Abstract
Recent observations from Africa have rekindled interest in the role of serum bactericidal antibodies in protecting against systemic infection with Salmonella enterica serovar Typhimurium. To determine whether the findings are applicable to other populations, we analyzed serum samples collected from healthy individuals in the United States. We found that all but 1 of the 49 adult samples tested had robust bactericidal activity against S. Typhimurium in a standard in vitro assay. The activity was dependent on complement and could be reproduced by immunoglobulin G (IgG) purified from the sera. The bactericidal activity was inhibited by competition with soluble lipopolysaccharide (LPS) from S. Typhimurium but not from Escherichia coli, consistent with recognition of a determinant in the O-antigen polysaccharide. Sera from healthy children aged 10 to 48 months also had bactericidal activity, although it was significantly less than in the adults, correlating with lower levels of LPS-specific IgM and IgG. The lone sample in our collection that lacked bactericidal activity was able to inhibit killing of S. Typhimurium by the other sera. The inhibition correlated with the presence of an LPS-specific IgM and was associated with decreased complement deposition on the bacterial surface. Our results indicate that healthy individuals can have circulating antibodies to LPS that either mediate or inhibit killing of S. Typhimurium. The findings contrast with the observations from Africa, which linked bactericidal activity to antibodies against an S. Typhimurium outer membrane protein and correlated the presence of inhibitory anti-LPS antibodies with human immunodeficiency virus infection.
INTRODUCTION
Gastroenteritis caused by nontyphoidal Salmonella (NTS) infection is a major public health problem in Africa, with an estimated disease burden of 2.5 million cases and 4,100 deaths per year (1–3). Moreover, invasive NTS disease, specifically, septicemia caused by Salmonella enterica serovar Typhimurium, is an important cause of infant morbidity and mortality in sub-Saharan Africa, with case fatality rates as high as 24% (1, 2). Understanding the immunological mechanisms that protect against invasive disease caused by NTS is essential to developing effective vaccines against this potentially devastating infection. Although protection is usually thought to depend mainly on cell-mediated immunity (4), recent clinical data have rekindled interest in the role played by antibodies. Serum antibodies that are able to mediate in vitro, complement-dependent killing of various bacteria, including S. Typhimurium, have been studied for a long time, but evidence of their importance in protection against in vivo infection has been scanty (5). This issue has received renewed interest as a result of recent studies from Malawi. These studies demonstrated that most healthy individuals older than 16 months had serum IgM and IgG antibodies that killed S. Typhimurium in vitro in a complement-dependent fashion but that healthy children below this age lacked such bactericidal antibodies, observations that correlated with the declining occurrence of NTS bacteremia in patients above the age of 12 months (6). The anti-Salmonella antibodies also facilitated bacterial killing by promoting the oxidative burst activity of circulating phagocytes (7). Further evidence of the importance of serum bactericidal antibodies in protection against S. Typhimurium was provided by data showing that the effect of these antibodies was inhibited in human immunodeficiency virus (HIV)-infected African adults, a population that is unusually susceptible to NTS bacteremia (3, 8). The inhibition was shown to be mediated by high levels of IgG antibodies to the O antigen of S. Typhimurium lipopolysaccharide (LPS) present in the sera of the HIV-infected individuals, which were proposed to prevent the action of bactericidal anti-outer membrane protein antibodies present in HIV-uninfected adults (8, 9).
Although there have been many studies carried out to characterize the molecular basis of the susceptibility and resistance of S. Typhimurium to killing by human serum, most have made use of pooled sera and have focused on the role of complement (10). One of the few analyses of individual sera, apart from the recent ones from Malawi, compared bactericidal activities against S. Typhimurium in healthy controls and patients with sickle cell anemia but did not examine the antigenic target of the bactericidal antibodies (11). Thus, it is not clear whether the features of the anti-Salmonella bactericidal activity delineated in Malawi—its age-dependent increase, its mediation by anti-outer membrane protein antibodies in non-HIV-infected adults, and its inhibition by high levels of anti-LPS antibodies in HIV-infected adults (6, 8)—are peculiar to that country or whether they are applicable to other populations. It is of particular interest and importance to determine if the findings from Africa can be extended to a high-income country such as the United States, where the burden of NTS disease is considerably lower (about 1.4 million infections and 400 deaths per year) (12). Accordingly, we carried out a study to characterize serum bactericidal activity against S. Typhimurium in healthy individuals from the United States. Our results confirmed some of the observations from Africa, including the wide prevalence of the bactericidal activity and its increase with maturation from child to adult, but also revealed unexpected differences regarding the roles of anti-LPS antibodies in both mediating and inhibiting the killing of S. Typhimurium.
MATERIALS AND METHODS
Blood samples.
