Abstract
The aims of the present study were to assess the concentrations of different cytokines and chemokines in blood serum and cerebrospinal fluid (CSF) samples of patients with Lyme neuroborreliosis and to identify the possible marker(s) that would enable a distinction between clinically evident and suspected Lyme neuroborreliosis, as well as between Lyme neuroborreliosis and tick-borne encephalitis (TBE). Our additional interest was to evaluate the relationship between cytokine and chemokine concentrations and Borrelia burgdorferi sensu lato isolation from CSF, as well as intrathecal synthesis of specific borrelial antibodies. We found that higher concentrations of CXCL13 and lower concentrations of interleukin 10 (IL-10) in serum were associated with higher odds for clinically evident Lyme neuroborreliosis compared to suspected Lyme neuroborreliosis, as well as to TBE. The concentrations of IL-2, IL-5, IL-6, IL-10, and CXCL13 in the CSF were higher in patients with evident Lyme neuroborreliosis than in those who were only suspected to have the disease. A comparison of CSF cytokine and chemokine levels in patients with and without intrathecal synthesis of specific borrelial antibodies revealed that CXCL13 CSF concentration is significantly associated with intrathecal synthesis of borrelial antibodies. A comparison of the cytokine and chemokine CSF concentrations in patients with clinically evident Lyme neuroborreliosis according to CSF culture results revealed that higher concentrations of gamma interferon (IFN-γ) were associated with lower odds of Borrelia isolation. Although several differences in the blood serum and CSF concentrations of various cytokines and chemokines between the groups were found, the distinctive power of the majority of these findings is low. Further research on well-defined groups of patients is needed to appraise the potential diagnostic usefulness of these concentrations.
INTRODUCTION
Slovenia is an area where Lyme borreliosis, a zoonosis caused by the spirochete from the Borrelia burgdorferi sensu lato complex (1), is endemic. It may manifest in a wide range of clinical symptoms, including erythema migrans (EM), the initial cutaneous lesion. From a skin lesion, borreliae may disseminate to other organs, most often to the nervous system, joints, and heart. While the presence of typical EM enables a reliable clinical diagnosis, the other clinical manifestations, including Lyme neuroborreliosis, do not (2).
Lyme neuroborreliosis usually manifests through meningitis, radiculitis, and peripheral facial palsy (2). A diagnosis of Lyme neuroborreliosis is based on clinical symptoms and signs, cerebrospinal fluid (CSF) pleocytosis, and evidence of Borrelia central nervous system infection demonstrated by the isolation of B. burgdorferi sensu lato from CSF, borrelial DNA in CSF samples, and/or (most frequently) the presence of borrelial intrathecal antibody production (3). The latter demonstration is convenient for routine laboratory work, but the synthesis may not be detectable during the first few weeks of infection (4, 5), and a positive test does not distinguish between acute and past infections (6).
Borreliae that enter the central nervous system are recognized by monocytes, macrophages, or dendritic cells, which produce proinflammatory cytokines; additionally, several chemokines are induced to attract other immune cells. High concentrations of interleukin 6 (IL-6), IL-8, IL-12, IL-18, gamma interferon (IFN-γ), and CXCL13 have been found in the CSF samples of patients suffering from Lyme neuroborreliosis (7–11). Interleukin 6 induces the final maturation of B cells into plasma cells, IL-8 is a chemotactic factor for immune cells, IL-12 induces the Th1 response, which results in a strong inflammatory reaction caused by IFN-γ and tumor necrosis factor beta (TNF-β), and IL-18 is an important regulator of the immune response, which in synergy with IL-12 increases the secretion of IFN-γ (7, 8). CXCL13 is produced by stromal cells and influences the migration of B cells (12). Previous studies have shown elevated concentrations of CXCL13 in the CSF samples of patients with Lyme neuroborreliosis, multiple sclerosis, and some other inflammatory diseases (11, 13–16), suggesting that CXCL13 is a diagnostic marker for Lyme neuroborreliosis (17, 18). As has been indicated by some reports, elevated concentrations of CXCL13 in the CSF may appear several days before intrathecal borrelial antibody synthesis occurs (18, 19).
The aim of the present study was to assess the concentrations of different cytokines and chemokines in the blood serum and CSF samples of patients with Lyme neuroborreliosis and to identify the possible marker(s) that would enable a distinction between clinically evident and suspected Lyme neuroborreliosis, as well as between Lyme neuroborreliosis and tick-borne encephalitis (TBE). Our additional interest was to evaluate the relationship between cytokine and chemokine concentrations and B. burgdorferi sensu lato isolation from the CSF, as well as between cytokine and chemokine concentrations and intrathecal synthesis of specific borrelial antibodies.
MATERIALS AND METHODS
Patient groups. (i) Lyme neuroborreliosis.
In the present study, we included 46 patients (25 men and 21 women, with an average age of 48 years [range, 18 to 81 years]) with a working clinical diagnosis of evident Lyme neuroborreliosis (evidenced by erythema migrans within 4 months before the appearance of neurological signs or symptoms, including radicular pain and/or peripheral facial palsy, and pleocytosis) and 60 patients (27 men and 33 women, with an average age of 49 years [range, 18 to 84] years) with a working clinical diagnosis of suspected Lyme neuroborreliosis (evidenced by erythema migrans within 4 months before the appearance of neurological symptoms or signs but no pleocytosis). Some of the patient case studies have been presented elsewhere (20). At the time of inclusion in the study (at initial examination), the median duration of neurological signs or symptoms was 10 days (range, 1 to 365 days) in patients with clinically evident Lyme neuroborreliosis and 11 days (range, 1 to 100 days) in patients with suspected Lyme neuroborreliosis.
(ii) Tick-borne encephalitis.
This group consisted of 39 adult patients with TBE (evidenced by clinical signs or symptoms of meningoencephalitis, CSF pleocytosis, and serological indication of TBE virus infection demonstrated by the presence of specific serum IgM and IgG antibodies). At the time of their inclusion (time of initial examination), the median duration of the neurological signs or symptoms was 10 days (range, 2 to 32 days).
