Abstract
Glucan particles (GPs) are hollow porous Saccharomyces cerevisiae cell walls that are treated so that they are composed primarily of β-1,3-d-glucans. Our previous studies showed that GPs can serve as an effective vaccine platform. Here, we characterize CD4+ T-cell and antibody responses in immunized mice as a function of antigen (ovalbumin) encapsulation, antigen dose, particle numbers, time, immunization schedule, and trapping methods. Although we found that GPs served as an effective adjuvant when admixed with free antigens for IgG1 antibody production, stronger CD4+ T-cell and IgG2c antibody responses were stimulated when antigens were encapsulated inside GPs, suggesting that the GP platform acts as both an adjuvant and a delivery system. Vigorous T-cell and antibody responses were stimulated even at submicrogram antigen doses, as long as the number of GPs was kept at 5 × 107 particles per immunization. One prime and one boost were sufficient to elicit robust immune responses. In addition, strong antigen-specific antibody and T-cell responses prevailed up to 20 months following the last immunization, including those of gamma interferon (IFN-γ), interleukin 17A (IL-17A), and dual IFN-γ/IL-17A-secreting CD4+ T cells. Finally, robust immune responses were observed using generally recognized as safe (GRAS) materials (alginate and calcium, with or without chitosan) to trap antigens within GPs. Thus, these studies demonstrate that antigens encapsulated into GPs make an effective vaccine platform that combines adjuvanticity and antigen delivery to elicit strong durable immune responses at relatively low antigen doses using translationally relevant formulations.
INTRODUCTION
The development of vaccines is one of the greatest achievements in the history of medicine. Vaccines can be classified as live attenuated and inactivated. While live vaccines typically generate longer lasting immunity, they tend to have a poorer safety profile and are contraindicated in immunocompromised persons. Among the inactivated vaccines, subunit vaccines have the theoretical advantage of being safer, but the antigens used in them generally have little to no intrinsic antigenicity and therefore require administration with adjuvants (1).
The most commonly used adjuvant in clinical use, aluminum salts (alum), generally elicits strong antibody responses to coadministered antigens. However, there is a critical need for adjuvants that stimulate protective T-cell responses against those diseases for which antibodies alone are not protective. To this end, we have developed a glucan particle (GP)-based vaccine platform (2). GPs are derived from Saccharomyces cerevisiae and predominantly consist of β-1,3-d-glucans (3). β-1,3-d-Glucans are fungal cell wall pathogen-associated molecular patterns (PAMPs) (4, 5) that are recognized by host phagocytic β-1,3-d-glucan receptors, including Dectin-1 (6), CR3 (7), and scavenger receptors (8–10). β-1,3-d-Glucans also activate the alternative pathway of complement, with deposition of C3 fragments that are recognized by complement receptors (11).
We have shown that mice immunized three times with GPs containing ovalbumin complexed with yeast RNA develop robust antigen-specific antibody and Th1- and Th17-biased CD4+ T-cell responses (2). The goals of the present study were to address unanswered issues that are relevant to the development of GP-based vaccines for eventual use in humans. The importance of GPs as an adjuvant independent of its antigen delivery properties was determined by comparing immune outcomes when antigens were simply mixed with GPs versus being encapsulated inside GPs. Vaccines that are dose sparing and elicit rapid immune responses may be lifesaving in urgent situations, such as during influenza pandemics or in response to bioterrorism threats. Therefore, we examined the antibody and T-cell responses that occur when reducing the (i) amount of antigen loaded into GPs, (ii) number of GPs per immunization, and (iii) number of boosters. Long-term immunity is desirable for most vaccines but has been difficult to achieve with inactivated vaccines. Here, we studied the durability of immune responses up to 20 months postimmunization. Finally, we addressed issues of safety in and translation to human use by examining the immunogenicity of GPs formulated with antigen-entrapping materials (alginate, calcium, and chitosan) that are generally recognized as safe (GRAS) by the U.S. Food and Drug Administration.
MATERIALS AND METHODS
Chemicals and cell culture media.
Chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise noted. RPMI 1640 medium was purchased from Invitrogen Life Technologies (Carlsbad, CA). R10 medium, made of RPMI 1640 containing 10% fetal bovine serum (FBS) (Tissue Culture Biologicals, Tulare, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine (Invitrogen), and 55 μM 2-mercaptoethanol (2-ME) (Invitrogen) was also used. Chicken ovalbumin (OVA) containing ≤1 endotoxin unit per mg was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Mouse serum albumin was purchased from Equitech Bio (Kerrville, TX). Cells were incubated at 37°C in humidified air supplemented with 5% CO2.