Deidentified serum and plasma samples from the United States were provided by the Partners Biorepository under protocol number 2012-P-000484/1. The samples were collected from healthy adults (18 to 75 years of age) and children (6 months to 5 years of age) during routine medical checkups by their primary care providers at Massachusetts General Hospital. Aliquots of excess serum that otherwise would have been discarded were selected by the staff of the biorepository using International Statistical Classification of Diseases and Related Health Problems (ICD-9) codes V70.0 (“routine general medical examination at a health care facility”) and V20.2 (“routine infant or child health check”). Based on the time required for identification and selection, the samples spent 12 to 16 h at 4°C before being stored at −80°C until use. The protocol was reviewed by the Human Research Committee of Massachusetts General Hospital and deemed not to meet the definition of human subject research.
Bacterial strains.
The S. Typhimurium strains SL1344 (wild type, mouse and calf virulent), SL3201 (wild type, mouse virulent), F98 (wild type, chicken isolate), JS107 (wild-type derivative of strain 14028, a chicken isolate), JS93 (the isogenic oafA-deficient derivative of JS107), and the S. enterica serovar Dublin strain 2229 have all been described previously (13–17). SL1344, SL3201, and 2229 were originally obtained from Beth McCormick, University of Massachusetts Medical Center, Worcester, MA, and F98 was obtained from Edouard Galyov, Institute for Animal Health, Berkshire, United Kingdom. JS107 and JS93 were provided for this study by James Slauch, University of Illinois, Urbana, IL. In addition, a human isolate of S. Typhimurium DT104, strain ATCC BAA-189, was obtained from the American Type Culture Collection, Manassas, VA (18). Bacteria were grown overnight in Luria-Bertani (LB) broth at 37°C with vigorous agitation on a rotary shaker. The saturated cultures were back-diluted 1/100 into fresh LB broth and then grown to an optical density at 600 nm of 0.1 to 0.15. The bacteria were harvested by centrifugation, washed with phosphate-buffered saline (PBS), and resuspended in PBS at a density of approximately 2 × 106 CFU per ml based on preliminary titering experiments.
Bactericidal assay.
Killing of Salmonella by serum or plasma was assessed essentially as described by MacLennan et al. (6). In brief, 5 μl of bacterial suspension prepared as described above (containing approximately 104 CFU) was incubated with 22.5 μl of serum or plasma and 22.5 μl of PBS at 37°C for 1 h. The number of bacteria surviving at the end of that period was determined by serial dilution of the reaction mix and plating on LB agar and are displayed as log10 numbers of CFU in the figures. LPS competition was carried out as described previously (8) by preincubating sera or plasma for 30 to 60 min at room temperature with 100 μg/ml of purified LPS from either S. Typhimurium or Escherichia coli (O111:B4) before they were tested for bactericidal activity. Both LPS preparations were obtained from List Biological Laboratories, Inc., Campbell, CA (catalog number 225 or 201, respectively). To test the role of antibody in the bactericidal activity, IgG was purified from 100-μl aliquots of individual sera using the Melon Gel IgG purification kit (Thermo Scientific, Rockford, IL) according to the manufacturer's directions. Approximately 90% pure IgG was obtained as indicated by protein staining (not shown). The purified IgGs were used in the bactericidal assay at concentrations comparable to those of the parental sera along with a source of human complement (nonbactericidal serum depleted of anti-Salmonella antibodies as described below). To test for inhibitory activity, 22.5 μl of bactericidal serum or plasma was mixed with an equal volume of the nonbactericidal sample and then incubated at 37°C for 1 h with 5 μl of bacterial suspension. The number of surviving bacteria was determined as described above.
Hemolytic complement assay.
We estimated hemolytic complement activity in our samples using a published microassay that has been shown to have good correlation with the traditional CH50 titration method (19). In brief, 50-μl aliquots of serum or plasma were incubated with 150 μl of antibody-coated sheep erythrocytes suspended at 5 × 108 cells per ml in gelatin-veronal buffer (Complement Technology Inc., Tyler, TX) for 30 min at 37°C in a microtiter plate. In addition, 50 μl of human serum complement (Sigma-Aldrich, St. Louis, MO; derived from pooled normal human plasma; CH50 of 41 units/ml) was assayed in parallel to provide a reference hemolytic activity. The negative control consisted of 50 μl of gelatin-veronal buffer instead of serum or plasma, while 50 μl of serum plus 150 μl of buffer was used as the blank. At the end of 30 min, the plate was vortexed briefly and the absorbance at 700 nm (A700) read as an indicator of the number of nonhemolyzed erythrocytes left in the reaction. The difference in A700 between the negative control and the test or reference sample provides a measure of hemolytic activity. The hemolytic activity of the individual serum or plasma samples was calculated as a percentage of the activity of the reference sample as follows: A700 of negative control – A700 of test sample/A700 of negative control – A700 of reference sample × 100.