(iii) Other neurological diseases and blood donors.
The control group consisted of 28 patients with neurological diseases (multiple sclerosis, vascular disease of CNS, depression, and cervical myelopathy) and 75 healthy blood donors.
Patients with Lyme neuroborreliosis and TBE were diagnosed at the Department of Infectious Diseases, and patients with neurological diseases were diagnosed at the Department of Neurology, University Medical Center Ljubljana, in the years 2009 and 2010. The blood donors presented to the Blood Transfusion Centre of Slovenia.
None of the persons included in the study reported recent treatment with antibiotics, and none had received a Lyme disease vaccine.
The study approach was approved by the Medical Ethics Committee of the Ministry of Health of the Republic Slovenia (no. 35/08/06 and 33/06/10).
Methods.
Blood and CSF samples were taken simultaneously at the initial examination from patients with working clinical diagnoses of either evident or suspected Lyme neuroborreliosis, TBE, and patients with other neurological diseases belonging to the control group. Only blood samples were obtained from the blood donors.
Serum antibodies to B. burgdorferi sensu lato were determined using IDEIA Borrelia burgdorferi (Oxoid, Cambridgeshire, United Kingdom) according to the manufacturer's instructions.
Intrathecal synthesis of specific antibodies was determined using the IDEIA Lyme neuroborreliosis kit (Oxoid, Cambridgeshire, United Kingdom) with Borrelia afzelii flagellar antigen. We followed the manufacturer's instructions for test performance and interpretation of the results. Accordingly, an index value of ≥0.3 indicated intrathecal synthesis of specific borrelial antibodies. Intrathecal antibody production was performed for patients with working clinical diagnoses of Lyme neuroborreliosis, TBE, and other neurological diseases.
Isolation of Borrelia strains from blood and cerebrospinal fluid.
One milliliter of CSF, obtained by lumbar puncture, was immediately inoculated into a tube with 5 ml of modified Kelly-Pettenkofer (MKP) medium and promptly transported to the laboratory (21). Five milliliters of blood obtained by venipuncture was placed in a tube containing sodium citrate and centrifuged at 100 × g for 5 min; the supernatant was inoculated into two or more tubes of MKP medium (21). The samples were cultivated at 33°C and were examined once a week for the presence of spirochetes using dark-field microscopy, as described previously (21). The samples were considered negative if no growth was detected after 9 weeks of incubation for CSF and after 12 weeks for blood (21). The isolation of borreliae was done only from specimens obtained from patients with working clinical diagnoses of evident and suspected Lyme neuroborreliosis and patients with TBE, but not from those with other neurological diseases and blood donors.
Genotypic characterization of isolated strains.
Borrelial DNA from positive cultures was isolated by the gel insert method, as previously reported (21). For species identification, borrelial DNA was digested overnight at 37°C with the restriction endonuclease MluI, and the restricted fragments were separated by pulsed-field gel electrophoresis (PFGE), as described elsewhere (21).
The cytokine and chemokine concentrations in blood and cerebrospinal fluid (CSF) samples were determined with the human Th1/Th2 cytokine kit, human inflammatory cytokines kit (BD Biosciences, San Jose, CA), and the Quantikine human CXCL13/BLC/BCA-1 (R&D Systems, Abingdon, United Kingdom), performed according to the manufacturers' instructions. We determined the CSF and blood serum concentrations of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, TNF-α, and IFN-γ cytokines and IL-8 and CXCL13 chemokines.
Statistical analysis.
Data were analyzed using multinomial logistic regression with the concentrations of cytokines and chemokines as predictors and group membership (working clinical diagnosis of evident Lyme neuroborreliosis, suspected Lyme neuroborreliosis, and TBE) as the dependent variable. Bivariate logistic regression, followed by multivariate logistic regression, was performed to test which markers distinguish between clinically evident Lyme neuroborreliosis and clinically suspected Lyme neuroborreliosis, as well as between clinically evident Lyme neuroborreliosis and TBE. Odds ratios with 95% confidence limits were calculated and corresponding P values were given. Multicolinearity between the predictors was assessed using Spearman's correlation coefficient. Significance tests were two-sided. Bonferroni correction for multivariate logistic regression was used and P values of ≤0.025 were considered statistically significant. The analyses were performed using SPSS version 19.0 software. Graphs were drawn using GraphPad Prism 6.0 software (GraphPad Software, Inc., San Diego, CA).
RESULTS
Demonstration of Borrelia infection.
In all (46/46) patients with a working clinical diagnosis of evident Lyme neuroborreliosis, borrelial serum IgM and/or IgG antibodies were present; the corresponding ratio for patients with suspected Lyme neuroborreliosis was 53/60 (88.3%).
Intrathecal synthesis of specific borrelial IgM and/or IgG antibodies was established in 21/46 (45.7%) patients with a working clinical diagnosis of evident Lyme neuroborreliosis and in none of the other groups. The proportion of patients with detectable intrathecal synthesis increased with the duration of neurological symptoms. Of 20 patients with a duration of neurologic symptoms of ≤7 days, only six (30%) had intrathecal synthesis of borrelial antibodies, while the corresponding ratio for patients with a duration of ≥2 weeks was 15/26 (57.7%). In 5 (23.8%) out of 21 patients with intrathecal synthesis of specific antibodies, borreliae were isolated either from blood (2/5) or CSF (4/5).
In the group of patients with a working clinical diagnosis of evident Lyme neuroborreliosis, borreliae were isolated from 2/46 (4.3%) blood and 8/46 (17.4%) CSF specimens; in one patient, borreliae were isolated from blood and CSF simultaneously. The isolation rate in patients with a working clinical diagnosis of suspected Lyme neuroborreliosis was 2/60 (3.3%) for blood and also 2/60 (3.3%) for CSF. All cultures of blood and CSF specimens obtained from patients with TBE remained Borrelia negative.
Eight isolates (1 from blood and 7 from CSF) from patients with a working clinical diagnosis of evident Lyme neuroborreliosis were found to be from Borrelia garinii (type Mlg2), and two (1 blood and 1 CSF isolate) were from B. afzelii (type Mla1). All four strains isolated from patients with a working clinical diagnosis of suspected Lyme neuroborreliosis were from B. afzelii (type Mla1).
