Abstract
Infection with Vibrio cholerae and oral cholera vaccines (OCVs) induce transient circulating plasmablast responses that peak within approximately 7 days after infection or vaccination. We previously demonstrated that plasmablast responses strongly correlate with subsequent levels of V. cholerae-specific duodenal antibodies up to 6 months after V. cholerae infection. Hence, plasmablast responses provide an early window into the immunologic memory at the mucosal surface. In this study, we characterized plasmablast responses following V. cholerae infection using a flow cytometrically defined population and compared V. cholerae-specific responses in adult patients with V. cholerae O1 infection and vaccinees who received the OCV Dukoral (Crucell Vaccines Canada). Among flow cytometrically sorted populations of gut-homing plasmablasts, almost 50% of the cells recognized either cholera toxin B subunit (CtxB) or V. cholerae O1 lipopolysaccharide (LPS). Using a traditional enzyme-linked immunosorbent spot assay (ELISPOT), we found that infection with V. cholerae O1 and OCVs induce similar responses to the protein antigen CtxB, but responses to LPS were diminished after OCV compared to those after natural V. cholerae infection. A second dose of OCV on day 14 failed to boost circulating V. cholerae-specific plasmablast responses in Bangladeshi adults. Our results differ from those in studies from areas where cholera is not endemic, in which a second vaccination on day 14 significantly boosts plasmablast responses. Given these results, it is likely that the optimal boosting strategies for OCVs differ significantly between areas where V. cholerae infection is endemic and those where it is not.
INTRODUCTION
Vibrio cholerae serogroups O1 and O139 are the major causes of cholera, a disease that can manifest as life-threatening severe watery diarrhea. Cholera is endemic in >50 countries and results in 100,000 to 130,000 deaths per year (1). V. cholerae O1 organisms are further divided into the El Tor or classical biotypes based on biochemical and phenotypic differences; more recently, an altered variant of El Tor strains which harbor the classical type of cholera toxin was also found. The increasing number of cases of cholera over the last decade and the emergence of increasingly pathogenic variants of V. cholerae O1 El Tor suggest that an emphasis on preventative strategies, such as vaccination, is warranted (2, 3).
There are two current internationally licensed oral cholera vaccines (OCVs): a killed V. cholerae O1 vaccine supplemented with recombinant cholera toxin B subunit (CtxB) (Dukoral; Crucell Vaccines Canada) and a bivalent killed V. cholerae O1/O139 vaccine that does not contain additional CtxB (Shanchol; Shantha Biotechnics) (1). Both vaccines are safe and induce significant protection when administered in a two- or three-dose schedule. In 2010, the WHO recommended the use of these vaccines in conjunction with other preventative strategies in areas where cholera is endemic (1).
Despite their clear benefits in preventing cholera, current OCVs have relative immunological limitations compared to natural infection. The requirement for multiple doses is one limitation, particularly in settings experiencing epidemic cholera. In addition, the protective immunity afforded by OCVs may wane more rapidly than the protection afforded by V. cholerae infection. Dukoral provides an initial protection of 60 to 85% in older children and adults, but protection wanes within 2 years (4). Dukoral also provides limited protection in children younger than 5 years (4). This is important since children are most affected by cholera in regions where it is endemic (5). In contrast, a single symptomatic infection with pathogenic V. cholerae has been shown to induce protection against the recurrence of moderate-to-severe illness for 5 to 10 years in both adults and children (6–8). Shanchol has not been as extensively studied as Dukoral, although a phase III trial in Kolkata, India, suggests that Shanchol may provide longer-lasting immunity than Dukoral (9).
We previously compared V. cholerae-specific antibody and memory B-cell responses in patients with severe V. cholerae infection and recipients of Dukoral (10). While patients and vaccinees had comparable antibody responses, only the patients developed longer-lasting memory B-cell responses (10). This suggests a potential mechanism for the longer duration of immunity following V. cholerae infection than that from OCVs, as memory B cells may provide long-term protection upon re-exposure to toxigenic V. cholerae (11). Given this finding, it is likely that B-cell responses diverge at an early juncture in response to natural V. cholerae infection or inactivated OCVs. Understanding the early events that lead to long-term memory B-cell responses may point to novel strategies for improving current cholera vaccines.