Absorption of anti-Salmonella antibodies from serum.
One and one-half milliliter of an overnight saturated culture of either SL1344 or 2229 was spun down, and the bacterial pellet was washed with PBS and resuspended in 0.5 ml of the serum sample. The samples were incubated for 1 h at 4°C with gentle rotation. After the bacteria were spun down, the supernatant serum was filter sterilized by passage through a 0.22-μm filter and stored at −80°C until use.
Enzyme-linked immunosorbent assay (ELISA) for serum antibodies.
To detect S. Typhimurium LPS-specific antibodies, we used procedures that we have described in detail earlier (20). Immulon II microtiter plates were coated with S. Typhimurium LPS diluted in PBS at a concentration of 10 μg/ml, washed, blocked, and then incubated overnight at 4°C with triplicate aliquots of sera diluted 500-fold in PBS. The plates were developed with horseradish peroxidase-conjugated secondary antibodies specific for human IgM or human IgG (BD Biosciences, San Jose, CA), followed by an o-phenylenediamine substrate. The intensity of color change was measured at 490 nm in a plate reader.
Total serum IgM, IgG, and IgA were measured using ELISA kits from eBioscience (San Diego, CA) and by following the manufacturer's guidelines.
Flow cytometry of bacteria.
To examine IgM binding to bacteria, 5 μl of PBS containing 107 CFU of mid-log-phase SL1344 was incubated for 30 min on ice with 12.5 μl of undiluted serum and 12.5 μl of PBS. After the bacteria were washed, bound IgM was detected by a biotinylated anti-human IgM antibody followed by Cy5-conjugated streptavidin (BD Biosciences, San Jose, CA). Analysis of fluorescence was carried out on an Accuri C6 flow cytometer. Examination of complement deposition on the bacterial surface was carried out similarly except that the initial incubation with serum was at 37°C for 15 min. The presence of activated complement on the bacteria was detected using a mouse antibody specific for the activated terminal complement components C5b to 9 (aE11; Abcam, Cambridge, MA) followed by a fluorescein-conjugated anti-mouse IgG antibody and flow cytometric analysis.
Statistical analysis.
Results from groups of serum or plasma samples are displayed as scattergrams, with each symbol representing an individual sample and a horizontal line the median of the group. Intergroup differences were analyzed by the Mann-Whitney U test using Prism v3.0 (GraphPad Software, Inc., La Jolla, CA). A P value of <0.05 was considered to be significant. Specific P values corresponding to the asterisks in the figures are provided in the figure legends.
RESULTS
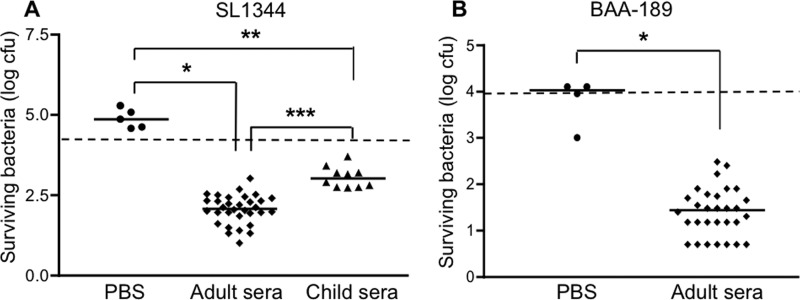
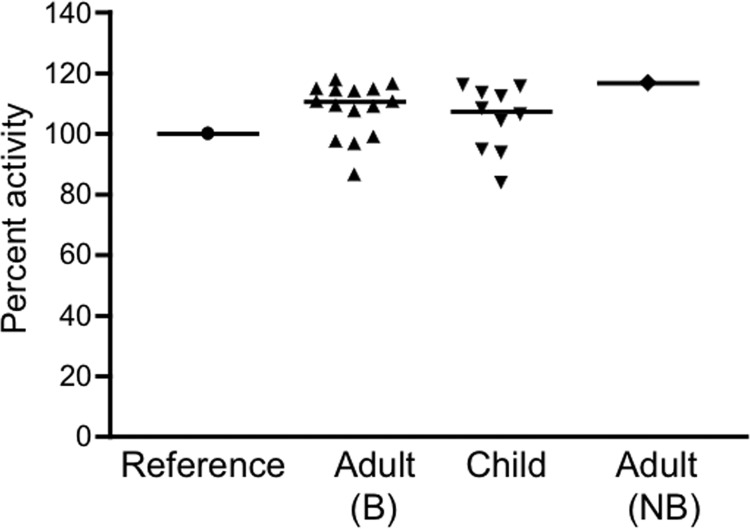
Deidentified serum or plasma samples from healthy adults (18 to 75 years old), obtained during routine health maintenance visits at a Boston hospital, were used to assess bactericidal activity against the S. Typhimurium strain SL1344 as described in Materials and Methods. Although SL1344 has been reported to be resistant to the bactericidal activity of human serum (21), the results from the first 32 of our samples clearly demonstrated that incubation of the bacteria with the serum or plasma for 1 h resulted in a statistically significant 1- to 2-log drop in the number of CFU (relative to the starting inoculum of 104 CFU), whereas incubation with PBS alone or human complement alone (serum depleted of anti-Salmonella antibodies by preabsorption) did not lead to a decrease in bacterial number (Fig. 1A and data not shown). Similar bactericidal activity was obtained with an additional 16 out of 17 samples examined