Cytokine and chemokine concentrations.
The concentrations of the cytokines IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, TNF-α, and IFN-γ and the chemokines IL-8 and CXCL13 were determined in the clinical specimens from patients with a working clinical diagnosis of evident Lyme neuroborreliosis, suspected Lyme neuroborreliosis, or TBE, from patients with other neurological diseases, and from blood donors are presented in Table 1. The median serum concentrations of cytokines and chemokines in blood donors were low and were comparable with the corresponding concentrations from patients with other neurological diseases, except for that of IL-8 (Table 1). Patients with working clinical diagnoses of evident Lyme neuroborreliosis, suspected Lyme neuroborreliosis, and TBE had higher concentrations of all tested blood serum cytokines and chemokines than did the blood donors, except for the concentration of CXCL13 in patients with suspected Lyme neuroborreliosis and in patients with other neurological diseases, in whom concentrations of this chemokine were lower.
Table 1.
Concentrations of cytokines and chemokines in blood serum and CSF of patients with a working clinical diagnosis of evident Lyme neuroborreliosis and suspected Lyme neuroborreliosis, tick-borne encephalitis, or other neurological diseases and in blood donors
| Sample and groupa | Concn (median [range], mean) (pg/ml) of the indicated cytokines/chemokines |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-8 | IL-10 | IL-12 | CXCL13 | IFN-γ | TNF-α | |
| Blood | |||||||||||
| LNB | 1.5 (0–12.0), 2.3 | 5.8 (0–39.0), 6.5 | 4.0 (0–161), 12.9 | 5.0 (0–68.2), 8 | 3.0 (1–311), 15.8 | 24.9 (2–7,343), 431.6 | 1.0 (0–4.2), 1.2 | 2.0 (0–8.0), 2.0 | 88.1 (21.2–452), 110 | 4.2 (1.7–17.5), 5.4 | 5.7 (0–33.0), 6.7 |
| sLNB | 1.1 (0–25.9), 2.9 | 6.0 (0–20.0), 6.4 | 4.0 (2–150), 11.4 | 5.0 (0–18.6), 5.5 | 3.0 (1–358), 27.1 | 23.5 (0–3,985), 410.7 | 1.5 (0–6.1), 1.6 | 2.8 (0–34.0), 2.5 | 61.3 (18.0–241), 67 | 4.0 (0–14.0), 4.8 | 6.0 (0–23.0), 6.8 |
| TBE | 1.6 (0–39.7), 3.4 | 6.0 (0–35.0), 6.3 | 4.0 (1.3–7.2), 3.9 | 5.0 (3–15.0), 5.5 | 4.6 (0–25.0), 6.6 | 54.8 (0–4,951), 364 | 2.0 (0–18.0), 2.5 | 2.0 (0–31.0), 3.4 | 85.4 (31.0–173), 87 | 4.0 (0–10.0), 4.5 | 5.0 (0–10.0), 4.8 |
| OND | 0.0 (0–3.0), 0.5 | 3.0 (1.6–16.8), 4.5 | 3.0 (2–68.0), 6.7 | 1.5 (1–8.2), 3.1 | 3.0 (1.7–33.0), 6.1 | 27.2 (8.5–498), 67.5 | 0 (0–2.9), 0.7 | 2.0 (0–3.3), 1.6 | 50.2 (20.0–513), 82 | 4.0 (2.0–9.0), 4.0 | 3.0 (0–14.0), 4.4 |
| BD | 1.4(0–3.0), 1.1 | 2.0 (0–4), 2.2 | 2.0 (0–5.0), 2.3 | 1.0 (0–4.3), 1.2 | 1.7 (0–3.0), 1.6 | 3.0 (1.4–377), 8.6 | 1 (0–18.9), 1.1 | 1.1 (0–3.4), 1.2 | 70.3 (21.0–545), 88 | 3.0 (0–7.0), 3.8 | 1.7 (0–6.0), 2.0 |
| CSF | |||||||||||
| LNB | 1.0 (0–3.0), 0.9 | 2.0 (0–226), 10.2 | 2.9 (0–7.2), 2.9 | 6.9 (0–193), 25.6 | 7.6 (1–247), 27.8 | 97.3 (3.0–1,279), 177 | 3.0 (0–86.0), 12.6 | 2.0 (0–4.2), 1.8 | 113.3 (0.3–963), 275 | 4.5 (0–168), 8.7 | 1.0 (0–3.1), 1.0 |
| sLNB | 0 (0–3.0), 0.6 | 1.5 (0–13.7), 2.4 | 3.1 (0–7.0), 3.1 | 3.5 (0–6.9), 3.2 | 2.4 (1–11.9), 2.9 | 24.1 (3.5–141), 26.8 | 1.0 (0–3.0), 1.0 | 1.2 (0–4.6), 1.4 | 4.9 (0–39.8), 6.3 | 3.5 (0–8.1), 3.7 | 1.0 (0–7.0), 1.0 |
| TBE | 1.2 (0–5.6), 1.3 | 15.8 (0–306), 33.4 | 0 (0–3.9), 1.0 | 5.0 (0–11.6), 4.9 | 638 (4.8–4,442), 976 | 350 (37–1,811), 447 | 2.0 (1–5.2), 2.4 | 2.0 (1–5.2), 2.0 | 14.7 (0–118), 25.7 | 2.0 (0–6.0), 2.5 | 1.0 (0–3.7), 1.1 |
| OND | 0 (0–5.0), 0.6 | 1.8 (1.6–7.9), 3.2 | 2.0 (1–6), 2.7 | 1.0 (1–6.4), 2.5 | 3.1 (1.9–72.0), 6.5 | 24.3 (12–171), 32 | 0 (0–2.0), 0.4 | 1.5 (0–3.0), 1.4 | 8.6 (0–44.9), 12.8 | 3.0 (1.0–6.9), 3.0 | 0.6 (0–2.0), 0 |
LNB, working clinical diagnosis of evident Lyme neuroborreliosis; sLNB, working clinical diagnosis of suspected Lyme neuroborreliosis; TBE, tick-borne encephalitis; OND, other neurological diseases; BD, blood donors.