Mice.
Wild-type specific-pathogen-free C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Wilmington, MA). The protocols for the mouse experiments were approved by The University of Massachusetts Medical School Institutional Use and Care of Animals Committee.
Mouse BMDCs.
Murine bone marrow-derived dendritic cells (BMDCs) were generated by culturing bone marrow cells for 8 days in R10 medium supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) followed by positive selection with CD11c+ magnetic beads, as in our previous studies, with slight modification (2). In some experiments, 5 ng/ml (final concentration) recombinant murine GM-CSF (PeproTech, Rocky Hill, NJ) was used instead of GM-CSF conditioned medium. The proliferation of BMDCs was irreversibly inhibited by treatment with 50 μg/ml mitomycin C in RPMI 1640 for 30 min at 37°C.
GP-OVA/tR, GP-OVA/AC, GP-OVA/ACC, and GP-MSA/tR.
GPs were purified from baker's yeast using a series of chemical and organic extractions as described previously (12). GPs containing complexed OVA and yeast tRNA (GP-OVA/tR) or mouse serum albumin (MSA) and tR (GP-MSA/tR) were formulated as described previously (12). GPs containing OVA trapped with alginate and calcium (GP-OVA/AC) were synthesized by incubating dry GP OVA-loaded particles in a subhydrodynamic volume of alginate (10 mg/ml) and allowing the alginate to swell and enter the GPs. Following lyophilization, the GP-OVA-alginate particles were incubated in a subhydrodynamic volume of 18 mM CaCl2 to ionically cross-link the alginate, trapping the OVA in a calcium alginate gel inside the GPs. GPs containing OVA trapped with alginate-calcium-chitosan (GP-OVA/ACC) were synthesized by incubating dry GP-OVA/AC in a subhydrodynamic volume of chitosan (10 mg/ml in 10 mM acetic acid) and allowing the chitosan to swell and enter the GPs. Following lyophilization, the GP-OVA-alginate-calcium-chitosan particles were incubated in a subhydrodynamic volume of phosphate-buffered saline (PBS) to neutralize the chitosan and induce its precipitation inside GPs. The OVA loading efficiency was >90% as measured using a fluorescent OVA tracer.
Immunizations.
GP-based formulations described above were administered to mice (6 to 12 weeks old, 4 mice per group) subcutaneously in a 100-μl volume of 0.9% saline. Unimmunized control mice received PBS alone. The particle numbers, antigen dose, number of immunizations, and intervals used are indicated in the figure legends.
T-cell proliferation, ELISpot, and OVA-specific Ab ELISAs.
The assays were performed as previously described (2). Briefly, the proliferation of purified CD4+ T cells stimulated with media, concanavalin A (ConA), or OVA was measured by [3H]thymidine incorporation with mitomycin C-treated BMDCs as antigen-presenting cells (APCs). Similarly, for the enzyme-linked immunosorbent spot assay (ELISpot), purified CD4+ T cells, mitomycin C-treated BMDCs, and stimuli were incubated in filtered plates coated with capture antibodies (Abs) specific for each cytokine. Secreted cytokines were then captured in situ and incubated with labeled cytokine detection Abs, and spots were developed following the enzymatic reaction of the substrate. Enzyme-linked immunosorbent assays (ELISAs) to detect IgG1 and IgG2c OVA-specific antibodies in immunized mouse serum samples were performed as described previously (13). Briefly, serially diluted mouse serum samples were incubated in OVA-coated plates, and the IgG1 and IgG2c subclass Abs from mouse serum samples were distinguished using biotin-conjugated rat anti-mouse IgG1 and IgG2c. Plates were then incubated with streptavidin-conjugated horseradish peroxidase (eBioscience) and developed with 3,3′,5,5′-tetramethylbenzidine solution (eBioscience), and the optical density at 450 nm (OD450) was measured. The Ab titer was defined as the dilution factor that was 2-fold greater than the inflection point.
FACS analysis.