Previously, it has been demonstrated that both infection with V. cholerae and OCVs induce transiently circulating antibody-secreting cell (ASC) responses that peak approximately 7 days after infection or vaccination. Many of these circulating ASCs express gut-homing markers (12), suggesting that upon terminal differentiation, some of these cells ultimately reside as long-lived blood plasma cells that may be the main effectors of immunologic memory in the gut. In support of this, we have demonstrated that these early (day 7 postinfection/postvaccination) lipopolysaccharide (LPS)-specific ASC responses strongly correlate with subsequent levels of antibodies in the small intestinal lamina propria (r = 0.78, P = 0.008), as well as numbers of duodenal plasma cells (r = 0.77, P = 0.04) up to 6 months after V. cholerae infection (13). Hence, these transiently circulating gut-homing ASCs may provide an early window into the immunologic memory at the mucosal surface.
Despite their importance, a direct comparison of the V. cholerae-antigen-specific ASC responses in patients and vaccinees has not been previously reported. To understand if these early B-cell responses differ between V. cholerae infection and vaccination, we directly measured and compared circulating V. cholerae-antigen-specific antibody-secreting cell (ASC) responses in patients with severe V. cholerae O1 infection and in vaccinees who received Dukoral in adults from Bangladesh.
MATERIALS AND METHODS
Study design and subject enrollment.
The study was conducted at the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh, between October 2011 and March 2012. We enrolled adult patients with cholera who presented to the Dhaka icddr,b hospital with severe acute watery diarrhea, as well as a cohort of adult vaccinees from Dhaka, Bangladesh, who were participants in a study of immune responses to OCVs (trade name Dukoral). For patients with culture-confirmed V. cholerae O1 infection, blood samples were obtained during an acute infection phase. The acute sample is designated day 2, referring to the day of culture confirmation. Additional patient blood samples were obtained during convalescence on days 7 and 30. The vaccinees were administered two doses of the OCV on days 0 and 14, according to the manufacturer's guidelines. We obtained preimmunization blood samples from vaccinees (day 0) and again on days 7 and 21 (7 days after the first and second immunizations, respectively). The study was approved by the Research Review Committee and Ethical Review Committee of the icddr,b, Dhaka, Bangladesh, and the Institutional Review Board (IRB) of the Massachusetts General Hospital. Written informed consent was obtained from each of the patients and vaccinees.
Isolation of PBMCs.
Peripheral blood mononuclear cells (PBMCs) were isolated by differential centrifugation on Ficoll-Isopaque (Pharmacia, Piscataway, NJ). The separated PBMCs were then washed, counted, and suspended at a concentration of 1 × 107 cells/ml in RPMI complete medium (Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT).
Quantification of circulating IgG, IgA, and IgM ASCs.
To enumerate circulating IgG, IgA, and IgM ASCs, we used a previously described enzyme-linked immunosorbent spot (ELISPOT) assay (14, 15). Nitrocellulose-bottom plates (Mahan-4550; Millipore, Bedford, MA) were coated with GM1 ganglioside (3 nM/ml), LPS (25 μg/ml), affinity-purified goat anti-human Ig (total Ig) (Jackson ImmunoResearch, West Grove, PA) (5 μg/ml), and keyhole limpet hemocyanin (KLH) (a negative-control antigen, 2.5 μg/ml; Pierce Biotechnology, Rockford, IL). The plates were incubated overnight at 4°C. On the next day, recombinant CtxB was applied to the GM1-coated plates followed by 1 h incubation at 37°C. All plates were then blocked with RPMI 1640 containing 10% fetal bovine serum for 2 h at 37°C. A total of 5 × 105 PBMCs were added to the CtxB-, LPS-, and KLH-coated wells, whereas 1 × 105 PBMCs were added to total Ig-coated wells along with corresponding 10-fold serial dilutions to determine the total number of ASCs of each isotype. The plates were incubated for 3 h at 37°C with 5% CO2. Isotype-specific horseradish peroxidase-conjugated mouse anti-human antibodies (Hybridoma Reagent Laboratory, Baltimore, MD) were then added at a 1:500 dilution. After overnight incubation, the plates were washed and developed with 3-amino-9-ethylcarbazole (AEC). The ASCs were enumerated by two independent observers who were unaware of each other's data.