Assessment of patients with a working clinical diagnosis of evident Lyme borreliosis.
Patients with a working clinical diagnosis of evident Lyme neuroborreliosis were allocated into two subgroups of microbiologically confirmed and unconfirmed diagnoses, according to the presence or absence of intrathecal synthesis of borrelial antibodies and/or isolation of Borrelia organisms from CSF samples. Bivariate logistic regression analysis revealed no differences in the levels of cytokines and chemokines when comparing the two subgroups. The only exception was for the CXCL13 concentration in CSF, which was higher in the microbiologically confirmed subgroup (median, 409.7 pg/ml in the microbiologically confirmed versus 28.4 pg/ml in the microbiologically unconfirmed group; odds ratio [OR], 0.996 [95% confidence interval, 0.994-0.999]); however, an odds ratio with a 95% confidence interval very close to 1 indicated the low distinctive power of this marker. For further analysis, the two subgroups were treated as a single group of patients with a working clinical diagnosis of evident Lyme neuroborreliosis. Figures 1 and 2 represent the CXCL13 concentrations in serum and CSF samples from patients with clinically evident Lyme neuroborreliosis (including the subgroups with microbiologically confirmed and unconfirmed Lyme neuroborreliosis), clinically suspected Lyme neuroborreliosis, or TBE, from blood donors, and from patients with other neurological diseases.
Fig 1.
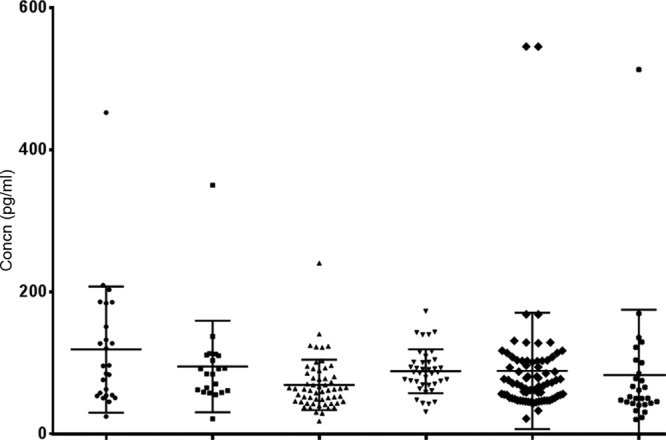
CXCL13 concentrations in sera from patients with clinically evident and microbiologically confirmed Lyme neuroborreliosis (circles), clinically evident but microbiologically unconfirmed Lyme neuroborreliosis (squares), clinically suspected Lyme neuroborreliosis (triangles), or tick-borne encephalitis (inverted triangles), from blood donors (diamonds), and from patients with other neurological diseases (asterisks). The horizontal lines represent the medians and the interquartile ranges.
Fig 2.
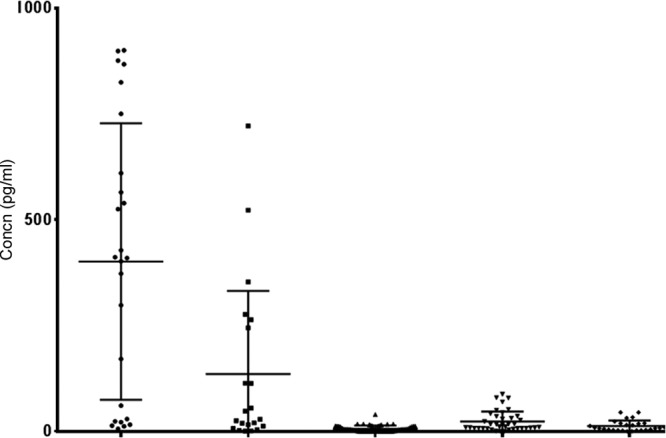
CXCL13 concentrations in CSF from patients with clinically evident and microbiologically confirmed Lyme neuroborreliosis (circles), clinically evident but microbiologically unconfirmed Lyme neuroborreliosis (squares), clinically suspected Lyme neuroborreliosis (triangles), or tick-borne encephalitis (inverted triangles) and from patients with other neurological diseases (diamonds). The horizontal lines represent the medians and the interquartile ranges.
Serum.
Bivariate analyses showed that the serum levels of four markers (CXCL13, IL-10, TNF-α, and IL-4) differed between individual groups (Table 2). These four markers were included in the multivariate model; the analysis showed that higher concentrations of CXCL13 were associated with higher odds of clinically evident Lyme neuroborreliosis than of clinically suspected Lyme neuroborreliosis, while higher levels of IL-10 were associated with higher odds of TBE than of clinically evident Lyme neuroborreliosis (Table 2).
Table 2.
Blood serum marker levels for prediction of disease type: results of bivariate and multivariate multinomial logistic regression analysesa
| Cytokine or chemokine | Analysis of suspected vs evident Lyme neuroborreliosis |
Analysis of TBE vs evident Lyme neuroborreliosisb |
||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate |
Multivariate |
Bivariate |
Multivariate |
|||||
| OR (95% CI)c | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| CXCL13 | 0.98 (0.97–0.99) | < 0.001 | 0.98 (0.97–0.99) | < 0.001 | 0.99 (0.99–1) | 0.18 | 0.99 (0.98–0.99) | 0.05 |
| IL-8 | 1 (1–1) | 0.35 | 1 (1–1) | 0.63 | ||||
| IL-1β | 1.05 (0.95–1.16) | 0.35 | 1.06 (0.95–1.17) | 0.29 | ||||
| IL-6 | 1 (1–1.01) | 0.35 | 0.99 (0.97–1.01) | 0.34 | ||||
| IL-10 | 1.29 (0.91–1.82) | 0.16 | 1.62 (1.06–2.46) | 0.03 | 1.72 (1.18–2.5) | 0.004 | 1.96 (1.26–3.04) | <0.001 |
| IL-12p70 | 1.09 (0.89–1.33) | 0.41 | 1.12 (0.92–1.37) | 0.26 | ||||
| IFN-γ | 0.93 (0.81–1.07) | 0.31 | 0.9 (0.76–1.06) | 0.22 | ||||
| TNF-α | 1 (0.93–1.08) | 0.98 | 1.05 (0.9–1.21) | 0.54 | 0.86 (0.75–1) | 0.04 | 1.01 (0.83–1.23) | 0.93 |
| IL-5 | 0.94 (0.86–1.02) | 0.15 | 0.96 (0.88–1.04) | 0.32 | ||||
| IL-4 | 1 (0.98–1.01) | 0.77 | 1 (0.98–1.01) | 0.67 | 0.85 (0.73–1) | 0.05 | 0.87 (0.71–1.07) | 0.20 |
| IL-2 | 0.99 (0.93–1.06) | 0.86 | 1.01 (0.94–1.08) | 0.89 | ||||
Statistically significant odds ratios (ORs) are in bold type.