Single-cell suspensions from combined lymph nodes and splenocytes (red blood cells were removed) from immunized or naive mice were left unstimulated or cultured with OVA (final concentration, 100 μg/ml) for 24 to 28 h. Brefeldin A was added for the last 5 h of incubation. The cells were collected and stained with LIVE/DEAD blue fixable dead cell stain kit (Invitrogen), according to the manufacturer's protocol. Then the cells were blocked with Fc-blocking antibodies (BD Biosciences) and stained for surface antigens (CD3 [fluorescein isothiocyanate {FITC}], CD4 [V500], and CD8 [V450]). After fixation and permeabilization using the Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences), the cells were stained with labeled antibodies (BD Biosciences) against mouse IFN-γ (Alexa 700) and interleukin 17A (IL-17A) (phycoerythrin [PE]). Single-color controls for LIVE/DEAD blue were prepared using amine reactive compensation (ArC) beads (Invitrogen) according to the manufacturer's protocol. Single-color controls for CD3, CD4, CD8, IFN-γ, and IL-17A were prepared using CompBeads (BD Biosciences) according to the manufacturer's protocol. Fluorescence-activated cell sorter (FACS) data were collected using an LSR II flow cytometer (BD) and analyzed using FlowJo software (Tree Star, Ashland, OR). Briefly, the dead cells were excluded using the LIVE/DEAD blue signal, the CD4+ CD8− population was selected from the CD3+ population, and finally the expression of IFN-γ and IL-17A on the live CD3+ CD4+ CD8− gated population was examined.
Statistics.
Data were analyzed and the figures prepared using GraphPad Prism software. For lymphoproliferation and ELISpot results, we performed a one-way analysis of variance (ANOVA) with the Tukey multiple comparison for comparisons of three or more groups; comparisons of two groups were performed by two-tailed unpaired Student's t test with a Bonferroni correction applied for multiple comparisons. For antibody titer data, nonparametric analyses were performed. Specifically, Kruskal-Wallis analysis was performed for comparisons of three or more groups and Mann-Whitney analysis for comparisons of two groups. Significance was defined as a P value of <0.05.
RESULTS
Effect of antigen encapsulation on stimulation of immune responses.
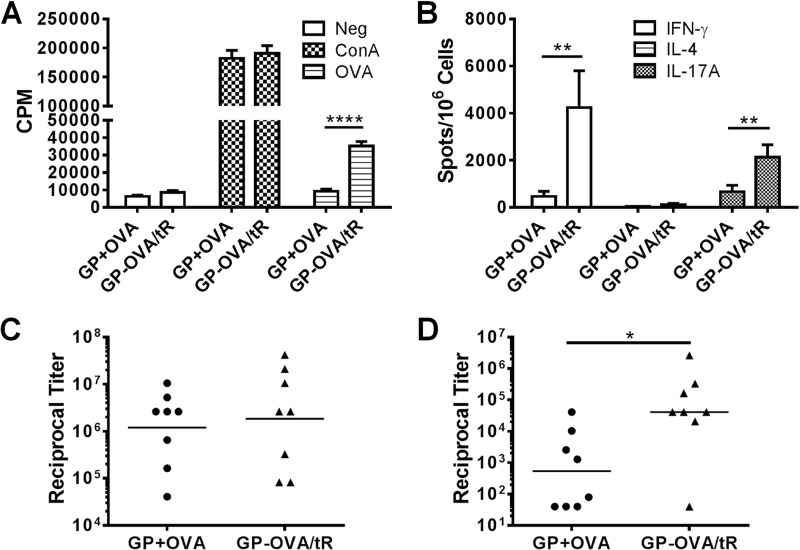
β-Glucans have been exploited in experimental vaccines for both their adjuvant and delivery properties (14, 15). To evaluate the relative contributions of these properties to the immunogenicity of GP-based vaccines, we compared the antigen-specific responses in mice immunized with OVA complexed inside GPs (i.e., GPs acting as both a delivery system and adjuvant) versus OVA and GPs that were admixed (i.e., GPs acting only as an adjuvant). Thus, mice received either a mixture of GPs (GP-MSA/tR) and free OVA (GP+OVA) or OVA encapsulated inside GPs (GP-OVA/tR). Only GP-OVA/tR stimulated CD4+ T-cell responses, as measured by the lymphoproliferation assays (Fig. 1A) and ELISpot (Fig. 1B). As in our previous studies (2), the Th1 and Th17 responses greatly exceeded the Th2 response, as judged by the number of IFN-γ- and IL-17A- versus IL-4-positive “spots.” Both formulations stimulated comparable IgG1 antibody titers (Fig. 1C), but GP-OVA/tR stimulated significantly higher levels of IgG2c antibodies (Fig. 1D). These results suggest that although GPs have some adjuvanticity when mixed with antigens, GPs with encapsulated antigens stimulate much stronger overall immune responses. This was particularly pronounced for CD4 cellular immunity.
Fig 1.
