NcSUB1t and NcSUB1tr was higher than that of N54, suggesting a positive association between the copy number of the conserved repeats and specific antibody production. Moreover, we revealed that anti-NcSUB1 IgG1 antibodies were detectable at higher levels from 1 to 2 months after the infection with N. caninum.
Comparison of the performances of the different antigens in the ELISAs on the bovine serum samples from a farm with confirmed Neospora-related abortions revealed that in terms of their sensitivity and specificity, NcSUB1 containing five copies of the tandem repeats was the reliable antigen, while N54 containing a single copy was the least reliable. The NcSUB1t protein consistently yielded higher OD values than the reference ELISA using NcSAG1t, as shown in Fig. 4. This implies that NcSUB1t has the potential to perform better than N54 and reference standard NcSAG1t. Therefore, NcSUB1t will be a useful serodiagnostic tool for detection of N. caninum-specific antibodies in field samples. The IgG antibody production dynamics in pregnant cattle were similar for NcSUB1t and NcSAG1t. Interestingly, the antibody levels against both of these antigens increased dramatically during parturition (Fig. 2C). Moreover, antibody transfer related to both of these antigens was detected in the calf. Thus, sampling periods during pregnancy should be considered in diagnoses using ELISA with the recombinant proteins NcSUB1 and NcSAG1.
As N. caninum can cause significant economic losses to cattle farmers, specific and sensitive detection of this pathogen is very important. Currently, several antigenic proteins, such as NcSAG1t and NcGRA7, are used in serodiagnostic assays for bovine neosporosis (21). The present study illustrates the diagnostic potential of NcSUB1, which contains five copies of tandem repeat elements, to complement or improve current serodiagnosis.
ACKNOWLEDGMENTS
We thank J. P. Dubey (United States Department of Agriculture, Agriculture Research Service, Livestock and Poultry Sciences Institute, and Parasite Biology and Epidemiology Laboratory) for the gift of the Neospora caninum Nc-1 isolate to us. We also thank Adrian P. Ybañez (United Graduate School of Veterinary Sciences, Gifu University) for excellent technical assistance.
This research was supported by the Japan Society for the Promotion of Science (JSPS) through the Funding Program for Next Generation World-Leading Researchers (NEXT Program), initiated by the Council for Science and Technology Policy (CSTP) (2011/LS003).
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Dubey JP, Lindsay DS. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1–59 [DOI] [PubMed] [Google Scholar]
- 2.Hemphill A. 1999. The host-parasite relationship in neosporosis. Adv. Parasitol. 43:47–104 [DOI] [PubMed] [Google Scholar]
- 3.Dubey JP, Hattel AL, Lindsay DS, Topper MJ. 1988. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J. Am. Vet. Med. Assoc. 193:1259–1263 [PubMed] [Google Scholar]
- 4.Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. 1988. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 192:1269–1285 [PubMed] [Google Scholar]
- 5.Dubey JP, Schares G. 2006. Diagnosis of bovine neosporosis. Vet. Parasitol. 140:1–34 [DOI] [PubMed] [Google Scholar]
- 6.Dubey JP. 2005. Neosporosis in cattle. Vet. Clin. North Am. Food Anim. Pract. 2:473–483 [DOI] [PubMed] [Google Scholar]
- 7.Dubey JP, Jenkins MC, Adams DS, McAllister MM, Anderson-Sprecher R, Baszler TV, Kwok OCH, Lally NC, Bjorkman C, Uggla A. 1997. Antibody responses of cows during an outbreak of neosporosis evaluated by indirect fluorescent antibody test and different enzyme-linked immunosorbent assays. J. Parasitol. 83:1063–1069 [PubMed] [Google Scholar]
- 8.Jenkins M, Baszler T, Bjorkman C, Schares G, Williams D. 2002. Diagnosis and seroepidemiology of Neospora caninum-associated bovine abortion. Int. Parasitol. 32:631–636 [DOI] [PubMed] [Google Scholar]
- 9.Baszler TV, Knowles DP, Dubey JP, Gay JM, Mathison BA, McElwain TF. 1996. Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 34:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang P, Liao M, Zhang H, Lee EG, Nishikawa Y, Xuan X. 2007. Dense-granule protein NcGRA7, a new marker for the serodiagnosis of Neospora caninum infection in aborting cows. Clin. Vaccine Immunol. 14:1640–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins MC, Fetterer R, Schares G, Bjorkman C, Wapenaar W, McAllister M, Dubey JP. 2005. HPLC purification of recombinant NcGRA6 antigen improves enzyme-linked immunosorbent assay for serodiagnosis of bovine neosporosis. Vet. Parasitol. 131:227–234 [DOI] [PubMed] [Google Scholar]
- 12.Howe DK, Tang K, Conrad PJPA, Sverlow K, Dubey JP, Sibley LD. 2002. Sensitive and specific identification of Neospora caninum infection of cattle based on detection of serum antibodies to recombinant Ncp29. Clin. Diagn. Lab. Immunol. 9:611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp DJ, Coppel RL, Anders RF. 1987. Repetitive proteins and genes of malaria. Annu. Rev. Microbiol. 41:181–208 [DOI] [PubMed] [Google Scholar]
- 14.Goto Y, Coler RN, Guderian J, Mohamath R, Reed SG. 2006. Cloning, characterization, and serodiagnostic evaluation of Leishmania infantum tandem repeat proteins. Infect. Immun. 74:3939–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakhaiyar P, Nagalakshimi Y, Aruna B, Murthy KJR, Katoch VM, Hasnain SE. 2004. Regions of high antigenicity within the hypothetical PPE major polymorphic tandem repeat open reading frame, RV2608. J. Infect. Dis. 190:1237–1244 [DOI] [PubMed] [Google Scholar]
- 16.Louie K, Conrad PA. 1999. Characterization of a cDNA encoding a subtilisin-like serine protease (NC-p65) of Neospora caninum. Mol. Biochem. Parasitol. 103:211–223 [DOI] [PubMed] [Google Scholar]
- 17.Louie K, Sverlow KW, Barr BC, Anderson ML, Conrad PA. 1997. Cloning and characterization of two recombinant Neospora protein fragments and their use in serodiagnosis of bovine neosporosis. Clin. Diagn. Lab. Immunol. 4:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chahan B, Gaturaga I, Huang X, Liao M, Fukumoto S, Hirata H, Nishikawa Y, Suzuki H, Sugimoto C, Nagasawa H, Fujisaki K, Igarashi I, Minami T, Xuan X. 2003. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet. Parasitol. 118:177–185 [DOI] [PubMed] [Google Scholar]
- 19.Nishimura M, Kohara J, Hiasa J, Muroi Y, Yokoyama N, Kida K, Xuan X, Furuoka H, Nishikawa Y. 2013. Tissue distribution of Neospora caninum in experimentally infected cattle. Clin. Vac. Immunol. 20:309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiasa J, Nishimura M, Itamoto K, Xuan X, Inokuma H, Nishikawa Y. 2012. ELISAs based on Neospora caninum dense granule protein 7 and profilin for estimating the stage of neosporosis. Clin. Vaccine Immunol. 19:411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiasa J, Kohara J, Nishimura M, Xuan X, Tokimitsu H, Nishikawa Y. 2012. ELISAs based on rNcGRA7 and rNcSAG1 antigens as an indicator of Neospora caninum activation. Vet. Parasitol. 187:379–385 [DOI] [PubMed] [Google Scholar]
- 22.Viera AJ, Garrett JM. 2005. Understanding interobserver agreement: the kappa statistic. Fam. Med. 37:360–363 [PubMed] [Google Scholar]