TBE, tick-borne encephalitis.
CI, confidence interval.
CSF.
The results of bivariate logistic analysis are presented in Table 3. Higher concentrations of CXCL13, IL-6, IL-10, IL-5, and IL-2 were associated with significantly higher odds of clinically evident Lyme neuroborreliosis than of clinically suspected Lyme neuroborreliosis. Spearman's correlation coefficients for each pair of markers were calculated. High correlations were found between IL-6 and IL-8, TNF-α and IFN-γ, and TNF-α and IL-4 concentrations (ρ = 0.88, 0.77, and 0.7, respectively). Markers that were not highly correlated that best distinguished between evident Lyme neuroborreliosis and suspected Lyme neuroborreliosis and between evident Lyme neuroborreliosis and TBE in the bivariate analyses were included as predictors in the multivariate logistic regression model. The results are shown in Table 3. Only CXCL13 significantly distinguished patients with evident Lyme neuroborreliosis from patients with suspected Lyme neuroborreliosis, with the higher concentrations of CXCL13 being associated with higher odds of evident Lyme neuroborreliosis. Blood serum and CSF concentrations of CXCL13 in 2 patients with a working clinical diagnosis of suspected Lyme neuroborreliosis from whom the isolation of borreliae from CSF was successful have not differed from those of the rest of the group. Serum CXCL13 concentrations were 124.2 pg/ml and 89.1 pg/ml, while the corresponding CSF concentrations were 0 pg/ml.
Table 3.
CSF marker levels for prediction of disease type: results of bivariate and multivariate multinomial logistic regression analyses
| Cytokine or chemokine | Analysis of suspected vs evident Lyme neuroborreliosisa |
Analysis of TBE vs evident Lyme neuroborreliosisa |
||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate |
Multivariate |
Bivariate |
Multivariate |
|||||
| OR (95% CI)b | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| CXCL13 | 0.9 (0.85–0.94) | <0.001 | 0.91 (0.86–0.97) | 0.01 | 0.99 (0.97–0.99) | 0.01 | 1 (0.98–1.01) | 0.88 |
| IL-8 | 1 (1–1) | 0.48 | 1 (1–1) | 0.28 | ||||
| IL-1β | 0.73 (0.44–1.21) | 0.23 | 1.99 (1.19–3.32) | 0.01 | ||||
| IL-6 | 0.66 (0.53–0.83) | <0.001 | 0.83 (0.63–1.08) | 0.16 | 1.02 (1.01–1.03) | <0.001 | 1.03 (0.99–1.07) | 0.10 |
| IL-10 | 0.41 (0.29–0.59) | <0.001 | 0.82 (0.41–1.63) | 0.56 | 0.83 (0.71–0.97) | 0.02 | 0.87 (0.67–1.13) | 0.29 |
| IL-12p70 | 0.8 (0.56–1.15) | 0.22 | 1.17 (0.8–1.7) | 0.43 | ||||
| IFN-γ | 0.91 (0.75–1.09) | 0.30 | 0.66 (0.53–0.84) | <0.001 | ||||
| TNF-α | 1.04 (0.91–1.2) | 0.55 | 0.67 (0.55–0.82) | <0.001 | ||||
| IL-5 | 0.72 (0.62–0.85) | <0.001 | 0.89 (0.74–1.08) | 0.23 | 0.88 (0.8–0.97) | 0.01 | 0.63 (0.37–1.09) | 0.10 |
| IL-4 | 1.08 (0.88–1.33) | 0.45 | 0.53 (0.39–0.71) | <0.001 | ||||
| IL-2 | 0.88 (0.82–0.95) | 0.001 | 1.03 (1–1.05) | 0.03 | ||||
Statistically significant odds ratios (ORs) are in bold type.
CI, confidence interval.
None of the several markers that show significant differences comparing clinically evident Lyme neuroborreliosis and TBE in the bivariate testing showed significant distinctive power in the multivariate analysis (Table 3).
Receiver operating characteristic (ROC) analysis revealed a CSF CXCL13 cutoff level of 15.6 pg/ml that resulted in 80% sensitivity and 91% specificity when using patients with suspected Lyme neuroborreliosis as a control group, and a cutoff level of 18.9 pg/ml resulting in 76% sensitivity and 83% specificity was used when the control group included patients with suspected Lyme neuroborreliosis, TBE, and other neurological diseases.
Cytokine and chemokine levels according to intrathecal synthesis of borrelial antibodies.
Bivariate logistic analyses disclosed comparable blood serum concentrations of cytokines and chemokines in patients with present versus absent intrathecal synthesis of borrelial antibodies. The corresponding findings for CSF revealed significantly higher CXCL13 levels in patients with a working clinical diagnosis of evident Lyme neuroborreliosis in whom intrathecal synthesis of borrelial antibodies was established than in a subgroup without such synthesis (Table 4). As indicated by the odds ratio with a 95% confidence interval that was very close to 1, the distinctive power of this marker was low.
Table 4.