Effect of antigen encapsulation in GPs on stimulation of immune responses. Mice were immunized three times at 2-week intervals with either control GP-MSA (5 × 107 GP particles containing 50 μg mouse serum albumin) plus 50 μg free OVA (GP+OVA) or GP-OVA/tR (GP-OVA/tR, 5 × 107 GP particles containing 50 μg OVA). CD4+ T-cell lymphoproliferation (A), ELISpot (B), IgG1 ELISA (C), and IgG2c ELISA (D) were performed 2 weeks after the 3rd immunization for one experiment and 4 weeks after for the other. Data shown are combined results from these two independent experiments, with four mice per group for each experiment. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
GP vaccines efficiently stimulate robust immune responses over a wide range of encapsulated antigen concentrations.
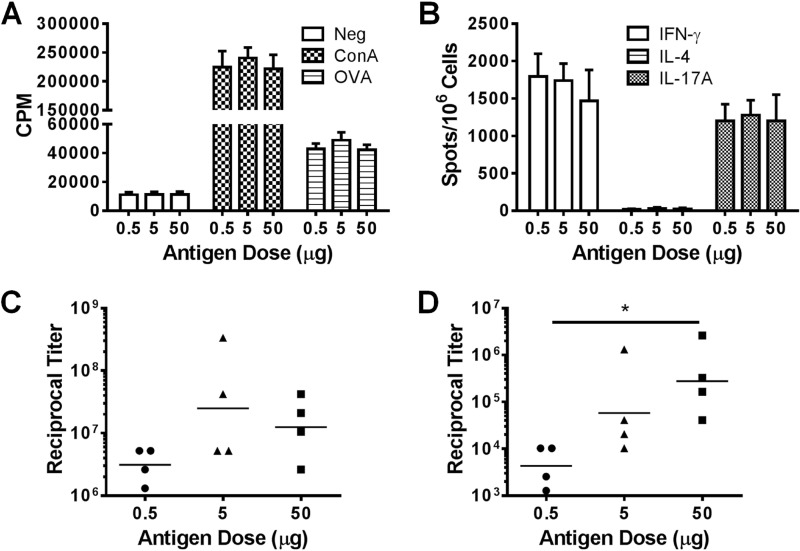
The antigen-sparing properties of vaccines are important when antigens are in short supply, such as might occur during an acute outbreak. We previously used a relatively high dose of antigen (50 μg) in order to establish the potential utility of GP-based vaccines (2). To determine the efficiency of the platform at lower antigen doses, we loaded various amounts of OVA (0.5, 5, and 50 μg) into 5 × 107 GPs and immunized the animals with these GP-OVA/tR formulations. Similar CD4 T-cell responses, as measured by CD4+ T-cell lymphoproliferation (Fig. 2A) and ELISpot (Fig. 2B), as well as IgG1 (Fig. 2C) antibody responses were stimulated. The lowest dose (0.5 μg) stimulated significantly lower IgG2c antibody responses than did the highest dose (50 μg) (Fig. 2D). These data establish that the GP vaccine platform is immunogenic even at submicrogram antigen doses.
Fig 2.
GP immunizations efficiently stimulate robust immune responses over a wide range of encapsulated antigen concentrations. Mice (four per group) were immunized three times at 2-week intervals with 5 × 107 GP-OVA/tR containing various amounts of OVA (0.5, 5, or 50 μg) per dose. Four weeks after the last immunization, CD4+ T-cell lymphoproliferation (A), ELISpot (B), IgG1 ELISA (C), and IgG2c ELISA (D) were performed. The results are from one experiment. Data for the 50 μg OVA group are also used in Fig. 1 (part of the GP-OVA/tR group). Similar results were obtained in an experiment in which mice were immunized three times at 2-week intervals with 5 × 107 GP-OVA/tR containing 0.33 μg, 3.3 μg, and 33 μg OVA per dose. *, P < 0.05.
GP numbers are important for mounting strong immune responses.