Analytical flow cytometry and cell sorting.
PBMCs were stained with CD3 AmCyan (BD Biosciences), CD19 PE-Texas Red (Invitrogen), CD20 fluorescein isothiocyanate (FITC) (Invitrogen), CD27 APC-Cy7 (BioLegend), CD38 PE-CY7 (BD Biosciences), and CCR9 Alexa Fluor 647 (BD Biosciences). The cells were incubated with the labeling antibodies for 45 min at 4°C. The total and gut-homing plasmablasts were gated as CD3− CD20−/low CD19+ CD27high CD38high and CD3− CD20−/low CD19+ CD27high CD38high CCR9high, respectively. The cells were gated on an extended lymphocyte gate to include blasting cells. Our flow cytometric procedure has been shown to separate live from dead cells in an efficient manner. Analyses of flow cytometric data were performed with FlowJo software (version 8.5.3; TreeStar, Inc.). The total and gut-homing plasmablasts were sorted into collection tubes containing 2% fetal calf serum (FCS) in phosphate-buffered saline (PBS) and used for ELISPOTs as described above.
CtxB- and LPS-specific IgA antibodies in vaccinees.
We measured the IgA-specific antibody responses in vaccinees by enzyme-linked immunosorbent assay (ELISA), as described previously (10, 16, 17). In brief, for LPS ELISAs, 96-well polystyrene plates (Nunc F; Nunc, Denmark) were coated with V. cholerae O1 serotype Ogawa LPS (250 ng/well). For the anti-CtxB ELISA, the plates were coated with ganglioside GM1 (0.3 nmol/ml) followed by recombinant CtxB subunit (50 ng/well). For both antigens, 100 μl of plasma (diluted 1:200 for CtxB and 1:50 for LPS) was added per well. Secondary antibodies (conjugated to horseradish peroxidase) to human IgA (Jackson ImmunoResearch, West Grove, PA) were then applied. The plates were developed with ortho-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.1% hydrogen peroxide. The plates were read kinetically at 450 nm for 5 min. The maximal rate of optical density change was expressed as milli-absorbance units per minute. The ELISA units were normalized by calculating the ratio of the test sample to a standard of pooled convalescent-phase sera from patients who had recovered from cholera.
Statistical analyses.
A comparison of the immunological responses at different time points was assessed by using the Wilcoxon signed-rank test, and a comparison between patients and vaccinees was performed using the Mann-Whitney U test. A two-tailed P value of ≤0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 5.0 (San Diego, CA).
RESULTS
Study population.
We enrolled nine adults presenting to the icddr,b hospital with acute watery diarrhea. All of the patients had a stool culture that was positive for V. cholerae O1 serotype Ogawa. All eight vaccinees who were enrolled received two doses of the OCV Dukoral 14 days apart. The demographic characteristics for all the study participants (patients and vaccinees) are presented in Table 1. The age and gender distributions of the patients and the vaccinees were similar, but the patients were more likely to possess the blood group O phenotype than the vaccinees.
Table 1.
Demographic and clinical characteristics of patients and vaccinees
| Characteristics | Patients (n = 9) | Vaccinees (n = 8) |
|---|---|---|
| Median age (range) (yr) | 25.6 (18–42) | 24.5 (23–28) |
| Gender (no. [%]) | ||
| Females | 4 (44.5) | 4 (50.0) |
| Males | 5 (55.5) | 4 (50.0) |
| Blood type (no. [%]) | ||
| A | 1 (11.1) | 2 (25) |
| B | 0 (0) | 5 (62.5) |
| O | 6 (66.7) | 1 (12.5) |
| AB | 2 (22.2) | 0 (0) |
Total and gut-homing ASC responses.