CSF marker levels for prediction of intrathecal synthesis of borrelial antibodies and prediction of Borrelia isolation from CSF in patients with clinically evident Lyme neuroborreliosisc
| Cytokine or chemokine | Present vs absent intrathecal synthesis |
Positive vs negative culture result |
||
|---|---|---|---|---|
| OR (95% CI)a | P | OR (95% CI) | P | |
| CXCL13 | 1.004 (1.001–1.006) | 0.004b | 1 (1–1) | 0.33 |
| IL-8 | 1 (1–1.01) | 0.12 | 1 (1–1) | 0.76 |
| IL-1β | 1.55 (0.77–3.13) | 0.22 | 0.78 (0.3–2.01) | 0.60 |
| IL-6 | 1 (0.98–1.01) | 0.66 | 1 (0.98–1.02) | 0.97 |
| IL-10 | 1.01 (0.98–1.04) | 0.45 | 1.01 (0.97–1.04) | 0.65 |
| IL-12p70 | 0.94 (0.54–1.63) | 0.83 | 0.97 (0.48–1.96) | 0.94 |
| IFN-γ | 0.99 (0.95–1.03) | 0.53 | 0.65 (0.44–0.97) | 0.03 |
| TNF-α | 1.21 (0.97–1.5) | 0.09 | 0.82 (0.62–1.08) | 0.16 |
| IL-5 | 1.01 (0.99–1.03) | 0.28 | 1.01 (0.99–1.02) | 0.31 |
| IL-4 | 1.12 (0.86–1.46) | 0.40 | 0.7 (0.47–1.05) | 0.08 |
| IL-2 | 0.99 (0.96–1.02) | 0.50 | 0.96 (0.84–1.09) | 0.53 |
CI, confidence interval.
Indicates a statistically significant odds ratio (OR).
Results of bivariate logistic regression.
Culture.
A comparison of cytokine and chemokine CSF concentrations in patients with a working clinical diagnosis of evident Lyme neuroborreliosis according to CSF culture result revealed that higher concentrations of IFN-γ were associated with lower odds of Borrelia isolation (Table 4).
DISCUSSION
The diagnosis of Lyme neuroborreliosis is complex and combines clinical features, laboratory indication of meningeal inflammation (i.e., lymphocytic pleocytosis), and a demonstration of borrelial infection of the central nervous system by the isolation of the etiological agent from CSF, demonstration of borreliae in CSF by PCR, and/or detection of intrathecal synthesis of specific antibodies. The latter is a well-recognized diagnostic marker for Lyme neuroborreliosis and is an essential criterion for the diagnosis of Lyme neuroborreliosis in Europe, although it may not be detectable during the first few weeks of the disease (3, 5, 22). However, in spite of this limitation, intrathecal synthesis of borrelial antibodies has a much higher sensitivity than that of the isolation of borreliae from CSF. In the present study, the proportion of patients with intrathecal production of specific antibodies was lower than expected, while the CSF culture results were relatively promising. Namely, in patients with proven Lyme neuroborreliosis, the reported recovery rate of B. burgdorferi sensu lato from CSF was, as a rule, <10% (23); the highest reported yield has been 17%, in a study by van Dam et al. (24). In the present study, isolation of Borrelia from the CSF samples of patients with a working clinical diagnosis of evident Lyme neuroborreliosis was successful in 8/46 (17.4%) samples. B. garinii was identified in 7/8 CSF isolates, which corroborates the results of previous studies regarding the predominance of B. garinii strains isolated from the CSF samples of European patients with Lyme neuroborreliosis (21, 23, 25). In contrast, all isolated strains from patients with a working clinical diagnosis of suspected Lyme neuroborreliosis (i.e., from patients who had Lyme borreliosis but normal CSF cell counts) were typed as B. afzelii Mla1; this finding substantiates previous results (21). On the other hand, we demonstrated intrathecal synthesis of borrelial IgM and/or IgG antibodies in 45.7% of patients with a working clinical diagnosis of evident Lyme neuroborreliosis. The proportion is rather low compared to that in previous studies, in which sensitivity of the Lyme borreliosis IDEIA kit was reported to be 79% (5), while the sensitivity of an enzyme-linked immunosorbent assay (ELISA) using the whole-cell antigen of B. afzelii PKo was 75 to 80% (26, 27). A possible explanation for this distinction might be the rather short duration of neurological symptoms (median, 10 days) in the present study. It has been recognized previously that the proportion of patients with established intrathecal synthesis of borrelial antibodies increases with the duration of neurological symptoms (20).
Borrelial infection stimulates the secretion of different cytokines and chemokines that are involved in the innate and adaptive immune responses. In the present study, we determined the levels of different cytokines and chemokines in the acute phase of Lyme neuroborreliosis prior to antibiotic treatment, with the aim of identifying possible serum and/or CSF marker(s) that would enable a distinction between evident and suspected Lyme neuroborreliosis, as well as between Lyme neuroborreliosis and other conditions. Additionally, in patients with evident Lyme neuroborreliosis, we sought to evaluate the cytokine and chemokine levels according to the B. burgdorferi sensu lato CSF culture result and the presence or absence of intrathecal synthesis of specific borrelial antibodies.
In addition to the finding that patients with a working clinical diagnosis of evident Lyme neuroborreliosis had higher blood serum concentrations of almost all tested cytokines and chemokines than those of the blood donors (Table 1), several other distinctions were found, the majority based on CSF-associated findings.
The results of the present study indicate that the serum levels of the tested cytokines and chemokines could not discriminate between clinically evident and suspected Lyme neuroborreliosis. The only exception was for CXCL13. However, even though the serum concentrations of CXCL13 were significantly higher in patients with clinically evident Lyme neuroborreliosis than in the group with suspected Lyme neuroborreliosis, the distinctive power (as indicated by the odds ratio and 95% confidence interval being very close to 1) was low, and the marker did not discriminate between clinically evident Lyme neuroborreliosis and TBE (Table 2). These findings are in concordance with previous results indicating that serum CXCL13 concentration is not an appropriate marker for diagnosing Lyme neuroborreliosis (11, 28).