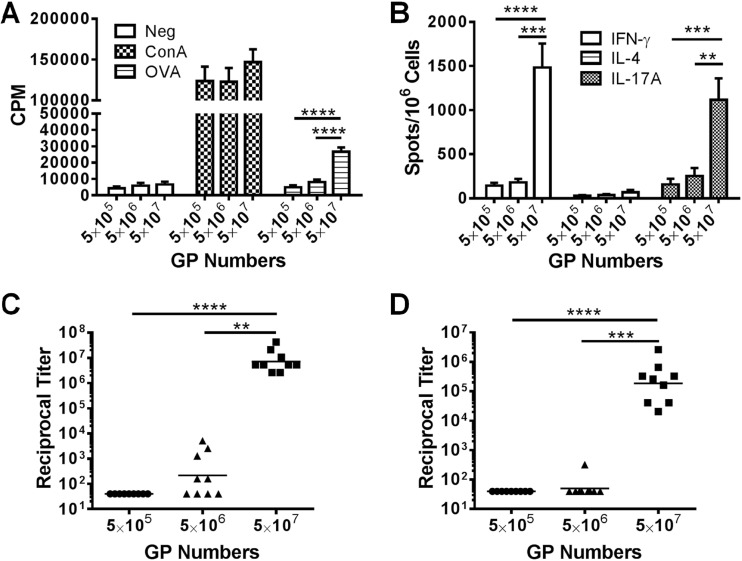
In the above-mentioned experiment, we varied the amount of antigens but kept the numbers of GPs per immunization constant. In the next set of experiments, we sought to determine the critical number of GPs needed to obtain vigorous immune responses. To do this, we immunized sets of mice with the same GP formulations (50 μg OVA per 5 × 107 GPs) but varied the number of GPs the mice received. We found that only the group receiving the largest amount of particles (5 × 107 GP-OVA/tR per dose, containing 50 μg OVA) stimulated robust immune responses (Fig. 3A to D). Groups receiving fewer particles (5 × 105 to 5 × 106, delivering 0.5 to 5 μg OVA) had little to no responses. By comparing the groups immunized with the same amount of OVA but different numbers of GPs (Fig. 2 and 3), it is apparent that a critical number of GPs is needed to elicit robust immune responses.
Fig 3.
Effect of GP number and antigen concentration on immune responses. Mice were immunized three times at 2-week intervals with the indicated number of GP-OVA/tR (containing 50 μg OVA per 5 × 107 particles; 5 μg OVA per 5 × 106 particles; 0.5 μg per 5 × 105 particles). Two or 4 weeks after the last immunization, CD4+ T-cell lymphoproliferation (A), ELISpot (B), IgG1 ELISA (C), and IgG2c ELISA (D) were performed. Data shown are combined results from two independent experiments with 4 or 5 mice per group for each experiment. Some of the data for the 5 × 107 GP group are also used in Fig. 1 (part of the GP-OVA/tR group) and Fig. 2 (50 μg OVA group). **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
One boost is sufficient to mount robust immune responses.
For the aforementioned immunization schemes, we gave two boosts following the priming dose. Given that reducing the number of boosts would have obvious benefits for vaccination programs, we compared the antigen-specific responses in mice immunized once (prime only), twice (prime plus boost), or three times (prime plus two boosts). We found that CD4+ T-cell proliferative responses and OVA-specific IgG1 and IgG2c antibodies did not significantly differ when comparing one boost with two boosts (Fig. 4A to C). However, immunization with only a prime did not elicit strong immune responses.
Fig 4.
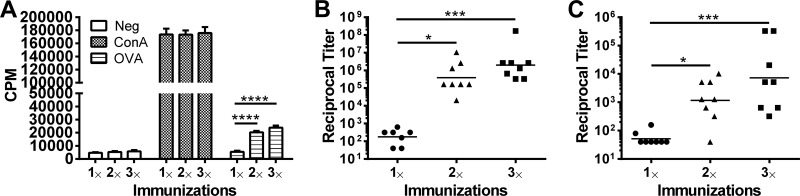
Effect of number of GP immunizations on immune responses. Mice were immunized once (prime only), twice (prime and one boost), or three times (one prime and two boosts) with 5 × 107 GP-OVA/tR (containing 5 μg OVA per 5 × 107 particles) per dose. Immunizations were at 2-week intervals for those mice that received boosts. Three weeks after the last immunization, CD4+ T-cell lymphoproliferation (A), IgG1 (B), and IgG2c (C) were performed. Data are combined results from two independently performed experiments, with four mice per group for each experiment. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
GP vaccines stimulate long-term durable responses.