and was abolished when the sample was heated to 56°C for 30 min (data not shown). To confirm that the handling and storage of our samples had not compromised their complement activity, we assayed their ability to lyse antibody-coated sheep erythrocytes. Most of the samples tested had hemolytic complement activity that was equal to or greater than the activity of the reference complement used in the assay (pooled human plasma), while the remainder had activity that ranged from 84 to 99% of the reference activity (Fig. 2). This distribution of activities is very similar to results obtained by others using randomly selected human serum samples (12). Taken together, our findings indicate that complement is functionally intact in our samples. Sera from 10 healthy Boston children aged 10 to 48 months were also able to kill S. Typhimurium, but the reduction in bacterial numbers was significantly less than that achieved by the adult sera (Fig. 1A). The reduced bactericidal activity of the pediatric samples was not related to a deficiency of complement, since the hemolytic complement activities of these samples did not differ significantly from those of the adults (Fig. 2) (P = 0.39). We also tested the sera against 3 additional strains of S. Typhimurium, JS107, SL3201, and F98, that have origins and virulence characteristics distinct from SL1344's (13–17) and obtained similar results (data for JS107 are shown in Fig. 5; SL3201 and F98 results are not shown). Moreover, when we tested 30 of the adult sera against a human isolate of S. Typhimurium (BAA-189 [18]), the bacteria were killed as effectively as were the laboratory strains by all of the samples (Fig. 1B). Adult sera and SL1344 were used in most of the experiments described here except where specifically indicated. To evaluate the role of antibody in the bactericidal activity, IgG was purified from 4 of the sera and tested in the presence of a source of complement (nonbactericidal human serum depleted of anti-Salmonella antibodies by preabsorption with SL1344 as described in Materials and Methods). All 4 purified IgGs were able to kill Salmonella in the presence of this source of complement (Fig. 3) but not when complement was inactivated by heating it to 56°C (data not shown).
Fig 1.
Serum bactericidal activity against S. Typhimurium. (A) S. Typhimurium strain SL1344 was incubated with PBS, adult sera, or child sera for 60 min at 37°C. The numbers of surviving bacteria were determined by plating serial dilutions of the reaction mixtures. *, P = 0.0004; **, P = 0.0007; ***, P < 0.0001. (B) The S. Typhimurium human isolate BAA-189 was incubated with PBS or adult sera for 60 min at 37°C. The numbers of surviving bacteria were determined by plating serial dilutions of the reaction mixtures. *, P = 0.0015. The dashed lines indicate the starting inocula.
Fig 2.
Serum hemolytic complement activity. Hemolytic complement activity against antibody-coated sheep erythrocytes was evaluated as described in Materials and Methods using bactericidal (B) adult samples, child samples, or the nonbactericidal (NB) adult sample. The results are shown as percentages of the activity of a commercial human complement used as a reference for this experiment.
Fig 5.
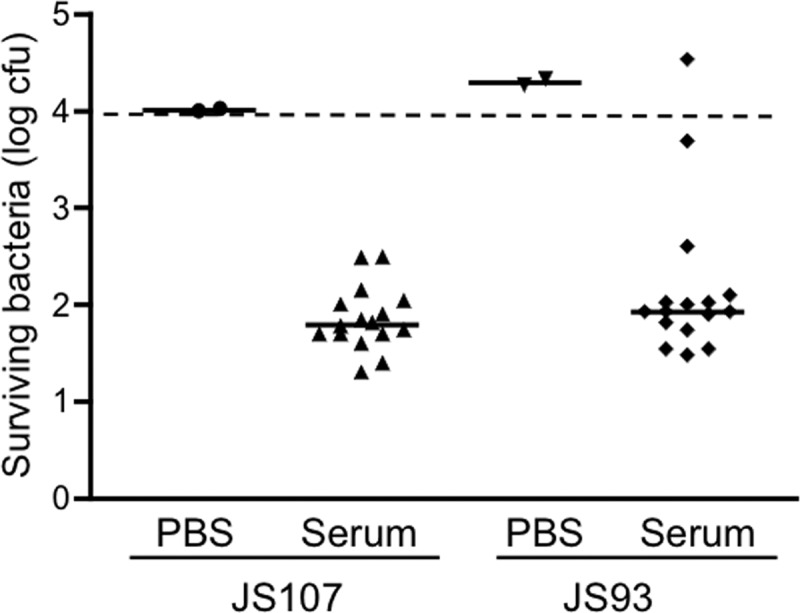
Comparison of serum bactericidal activities against S. Typhimurium strains JS107 (wild-type) and JS93 (oafA mutant). JS107 and JS93 were incubated at 37°C for 60 min with PBS or adult sera. The numbers of surviving bacteria were determined by plating serial dilutions of the reaction mixtures. The dashed line indicates the starting inoculum.