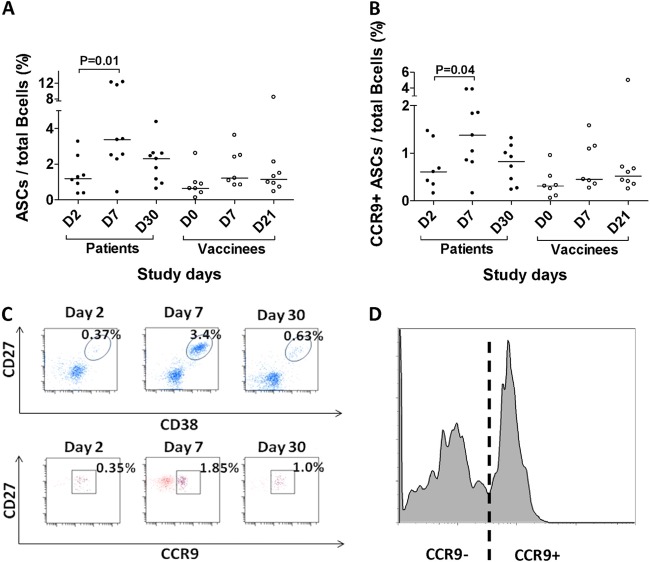
We compared the total circulating and gut-homing ASCs as a proportion of the B-cell compartment after V. cholerae infection and vaccination, as shown in Fig. 1. The proportion of circulating total and gut-homing ASCs increased significantly at day 7 following V. cholerae infection, but no relative increase in the total or gut-homing ASC populations was seen following vaccination (Fig. 1A and B). The increase in circulating ASCs after V. cholerae infection was transient, peaking at day 7 and returning to baseline at day 30 following infection. During the peak in patients on day 7, analytic flow cytometry demonstrated a distinct population of CD3− CD20−/low CD19+ CD27high CD38high cells consistent with a plasmablast population (Fig. 1C), as well as a clear bimodal distribution of CCR9 staining that was consistent with distinct intestinal homing and nonintestinal homing populations of plasmablasts in the peripheral circulation following V. cholerae infection (Fig. 1D).
Fig 1.
V. cholerae infection is associated with an increase in total and intestinal homing ASCs. The proportions of ASCs (CD3− CD20−/low CD19+ CD27high CD38high) (A) and intestinal homing ASCs (CD3− CD20−/low/CD19+ CD27high CD38high CCR9high) (B) relative to the total number of B cells (CD19+ CD20+) were compared in cholera patients and oral cholera vaccine (OCV) recipients at various time points. Patients with cholera demonstrated a significant increase in the proportion of ASCs and intestinal homing ASCs 7 days after acute infection, while one or two doses of OCV vaccine did not result in significant increases in ASCs as a fraction of the total circulating B cells. (C) The total and intestinal homing (inset ovals [top] and squares [bottom]) ASC responses are shown for a representative patient. (D) Bimodal distribution of CCR9 expression in ASCs from this patient showing a clearly demarcated intestinal homing population.
V. cholerae-specific ASC responses in PBMCs and correlation with serum IgA responses.
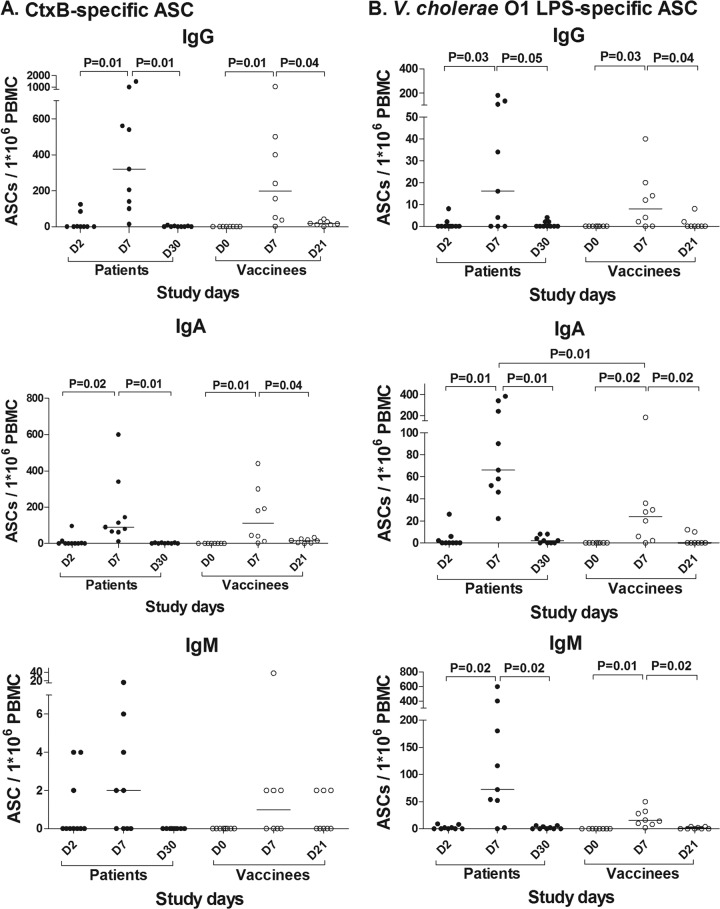
To measure V. cholerae-antigen-specific ASC responses following natural cholera and vaccination, we performed IgG, IgA, and IgM ELISPOTs, as shown in Fig. 2. For both patients and vaccinees, antigen-specific ASC responses peaked on day 7 after infection or vaccination, respectively. Although most patients had no detectable antigen-specific IgG, IgA, or IgM ASCs on day 2, some patients had detectable ASCs at this time point, suggesting these individuals developed early ASC responses to V. cholerae that are consistent with a rapid anamnestic response (18).