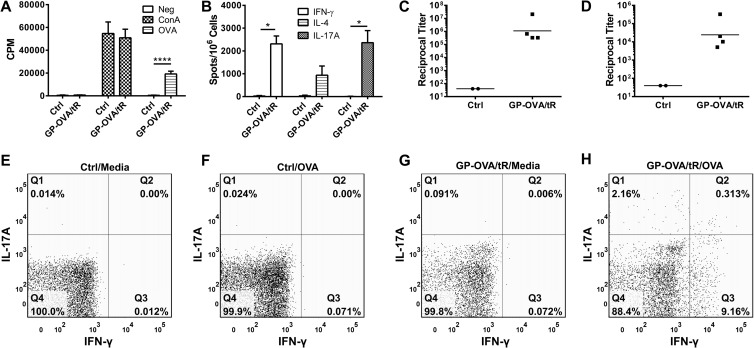
Ideal vaccines stimulate long-lasting immune responses. Therefore, we next evaluated the durability of the responses following GP-OVA/tR immunization (prime and two boosts). In the first set of experiments, OVA-specific CD4+ lymphoproliferation (Fig. 5A), ELISpot (Fig. 5B), IgG1 (Fig. 5C), and IgG2c (Fig. 5D) responses were found to be strong seven to eight months following the last immunization. We then examined intracellular IFN-γ and IL-17A staining in CD4+ T cells 18 to 20 months following the last immunization. We found that ex vivo OVA stimulation resulted in increases in IFN-γ-, IL-17A-, and dual IFN-γ/IL-17A-secreting CD4+ T cells from immunized, but not naive, mice (Fig. 5E to H). In addition, the OVA-specific reciprocal antibody titers from the two mice were 163,840 and 20,480 for IgG1 and 640 and 640 for IgG2c. These results suggest that GP vaccines might stimulate long-term durable immune responses.
Fig 5.
Durability of responses stimulated by GP immunizations. (A to D) Mice were immunized three times at 2-week intervals with 5 × 107 GP-OVA/tR containing 50 μg per dose. Control mice received PBS. Seven to 8 months after the last immunization, CD4+ T-cell lymphoproliferation (A), ELISpot (B), IgG1 ELISA (C), and IgG2c ELISA (D) were performed. Results are from two independent experiments, with a total of four mice per GP-OVA/tR group and two mice per control group. (E to H) Mice were left unimmunized (naive) or immunized three times at 2-week intervals with 5 × 107 GP-OVA/tR (containing 5 μg OVA per 5 × 107 particles) per dose. Eighteen to 20 months following the last immunization, intracellular expression of IFN-γ and IL-17A by CD4+ T cells from mixed lymph nodes and spleens was determined by FACS. Groups included naive mice stimulated with media (E) or OVA (F) and vaccinated mice stimulated with media (G) or OVA (H). Data shown are representative results from two similar experiments. *, P < 0.05; ****, P < 0.0001.
GP formulations using GRAS materials.
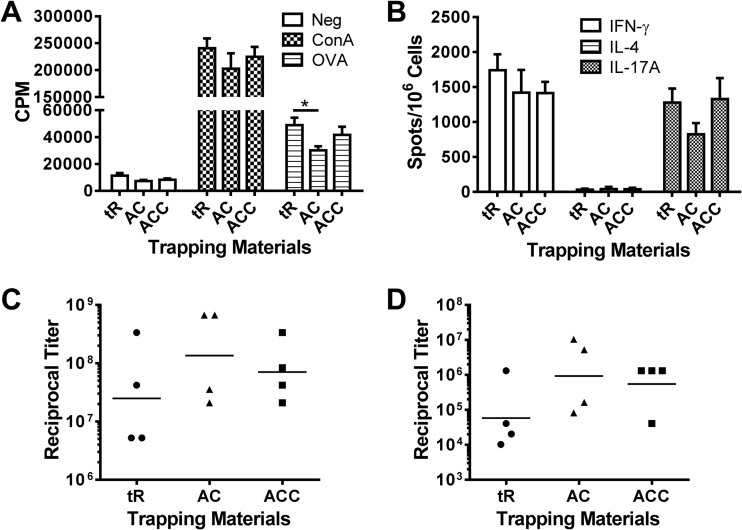
In our previous studies, yeast RNA was used to trap OVA in GPs because of its effectiveness in complexing serum albumins inside GPs. To increase the translational relevance of the GP platform, we tested several GRAS materials (alginate and calcium, with or without chitosan) as antigen-trapping agents. GP formulations containing either alginate and calcium or alginate, calcium, and chitosan were comparable to RNA-based formulations in their capacity to induce antigen-specific T-cell and antibody responses in mice (Fig. 6A to D).
Fig 6.