Fig 3.
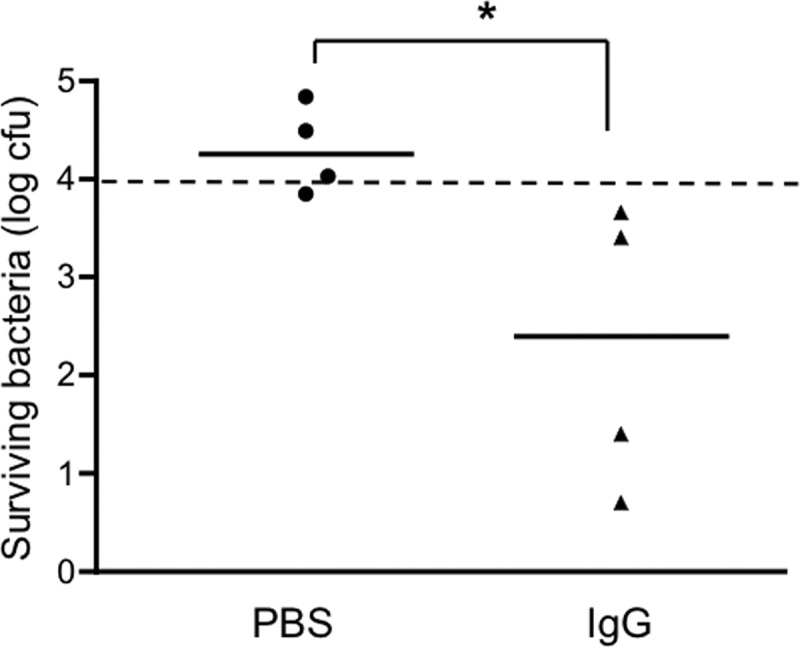
Results of a bactericidal assay using IgG purified from adult sera. S. Typhimurium strain SL1344 was incubated with PBS or with purified IgG and a source of complement for 60 min at 37°C. The numbers of surviving bacteria were determined by plating serial dilutions of the reaction mixtures. *, P = 0.029. The dashed line indicates the starting inoculum.
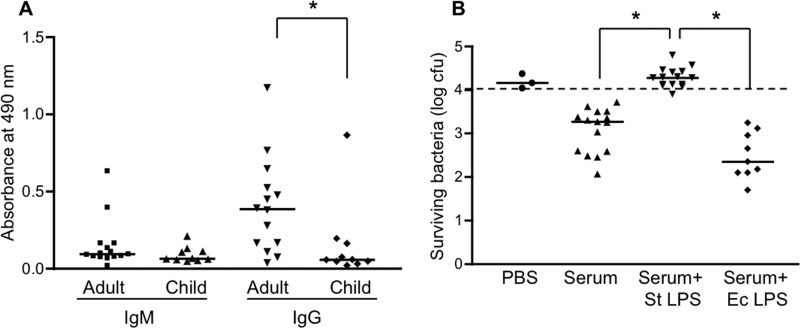
We considered the possibility that the antigenic target of the bactericidal activity was LPS since both IgM and IgG antibodies to S. Typhimurium LPS were readily detectable in the adult sera and were appreciably reduced in the pediatric samples (Fig. 4A). Consistent with this possibility, preincubation of the adult sera with an excess of purified S. Typhimurium LPS completely inhibited the bactericidal activity (Fig. 4B). LPS preincubation also inhibited the bactericidal activity of the pediatric samples against SL1344, as well as of the adult sera against the human isolate BAA-189 (data not shown). Interestingly, LPS from E. coli did not inhibit serum-mediated killing of Salmonella (Fig. 4B), suggesting that the bactericidal antibodies are specific for an epitope in the outer polysaccharide (O antigen), the region that structurally distinguishes different types of Gram-negative LPSs (22).
Fig 4.