Fig 2.
Both V. cholerae infection and OCV induce significant V. cholerae antigen-specific ASC responses. (A) Cholera patients and OCV recipients demonstrated significant and comparable increases in CtxB-specific ASC responses in both the IgG and IgA isotypes. There was no significant IgM ASC response to CtxB. (B) Cholera patients and OCV recipients demonstrated significant increases in V. cholerae O1 LPS-specific ASC responses across all isotypes. Notably, V. cholerae antigen-specific ASC responses were not seen on day 21, after a second dose of OCV administered on day 14, suggesting that a second dose does not further boost ASC responses in a population from an area where cholera has historically been endemic. Significant differences (values of P ≤ 0.05 in brackets) between different time points or groups are shown.
While almost all vaccinees demonstrated significant increases in circulating ASCs a week after their initial vaccination, ASC responses were not seen in the peripheral circulation a week after the second dose of OCV. Thus, a second dose of OCV on day 14 did not appear to boost ASC responses to V. cholerae antigens.
A comparison of the peak ASC responses in patients and vaccinees demonstrated that the groups had CtxB-specific ASCs responses that were of a similar magnitude (Fig. 2A). In contrast to CtxB responses, patients with V. cholerae infection appeared to have greater V. cholerae-specific LPS responses than vaccinees; however, this difference only attained statistical significance for IgA ASCs (Fig. 2B). No significant IgM ASC responses to the T-cell-dependent antigen CtxB were observed in either patients or vaccinees. Of note, we found that both CtxB IgA and LPS IgA antibody titers were strongly correlated with CtxB- and LPS-specific IgA ASC responses in serum (for CtxB, r = 0.78, P = 0.02; for LPS, r = 0.97, P < 0.001).
V. cholerae-specific ASC responses measured in sorted total and intestinal homing plasmablasts.
To compare the distributions of V. cholerae-antigen-specific ASCs in the intestinal homing and overall plasmablast populations, we sorted PBMCs on day 7 after V. cholerae infection into CD3− CD20−/low CD19+ CD27high CD38high (plasmablast) and CD3− CD20−/low CD19+ CD27high CD38high CCR9high (gut-homing plasmablast) populations (Fig. 3A). We then characterized V. cholerae antigen specificities and antibody isotypes of the sorted cells by ELISPOT, as shown in Fig. 3B.
Fig 3.