According to bivariate multinomial logistic regression, CSF concentrations of CXCL13, IL-10, IFN-γ, TNF-α, IL-5, IL-4, IL-6, and IL-1β might be useful for making a distinction between evident Lyme neuroborreliosis and TBE. Higher concentrations of CXCL13, IL-10, IFN-γ, TNF-α, IL-5, and IL-4 were associated with higher odds of evident Lyme neuroborreliosis, while higher levels of IL-1β and IL-6 were related to TBE (Table 4). These findings are not in accordance with the results of Cepok et al. (7), who reported nonsignificant differences in IL-6 and IL-8 CSF concentrations when they compared patients with Lyme neuroborreliosis and patients with viral meningitis.
CSF concentrations of IL-2, IL-5, IL-6, IL-10, and CXCL13 were higher in patients with evident Lyme neuroborreliosis than in those with suspected Lyme neuroborreliosis (Table 3). However, multivariate multinomial logistic regression revealed that only CXCL13 significantly distinguished between clinically suspected and clinically evident Lyme neuroborreliosis (Table 3), but the odds ratio of 0.91 and 95% confidence interval (0.86 to 0.97) being close to 1 indicated the limited distinctive power of this marker. While our results corroborate previous findings that CXCL13 CSF concentration is the most useful diagnostic marker for Lyme neuroborreliosis (11, 14, 17, 29), they show that it has a limited distinctive value.
Previously, numerous cutoff values of CXCL13 in CSF have been suggested as optimal, such as 61 pg/ml (30), 142 pg/ml (31), 250 pg/ml (17), 337 pg/ml (11), and 1,229 pg/ml (29). In the present study, cutoffs of 15.6 pg/ml and 18.9 pg/ml were determined, which are much lower than those in previously published results. Nevertheless, the sensitivity of the CXCL13 was higher than the intrathecal synthesis of specific antibodies.
Similarly, although an evaluation of CSF cytokine and chemokine levels in patients with and without intrathecal synthesis of specific borrelial antibodies revealed that CXCL13 CSF concentration was significantly associated with intrathecal synthesis of borrelial antibodies (Table 4), the odds ratio being very close to 1 indicates the low distinctive power of this marker.
A comparison of cytokine and chemokine concentrations in CSF among Borrelia-culture-positive and Borrelia-culture-negative patients (Table 4) revealed that the concentrations of IFN-γ were higher in culture-negative patients than in culture-positive patients. Previous studies on animal models have shown beneficial roles of IL-4 and IFN-γ in limiting spirochetal burden and eliminating the disease (32, 33), suggesting that culture negativity might be explained by the eradication of spirochetes by an increased Th1 immune response triggered by IFN-γ.
Conclusions.
A comparison of patients with clinically evident Lyme neuroborreliosis, suspected Lyme neuroborreliosis, TBE, or other neurologic diseases and blood donors revealed significant differences in the blood serum and CSF concentrations of several cytokines and chemokines between the groups. However, as indicated by the odds ratios being close to 1, the distinctive power of the majority of these findings was low. Additional research using well-defined groups of patients is needed to appraise the potential diagnostic usefulness of these markers. Our study suggests that CXCL13 CSF concentration alone may not have as excellent of a diagnostic value for the confirmation of Lyme neuroborreliosis diagnosis as has been reported previously.
ACKNOWLEDGMENT
This study was supported by the Slovenian Research Agency (grant no. Z3-2254-0381 [to T.C.] and grant no. P3-0296 and J3-3636 [to F.S.]).
Footnotes
Published ahead of print 14 August 2013
REFERENCES
- 1.Stanek G, Strle F. 2003. Lyme borreliosis. Lancet 362:1639–1647 [DOI] [PubMed] [Google Scholar]
- 2.Stanek G, Wormser GP, Gray J, Strle F. 2011. Lyme borreliosis. Lancet 379:461–473 [DOI] [PubMed] [Google Scholar]
- 3.Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, Kristoferitsch W, O'Connell S, Ornstein K, Strle F, Gray J. 2011. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 17:69–79. 10.1111/j.1469-0691.2010.03175.x [DOI] [PubMed] [Google Scholar]
- 4.Kaiser R, Rauer S. 1998. Analysis of the intrathecal immune response in neuroborreliosis to a sonicate antigen and three recombinant antigens of Borrelia burgdorferi sensu stricto. Eur. J. Clin. Microbiol. Infect. Dis. 17:159–166 [DOI] [PubMed] [Google Scholar]
- 5.Ljøstad U, Skarpaas T, Mygland A. Clinical usefulness of intrathecal antibody testing in acute Lyme neuroborreliosis. Eur. J. Neurol. 14:873–876 [DOI] [PubMed] [Google Scholar]
- 6.Hammers-Berggren S, Hansen K, Lebech AM, Karlsson M. 1993. Borrelia burgdorferi-specific intrathecal antibody production in neuroborreliosis: a follow-up study. Neurology 43:169–175 [DOI] [PubMed] [Google Scholar]
- 7.Cepok S, Zhou D, Vogel F, Rosche B, Grummel V, Sommer N, Hemmer B. 2003. The immune response at onset and during recovery from Borrelia burgdorferi meningoradiculitis. Arch. Neurol. 60:849–855 [DOI] [PubMed] [Google Scholar]
- 8.Grusell M, Widhe M, Ekerfelt C. 2002. Increased expression of the Th1-inducing cytokines interleukin-12 and interleukin-18 in cerebrospinal fluid but not in sera from patients with Lyme neuroborreliosis. J. Neuroimmunol. 131:173–178 [DOI] [PubMed] [Google Scholar]