Effect of LPS on serum bactericidal activity. (A) Adult and child serum samples were analyzed by ELISA to determine levels of IgM and IgG antibodies reactive with S. Typhimurium LPS. *, P = 0.011. (B) S. Typhimurium strain SL1344 was incubated with PBS or adult serum that had been preincubated or not preincubated with 100 μg/ml of S. Typhimurium (St) or E. coli (Ec) LPS. After 60 min at 37°C, the numbers of surviving bacteria were determined by plating serial dilutions of the reaction mixtures. *, P < 0.0001; **, P < 0.0001. The dashed line indicates the starting inoculum.
S. Typhimurium belongs to serogroup B and has an O antigen made up of repeats of the tetrasaccharide abequose-mannose-rhamnose-galactose (23). In some strains of S. Typhimurium, including SL1344, the 2-hydroxyl group of the abequose moiety in the O antigen is acetylated, causing conformational changes that can affect antibody binding (16, 24). To determine if the serum bactericidal activity might be affected by such acetylation, we tested adult sera against the wild-type S. Typhimurium strain JS107 and its isogenic mutant derivative JS93, which is deficient in the oafA locus required for abequose acetylation (16). All the tested sera were effective against JS107, whereas 2 of them had clearly reduced or no activity against JS93 (Fig. 5). The results indicate that abequose acetylation affected the bactericidal activity of only a small proportion of the sera.
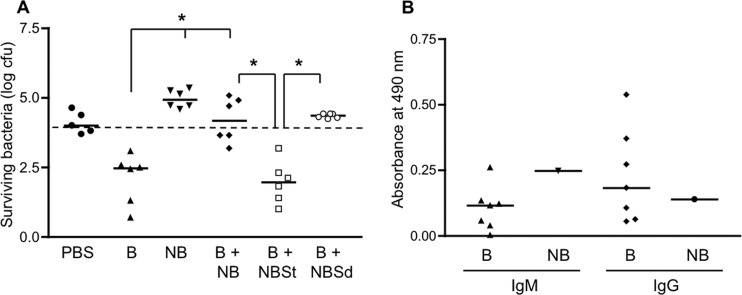
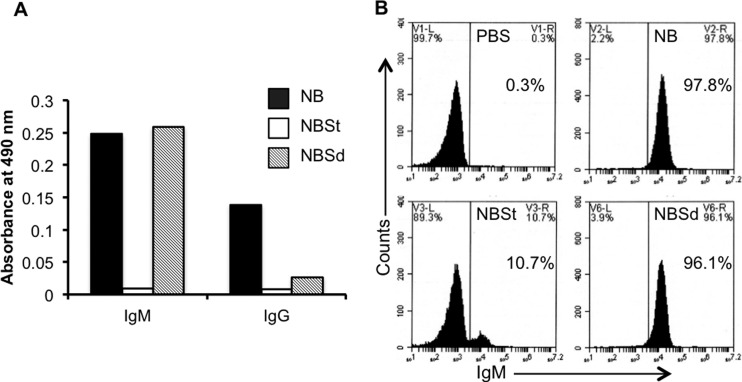
One sample in our collection (designated NB for “nonbactericidal”) completely lacked bactericidal activity against SL1344 (Fig. 6A) despite having S. Typhimurium LPS-specific IgM and IgG levels comparable to those in the bactericidal sera (Fig. 6B). This sample also had hemolytic complement activity that was similar to that of the bactericidal samples, as well as levels of complement that were sufficient to restore the killing of S. Typhimurium by heat-inactivated bactericidal sera (Fig. 2 and data not shown). Interestingly, NB was able to inhibit the activity of the bactericidal samples when equal volumes of the 2 types were mixed together (Fig. 6A). The inhibitory effect was abrogated when NB was depleted of anti-Salmonella antibodies by preabsorption with the S. Typhimurium strain SL1344 but not with the S. Dublin strain 2229 (Fig. 6A). We tested if the preabsorption with the 2 strains might have differential effects on the presence of antibodies to S. Typhimurium LPS, since one of the differences between S. Typhimurium and S. Dublin is in the compositions of their LPS O antigens. As mentioned above, S. Typhimurium has an O antigen made up of repeats of the tetrasaccharide abequose-mannose-rhamnose-galactose (Kaufmann-White O-antigen designation 1,4,5,12), whereas S. Dublin (serogroup D) has an O antigen with repeats of the tetrasaccharide tyvelose-mannose-rhamnose-galactose (Kaufmann-White O-antigen designation 1,9,12) (23). Indeed, we found by ELISA that preabsorption of NB with SL1344 produced a dramatic decrease in the levels of S. Typhimurium LPS-specific IgM but that preincubation with 2229 had little or no effect in this regard (Fig. 7A). Consistent with this result, we found corresponding differences in the amounts of IgM bound to the surface of SL1344 by flow cytometry (Fig. 7B). Equivalent reductions in S. Typhimurium LPS-specific IgG levels were observed after preabsorption with either of the 2 strains (Fig. 7A). Taken together, these findings suggest that it is the S. Typhimurium LPS-specific IgM that mediates the inhibitory effect of NB.