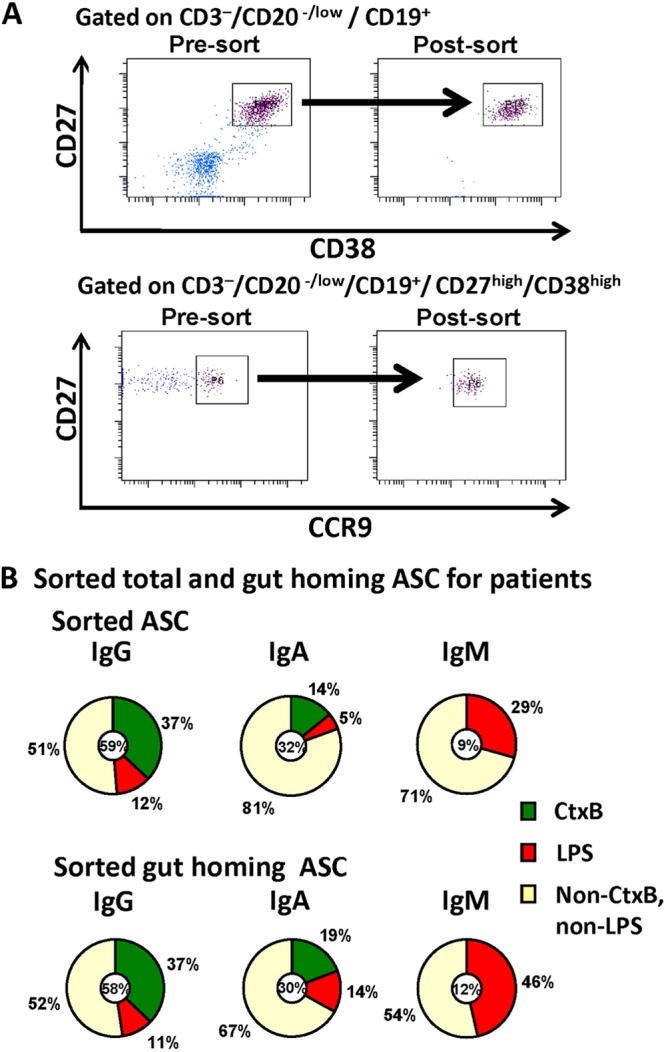
Isolation and characterization of sorted total and intestinal homing ASCs during the peak in the peripheral circulation on day 7 after cholera demonstrate a high proportion of circulating ASCs are V. cholerae-antigen specific. (A) Circulating ASCs and intestinal homing (inset squares/rectangles) ASCs were isolated from cholera patients by flow cytometry as shown, for characterization by ELISPOT assay. (B) The distribution of isotypes among sorted ASC populations is shown by the small circle at the center of each pie chart, which also indicates the proportion of ASCs that are specific for V. cholerae O1 LPS and CtxB. A high proportion of the isolated ASCs recognized these two immunodominant V. cholerae antigens. V. cholerae O1 LPS-specific IgA responses comprised a significantly greater proportion of the intestinal homing ASC responses (5% in all ASCs versus 14% in gut-homing ASCs) (P = 0.03).
Both the total plasmablast and intestinal homing plasmablast populations had similar isotype distributions, with the IgG isotype predominating (approximately 60%) in both populations. Antigen-specific responses to V. cholerae O1 LPS and CtxB comprised a remarkably high proportion of both the total and intestinal homing plasmablasts, with approximately 50% of the sorted plasmablasts recognizing these two antigens alone.
An analysis of antigen specificity of the sorted cells showed that the frequencies of cells secreting CtxB- and LPS-specific IgG isotype antibodies were comparable in both the total and gut-homing plasmablast populations. However, the percentage of cells secreting LPS-specific IgA isotype antibodies was higher in the gut-homing plasmablasts than that in the overall plasmablast population (P = 0.03).
DISCUSSION
Our study is the first, to our knowledge, to characterize the plasmablast response following V. cholerae infection using a flow cytometrically identified population. We found that even though V. cholerae is a noninvasive organism, infection with toxigenic V. cholerae still induces a remarkably large circulating plasmablast response when defined by flow cytometric parameters. Not only does V. cholerae O1 infection result in a transient increase in the total proportion of intestinal homing plasmablasts in the peripheral circulation a week after infection, but almost 50% of these plasmablasts recognize two V. cholerae antigens: CtxB and V. cholerae O1 LPS. Since there are many additional antigens in V. cholerae, the proportion that is specific to antigens in this organism overall is likely even higher.