GP formulations containing GRAS materials. Mice (four per group) were immunized three times at 2-week intervals with 5 × 107 particles containing 5 μg OVA per dose. Three methods of encapsulations were compared: yeast tRNA (tR) (GP-OVA/tR), alginate and calcium (AC) (GP-OVA/AC), and alginate, calcium, and chitosan (ACC) (GP-OVA/ACC). Mice were euthanized 4 weeks after the 3rd immunization, following which CD4+ T-cell lymphoproliferation (A), ELISpot (B), IgG1 ELISA (C), and IgG2c ELISA (D) were performed. Results are from one experiment. Data for the tR group are also used in Fig. 2 (part of the 5 μg OVA group). Comparable results were obtained from a similarly designed experiment (mice were immunized three times at 2-week intervals with 5 × 107 respective GP-OVA constructs containing 3.3 μg OVA per dose). *, P < 0.05.
DISCUSSION
Our previous studies established that antigens encapsulated in GPs stimulate potent CD4+ T- and B-cell responses when given subcutaneously to mice (2, 13). Here, we studied the properties of the GP-based vaccine platform, particularly focusing on aspects relevant to human development, including antigen sparing, dose optimization, memory responses, and formulation composition. We found that the GP platform stimulates strong and durable immune responses to encapsulated antigens, even at submicrogram doses. While a single boost following the prime was sufficient to mount strong responses, optimal T-cell responses required a critical number of GPs per immunization. Finally, GP formulations that incorporated GRAS materials as trapping components retained immunogenicity. These findings broaden our understanding of the GP platform and should enhance its prospects for future clinical development.
GPs target antigens to APCs bearing β-glucan and complement receptors (2, 13). In addition to their targeting properties, β-glucans have intrinsic adjuvant activity (14). Moreover, C5a, which is generated as a result of complement activation by GPs (11), has been used as an adjuvant (16). Therefore, it was of interest to distinguish the antigen-targeting and intrinsic adjuvanticity properties of GPs. Our data demonstrating that GP formulations containing encapsulated OVA elicited superior immune responses to those of immunizations consisting of a simple mix of GPs and OVA suggest that the antigen-targeting properties of the GP platform are essential for optimal immunogenicity. However, the finding that a mix of GPs and antigen still generated some responses attests to the intrinsic adjuvant properties of GPs. Interestingly though, mixing GPs and OVA generated significant antibody titers but not CD4+ T-cell responses. Consistent with our data, it has been reported that the host sees more “danger” when antigens are codelivered with PAMPs into the same phagocytic compartment (17).
T-cell activation is more efficient when clusters of peptide-major histocompatibility complex (MHC) molecules are displayed on the surfaces of APCs (18). All the antigens delivered to an APC by a single antigen-laden GP should traffic to a distinct phagolysosomal compartment. An antigen load of 0.5 μg of OVA in 5 × 107 GPs delivers >100,000 OVA molecules per GP. We speculate that the capability of the GP platform to induce potent cellular immune responses even at submicrogram antigen doses is due, at least in part, to preferential clustering when peptides are derived from same compartment.
Despite the ability of GPs to target antigen to APCs, immune responses declined when mice received <5 × 107 GPs per immunization. All the major phagocytic populations, including neutrophils, macrophages, and DCs express Dectin-1 and complement receptors and are able to phagocytize GPs. Our preliminary data (H. Huang, G. R. Ostroff, and S. M. Levitz, unpublished data) demonstrate that only a minority of GPs are phagocytosed by DCs at the subcutaneous injection site. As DCs are thought to be the primary cell type that initiates adaptive immune responses, modifying the GP platform to selectively target DCs might result in vaccines that require fewer GPs per dose. While clinical trials are needed to determine the optimal number of GPs in the context of human vaccination, it should be noted that the mass of 5 × 107 GPs is only 100 μg.
Ideal vaccines provide protective immunity after a minimum number of immunizations. Reducing the number of immunizations with GP-OVA from three doses to two did not significantly compromise antigen-specific antibody and CD4+ T-cell responses. Although T-cell responses were not detected after a single immunization with GP-OVA, modest antibody responses were observed. While such responses might be useful for protection against diseases that do not require high titers of antibody, future efforts will be directed to develop formulations that elicit more vigorous responses after only a single dose.
It is desirable that vaccination confers long-term protective immunity. We found that following immunization with GPs, antigen-specific responses prevailed for essentially the life span of the mouse. Moreover, even 18 to 20 months following the last immunization, approximately 10% and 2% of the CD4+ T-cell population stained positive for intracellular IFN-γ and IL-17A, respectively, following ex vivo stimulation with OVA. These data suggest that long-term Th1 and Th17 memory responses are induced using the GP platform. Although there is controversy regarding the longevity of Th17 cells, our results are consistent with a recent study showing long-lasting Th1 and Th17 memory cells following subunit vaccination with antigens administered with the cationic liposome adjuvant CAF01 (19). Interestingly, in both our study and the aforementioned study, a small but distinctive population of IFN-γ/IL-17A double-positive antigen-specific CD4+ T cells was detected. The development, plasticity, and functionality of these CD4+ T-cell lineages after immunization remain to be defined.