- 9.Widhe M, Grusell M, Ekerfelt C, Vrethem M, Forsberg P, Ernerudh J. 2002. Cytokines in Lyme borreliosis: lack of early tumour necrosis factor-alpha and transforming growth factor-beta1 responses are associated with chronic neuroborreliosis. Immunology 107:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rupprecht TA, Pfister HW, Angele B, Kastenbauer S, Wilske B, Koedel U. 2005. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65:448–450 [DOI] [PubMed] [Google Scholar]
- 11.Senel M, Rupprecht TA, Tumani H, Pfister HW, Ludolph AC, Brettschneider J. 2010. The chemokine CXCL13 in acute neuroborreliosis. J. Neurol. Neurosurg. Psychiatry 81:929–933 [DOI] [PubMed] [Google Scholar]
- 12.Brandes M, Legler DF, Spoerri B, Schaerli P, Moser B. 2000. Activation-dependent modulation of B lymphocyte migration to chemokines. Int. Immunol. 12:1285–1292 [DOI] [PubMed] [Google Scholar]
- 13.Khademi M, Kockum I, Andersson ML, Iacobaeus E, Brundin L, Sellebjerg F, Hillert J, Piehl F, Olsson T. 2011. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult. Scler. 17:335–343 [DOI] [PubMed] [Google Scholar]
- 14.Rupprecht TA, Plate A, Adam M, Wick M, Kastenbauer S, Schmidt C, Klein M, Pfister HW, Koedel U. 2009. The chemokine CXCL13 is a key regulator of B cell recruitment to the cerebrospinal fluid in acute Lyme neuroborreliosis. J. Neuroinflammation 6:42. 10.1186/1742-2094-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brettschneider J, Czerwoniak A, Senel M, Fang L, Kassubek J, Pinkhardt E, Lauda F, Kapfer T, Jesse S, Lehmensiek V, Ludolph AC, Otto M, Tumani H. 2010. The chemokine CXCL13 is a prognostic marker in clinically isolated syndrome (CIS). PLoS One 5:e11986. 10.1371/journal.pone.0011986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T, Berthele A, Hemmer B. 2012. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J. Neuroinflammation 9:93. 10.1186/1742-2094-9-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Burgel ND, Bakels F, Kroes AC, van Dam AP. 2011. Discriminating Lyme neuroborreliosis from other neuroinflammatory diseases by levels of CXCL13 in cerebrospinal fluid. J. Clin. Microbiol. 49:2027–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rupprecht TA, Koedel U, Angele B, Fingerle V, Pfister HW. 2006. Cytokine CXCL13–a possible early CSF marker for neuroborreliosis. Nervenarzt 77:470–473 (Article in German.) [DOI] [PubMed] [Google Scholar]
- 19.Ljøstad U, Mygland A. 2008. CSF B–lymphocyte chemoattractant (CXCL13) in the early diagnosis of acute Lyme neuroborreliosis. J. Neurol. 255:732–737 [DOI] [PubMed] [Google Scholar]
- 20.Cerar T, Ogrinc K, Strle F, Ružiæ-Sabljiæ E. 2010. Humoral immune responses in patients with Lyme neuroborreliosis. Clin. Vaccine Immunol. 17:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ružiæ-Sabljiæ E, Maraspin V, Lotriè-Furlan S, Jurca T, Logar M, Pikelj-Pecnik A, Strle F. 2002. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien. Klin. Wochenschr. 114:544–550 [PubMed] [Google Scholar]
- 22.Mygland A, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I, European Federation of Neurological Societies 2010. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur. J. Neurol. 17:8–16, e1–e4. 10.1111/j.1468-1331.2009.02862.x [DOI] [PubMed] [Google Scholar]
- 23.Strle F, Ružiæ-Sabljiæ E, Cimperman J, Lotriè-Furlan S, Maraspin V. 2006. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin. Infect. Dis. 43:704–710 [DOI] [PubMed] [Google Scholar]
- 24.van Dam AP, Kuiper H, Vos K, Widjojokusumo A, de Jongh BM, Spanjaard L, Ramselaar AC, Kramer MD, Dankert J. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708–717 [DOI] [PubMed] [Google Scholar]
- 25.Cerar T, Ogrinc K, Cimperman J, Lotriè-Furlan S, Strle F, Ružiæ-Sabljiæ E. 2008. Validation of cultivation and PCR methods for diagnosis of Lyme neuroborreliosis. J. Clin. Microbiol. 46:3375–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roux F, Boyer E, Jaulhac B, Dernis E, Closs-Prophette F, Puéchal X. 2007. Lyme meningoradiculitis: prospective evaluation of biological diagnosis methods. Eur. J. Clin. Microbiol. Infect. Dis. 26:685–693 [DOI] [PubMed] [Google Scholar]
- 27.Blanc F, Jaulhac B, Fleury M, de Seze J, de Martino SJ, Remy V, Blaison G, Hansmann Y, Christmann D, Tranchant C. 2007. Relevance of the antibody index to diagnose Lyme neuroborreliosis among seropositive patients. Neurology 69:953–958 [DOI] [PubMed] [Google Scholar]
- 28.Wutte N, Berghold A, Loffler S, Zenz W, Daghofer E, Krainberger I, Kleinert G, Aberer E. 2011. CXCL13 chemokine in pediatric and adult neuroborreliosis. Acta Neurol. Scand. 124:321–328 [DOI] [PubMed] [Google Scholar]
- 29.Schmidt C, Plate A, Angele B, Pfister HW, Wick M, Koedel U, Rupprecht TA. 2011. A prospective study on the role of CXCL13 in Lyme neuroborreliosis. Neurology 76:1051–1058 [DOI] [PubMed] [Google Scholar]
- 30.Bremell D, Mattsson N, Edsbagge M, Blennow K, Andreasson U, Wikkelsö C, Zetterberg H, Hagberg L. 2013. Cerebrospinal fluid CXCL13 in Lyme neuroborreliosis and asymptomatic HIV infection. BMC Neurol. 13:2–8. 10.1186/1471-2377-13-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tjernberg I, Henningsson AJ, Eliasson I, Forsberg P, Ernerudh J. 2011. Diagnostic performance of cerebrospinal fluid chemokine CXCL13 and antibodies to the C6-peptide in Lyme neuroborreliosis. J. Infect. 62:149–158 [DOI] [PubMed] [Google Scholar]
- 32.Keane-Myers A, Nickell SP. 1995. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020–2028 [PubMed] [Google Scholar]
- 33.Brown CR, Reiner SL. 1999. Experimental lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]