Thus, mucosal infection with V. cholerae generates a robust plasma cell response that is similar to that seen following systemic infection or vaccination. Although transient, circulating plasmablast responses to systemic infection are of substantial magnitude (19–21); for example, during acute dengue fever, virus-specific plasmablasts comprise an average of 30% of all circulating lymphocytes but then are undetectable a month after illness (20). Similarly, 7 days following influenza vaccination, as many as 80% of circulating plasmablasts are influenza virus specific (21). The characterization of these circulating plasmablast responses at the phenotypic and immunogenetic levels has revealed fundamental insights into the generation of B-cell responses following antigenic exposure (19–22). This suggests that additional characterization of the plasmablast population following V. cholerae mucosal infection and vaccination, particularly the distinct intestinal homing population we observed, may provide a similar window into how early mucosal B-cell responses are generated.
Comparing ASC responses in patients and vaccinees, we found that infection with V. cholerae O1 and a single dose of the OCV induce a similar magnitude of ASC responses to the T-cell-dependent antigen CtxB. This is consistent with our earlier results that circulating antibody responses to CtxB are similar after V. cholerae infection and Dukoral vaccination (10). However, unlike T-cell-dependent CtxB responses, we found that ASC responses to LPS were diminished after the first dose of OCV compared to in natural V. cholerae infection. Unlike responses to CtxB that are dependent on germinal center programming by T follicular helper cells, responses to LPS may be initiated directly by lamina propria B cells. Given that protective immunity to V. cholerae is largely dependent on the response to the O-specific polysaccharide (OPS) component of LPS, it may be most important to boost OPS-specific ASC responses after vaccination (16).
For this reason, an unexpected and significant finding in our study was that a second dose of the OCV Dukoral on day 14 failed to boost circulating ASC responses to both CtxB and LPS in Bangladeshi adults. Our results are different from those of a previous study in Swedish volunteers that demonstrated a significant boosting of CtxB responses after a second dose of a similar OCV (23). However, our results match those of a previous study in Bangladesh that demonstrated limited ASC responses after a second dose of OCV (they did not evaluate responses after the first dose) (17); they are also concordant with a study of ASC responses to an oral enterotoxigenic Escherichia coli (ETEC) vaccine in Bangladesh, where no significant boosting was seen after a second dose of the vaccine (24). We also observed significant LPS- and CtxB-specific IgA antibody responses after a single dose of oral cholera vaccine in Bangladeshi adults, which were closely correlated with IgA ASC responses. It is thus possible that high levels of pre-existing mucosal antibodies to V. cholerae vaccine antigens blunt the emergence of a detectable plasmablast response after the second vaccination.
Given our finding that ASC responses are not detectable at significant levels after the second dose of OCV, it appears that a boosting of the mucosal immune response does not occur in the majority of adults in areas where cholera is highly endemic after a 2-week dosing interval. Also, given the striking difference between our results and those of previous studies in Swedish volunteers, it is likely that optimal boosting intervals for OCV differ between areas where cholera is historically endemic and those where it is not, reflecting differences between naive and primed populations. Specifically, while a short 2-week boosting interval appears necessary to boost responses in an immunologically naive population, the optimal time between doses in adults living in areas where cholera is endemic should be reassessed by characterizing the ASC responses at different intervals. Alternatively, it is possible that only a single dose of vaccine is needed for immediate protection against cholera in primed populations in countries where cholera is endemic.
ACKNOWLEDGMENTS
This research was supported by the icddr,b and by grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (U01 AI058935 [to S.B.C. and E.T.R.], R03 AI063079 [to F.Q.], U01 AI077883 [to E.T.R.], and R01 AI099243 [to J.B.H.]), as well as Career Development awards (K01 TW07409 [to J.B.H.] and TW07144 [to R.C.L.]), the Charles Hood Foundation (to J.B.H.), and a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (to R.C.L.).