Regulatory agencies have rigorous safety requirements for new vaccines, including a requirement to test all component parts (1). Thus, translation from preclinical testing to clinical trials and eventual human approval is facilitated by the use of GRAS materials. As yeast RNA is not considered a GRAS material by the FDA, we studied whether combinations of alginate and calcium, with and without chitosan, three materials that are GRAS, could be substituted for RNA to trap OVA inside GPs. We found that the combination of alginate plus calcium was slightly inferior to the standard yeast RNA formulation at stimulating some T-cell responses. However, the addition of chitosan to alginate and calcium resulted in antibody and T-cell responses similar to those stimulated by RNA-based formulations. While chitosan has been shown to activate the inflammasome (20), additional studies will be needed to determine whether inflammasome activation contributes to the enhanced immunogenicity of chitosan-containing GPs.
Finally, our studies utilized responses to OVA to assess variables that affect the immunogenicity of the GP platform. It is appreciated that immune responses to model antigens, while informative, cannot be relied upon to predict vaccine-mediated protection. In this regard, in published (21) and unpublished works, we have begun to study GP-based vaccines in models of infection, with encouraging results.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant no. AI025780, AI093302, AI102618, HL112671, and AI094074 from the National Institutes of Health.
Footnotes
Published ahead of print 14 August 2013
REFERENCES
- 1.Levitz SM, Golenbock DT. 2012. Beyond empiricism: informing vaccine development through innate immunity research. Cell 148:1284–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. 2010. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 1(3):00164-10. 10.1128/mBio.00164-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. 2004. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 173:797–806 [DOI] [PubMed] [Google Scholar]
- 4.Bowman SM, Free SJ. 2006. The structure and synthesis of the fungal cell wall. Bioessays 28:799–808 [DOI] [PubMed] [Google Scholar]
- 5.Tsoni SV, Brown GD. 2008. Beta-glucans and dectin-1. Ann. N. Y. Acad. Sci. 1143:45–60 [DOI] [PubMed] [Google Scholar]
- 6.Brown GD, Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36–37 [DOI] [PubMed] [Google Scholar]
- 7.Beller DI, Springer TA, Schreiber RD. 1982. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J. Exp. Med. 156:1000–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezekowitz RA, Sim RB, Hill M, Gordon S. 1984. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J. Exp. Med. 159:244–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. 2009. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 206:637–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vera J, Fenutria R, Cañadas O, Figueras M, Mota R, Sarrias MR, Williams DL, Casals C, Yelamos J, Lozano F. 2009. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc. Natl. Acad. Sci. U. S. A.106:1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal S, Specht CA, Haibin H, Ostroff GR, Ram S, Rice PA, Levitz SM. 2011. Linkage specificity and role of properdin in activation of the alternative complement pathway by fungal glycans. mBio 2(5):00178-11. 10.1128/mBio.00178-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soto ER, Ostroff GR. 2008. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjug. Chem. 19:840–848 [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, Specht CA, Levitz SM. 2012. Relative contributions of dectin-1 and complement to immune responses to particulate beta-glucans. J. Immunol. 189:312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 [DOI] [PubMed] [Google Scholar]
- 15.Xie J, Guo L, Ruan Y, Zhu H, Wang L, Zhou L, Yun X, Gu J. 2010. Laminarin-mediated targeting to Dectin-1 enhances antigen-specific immune responses. Biochem. Biophys. Res. Commun. 391:958–962 [DOI] [PubMed] [Google Scholar]
- 16.Morgan EL, Thoman ML, Sanderson SD, Phillips JA. 2010. A novel adjuvant for vaccine development in the aged. Vaccine 28:8275–8279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blander JM, Medzhitov R. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808–812 [DOI] [PubMed] [Google Scholar]
- 18.Manz BN, Jackson BL, Petit RS, Dustin ML, Groves J. 2011. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc. Natl. Acad. Sci. U. S. A. 108:9089–9094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenstrøm T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. 2012. Vaccine-induced Th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 80:3533–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bueter CL, Lee CK, Rathinam VA, Healy GJ, Taron CH, Specht CA, Levitz SM. 2011. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 286:35447–35455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. 2012. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect. Immun. 80:3960–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]