Fig 6.
Inhibitory effect of the nonbactericidal sample. (A) The standard assay to test bactericidal activity against SL1344 was carried out using PBS, individual bactericidal samples (B), the nonbactericidal sample (NB; results from multiple experiments), or a mix of individual bactericidal samples with the nonbactericidal sample (B + NB). In some of the experiments, individual bactericidal samples were mixed with the nonbactericidal sample, which had been preabsorbed with either S. Typhimurium SL1344 (B + NBSt) or S. Dublin 2229 (B + NBSd). *, P = 0.0022. The dashed line indicates the starting inoculum. (B) Bactericidal (B) and nonbactericidal (NB) samples were analyzed by ELISA to determine levels of IgM and IgG antibodies reactive with S. Typhimurium LPS.
Fig 7.
Role of S. Typhimurium LPS-specific IgM antibodies in the inhibitory effect of the nonbactericidal sample. (A) Results of the ELISA for S. Typhimurium LPS-specific IgM and IgG in the nonbactericidal sample (NB), in NB preabsorbed with S. Typhimurium SL1344 (NBSt), or in NB preabsorbed with S. Dublin 2229 (NBSd). The histograms indicate the mean absorbances of triplicates. (B) Flow cytometric analysis of IgM binding to the surface of SL1344 bacteria following incubation with PBS, NB, NB preabsorbed with S. Typhimurium SL1344 (NBSt), or NB preabsorbed with S. Dublin 2229 (NBSd). Percentages of bacteria staining positively for IgM are indicated.
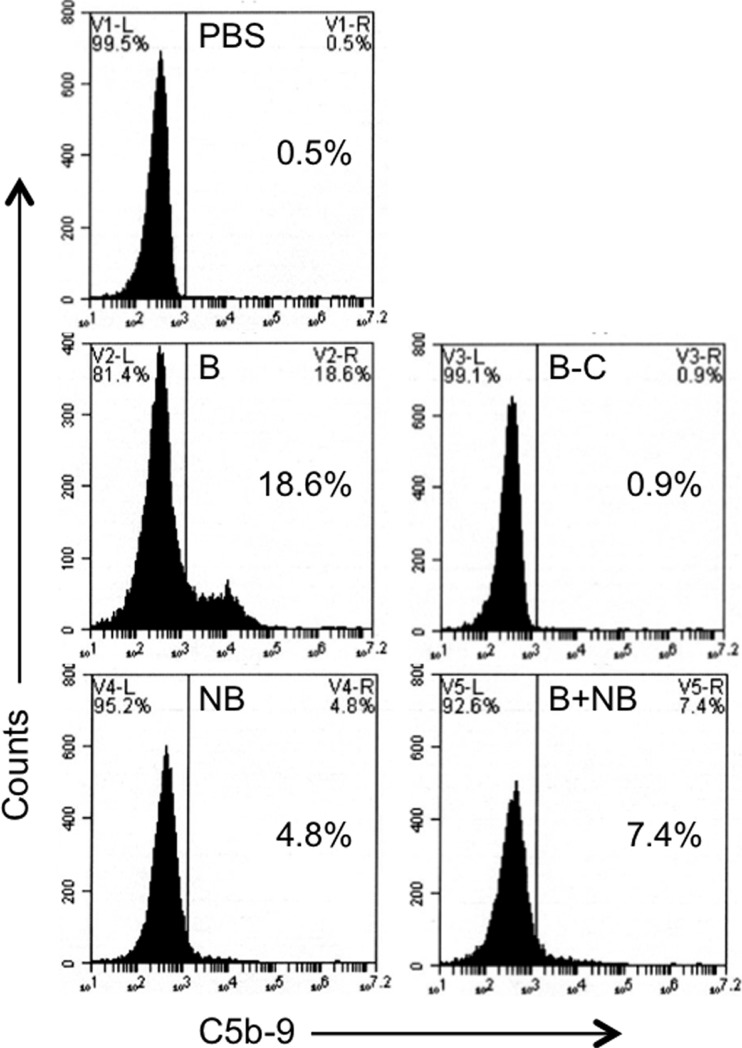
Finally, to elucidate the mechanism of the inhibitory effect, we used flow cytometry to examine the amount of complement deposited on the bacterial surface by bactericidal sera in the presence or absence of NB. The results for one of the bactericidal sera is shown in Fig. 8. They demonstrate clearly that, compared to PBS, the bactericidal serum alone induced the deposition of an appreciable amount of activated complement on the bacterial surface. This effect was almost completely lost when the serum was heat inactivated. NB alone did not induce deposition of complement and also inhibited complement deposition by the bactericidal serum. Similar results were obtained with 3 other bactericidal sera (data not shown). These findings suggest that the inhibitory effect of NB on bactericidal activity is the result of reduced deposition of activated complement on the bacterial surface.