Footnotes
Published ahead of print 14 August 2013
REFERENCES
- 1.World Health Organization 2010. Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:117–128 [PubMed] [Google Scholar]
- 2.Harris JB, Larocque RC, Charles RC, Mazumder RN, Khan AI, Bardhan PK. 2010. Cholera's western front. Lancet 376:1961–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan ET. 2011. The cholera pandemic, still with us after half a century: time to rethink. PLoS Negl. Trop. Dis. 5:e1003. 10.1371/journal.pntd.0001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N, Stanton BF, Kay E, Eeckels R, Walter S, Svennerholm AM, Holmgren J. 2009. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270–273 [DOI] [PubMed] [Google Scholar]
- 5.Deen JL, von Seidlein L, Sur D, Agtini M, Lucas MES, Lopez AL, Kim DR, Ali M, Clemens JD. 2008. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Negl. Trop. Dis. 2:e173. 10.1371/journal.pntd.0000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass RI, Becker S, Huq MI, Stoll BJ, Khan MU, Merson MH, Lee JV, Black RE. 1982. Endemic cholera in rural Bangladesh, 1966–1980. Am. J. Epidemiol. 116:959–970 [DOI] [PubMed] [Google Scholar]
- 7.Koelle K, Rodó X, Pascual M, Yunus M, Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700 [DOI] [PubMed] [Google Scholar]
- 8.Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818–820 [DOI] [PubMed] [Google Scholar]
- 9.Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702 [DOI] [PubMed] [Google Scholar]
- 10.Alam MM, Riyadh MA, Fatema K, Rahman MA, Akhtar N, Ahmed T, Chowdhury MI, Chowdhury F, Calderwood SB, Harris JB, Ryan ET, Qadri F. 2011. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin. Vaccine Immunol. 18:844–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, Chowdhury F, Khan AI, Weil AA, Aktar A, Nazim M, LaRocque RC, Ryan ET, Calderwood SB, Qadri F, Harris JB. 2012. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin. Vaccine Immunol. 19:842–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qadri F, Mäkelä PH, Holmgren J, Albert MJ, Mannoor K, Kantele A, Saha D, Salam MA, Kantele JM. 1998. Enteric infections in an endemic area induce a circulating antibody-secreting cell response with homing potentials to both mucosal and systemic tissues. J. Infect. Dis. 177:1594–1599 [DOI] [PubMed] [Google Scholar]
- 13.Uddin T, Harris JB, Bhuiyan TR, Shirin T, Uddin MI, Khan AI, Chowdhury F, LaRocque RC, Alam NH, Ryan ET, Calderwood SB, Qadri F. 2011. Mucosal immunologic responses in cholera patients in Bangladesh. Clin. Vaccine Immunol. 18:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qadri F, Jonson G, Begum YA, Wennerås C, Albert MJ, Salam MA, Svennerholm AM. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin. Diagn. Lab. Immunol. 4:429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qadri F, Ryan ET, Faruque AS, Ahmed F, Khan AI, Islam MM, Akramuzzaman SM, Sack DA, Calderwood SB. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 71:4808–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovác P, Calderwood SB, Qadri F, Ryan ET. 2012. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin. Vaccine Immunol. 19:1712–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamsuzzaman S, Ahmed T, Mannoor K, Begum YA, Bardhan PK, Sack RB, Sack DA, Svennerholm AM, Holmgren J, Qadri F. 2009. Robust gut associated vaccine-specific antibody-secreting cell responses are detected at the mucosal surface of Bangladeshi subjects after immunization with an oral killed bivalent V. cholerae O1/O139 whole cell cholera vaccine: comparison with other mucosal and systemic responses. Vaccine 27:1386–1392 [DOI] [PubMed] [Google Scholar]
- 18.Jayasekera CR, Harris JB, Bhuiyan S, Chowdhury F, Khan AI, Faruque AS, Larocque RC, Ryan ET, Ahmed R, Qadri F, Calderwood SB. 2008. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J. Infect. Dis. 198:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, Yoksan S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Ahmed R. 2012. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 86:2911–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, Dekker CL, Davis MM, Wilson PC, Greenberg HB, He XS. 2011. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J. Clin. Invest. 121:3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czerkinsky C, Svennerholm AM, Quiding M, Jonsson R, Holmgren J. 1991. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect. Immun. 59:996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qadri F, Wennerås C, Ahmed F, Asaduzzaman M, Saha D, Albert MJ, Sack RB, Svennerholm A. 2000. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine 18:2704–2712 [DOI] [PubMed] [Google Scholar]