Fig 8.
Effect of the nonbactericidal sample on complement deposition on S. Typhimurium. S. Typhimurium SL1344 bacteria were incubated with PBS, a bactericidal sample (B), the bactericidal sample heat inactivated to eliminate complement (B-C), the nonbactericidal sample (NB), or a mix of the bactericidal and nonbactericidal samples (B+NB). Flow cytometry was carried out to detect the presence of the activated terminal complement components C5b to 9 on the bacterial surface. Percentages of bacteria staining positively for C5b to 9 are indicated.
DISCUSSION
Like the findings from Africa (6, 8), our results indicate the widespread occurrence of complement-dependent, antibody-mediated bactericidal activity against S. Typhimurium in sera from healthy adults in the Boston area, as well as an age-dependent increase in this activity during maturation from child to adult. However, in contrast to the results from Africa, the bactericidal antibodies in our study appear to be directed against S. Typhimurium LPS rather than outer membrane proteins. Our data also indicate that the bactericidal antibodies discriminate between LPS from S. Typhimurium and E. coli, suggesting that the antibodies recognize a determinant in the outer polysaccharide of LPS (22, 23). The bactericidal activities of 2 of our samples appeared to be affected by acetylation of the abequose residue in the LPS outer polysaccharide, suggesting fine differences in antigen specificity within our collection. IgG purified from our samples was sufficient to recapitulate the bactericidal activity in the presence of complement, but it is likely that IgM also contributes to the activity, since LPS-specific antibodies of both isotypes were detected.
Our results, as well as those from Malawi, suggest that an age-dependent increase in exposure to a ubiquitous environmental antigen induces serum antibodies that react with S. Typhimurium. In developing countries such as Malawi, where the population may have limited access to protected food and water sources, such exposure may well correspond to subclinical or clinical infection with S. Typhimurium itself. Whether asymptomatic or symptomatic infection with S. Typhimurium accounts for the almost-universal presence of the bactericidal antibodies in our U.S. adult population is open to question, but it is a possibility that needs to be considered. Certainly, there are multiple animal reservoirs of NTS, including pet reptiles and amphibians, that may act as sources of human infection, particularly in children (25, 26), and both epidemic and sporadic gastroenteritis caused by S. Typhimurium is not uncommon in the United States (1). Asymptomatic carriage of Salmonella is also a potential outcome of infection, as has been documented in Sweden, although the exact prevalence of subclinical infection is not known (27, 28). An alternative possibility is that the bactericidal antibodies detected in our study develop as a result of exposure to a cross-reactive LPS. LPS is present widely in the external environment and is also a component of the commensal microflora present within the gastrointestinal tract (29, 30). Exposure and immunological sensitization to LPS is thus likely to be a characteristic of most populations. Indeed, serum IgM, IgA, and IgG antibodies to surface antigens of a number of different gut commensal bacteria have been observed in healthy individuals (31). Taking these facts into consideration, it seems plausible that the bactericidal antibodies detected in our study represent a response to some source of LPS present in either the external or the internal (commensal) environment and that these antibodies fortuitously cross-react with S. Typhimurium LPS. Evidence in support of this idea is provided by observations showing that human volunteers fed a commensal E. coli strain developed cross-reacting serum bactericidal anticapsular antibodies against Haemophilus influenzae type b (32, 33).
There may be a number of explanations, not mutually exclusive, for the intriguing differences between our findings and the data from Africa with respect to the antigen targeted by the bactericidal antibodies (LPS in the case of the United States and outer membrane proteins in Malawi). First, the S. Typhimurium strains circulating in Africa are genetically different from the ones circulating in the United States (34, 35). Second, as discussed above, it is possible that the bactericidal antibodies in the United States develop as a result of sensitization by environmental LPS but that they may develop as a result of subclinical infection with S. Typhimurium in Malawi. These circumstances may account for the difference in the antigen specificities of the bactericidal antibodies detected in the 2 locations. It should also be noted that different S. Typhimurium strains were used to test bactericidal activity in the 2 sets of studies. Microheterogeneities in the polysaccharide compositions of LPSs have been described even within the same serotype (36), and it is possible that such variations affect antibody binding and function, especially since the exact epitope to which antibody binds may alter bactericidal activity (5, 8). In this context, it may be relevant that recent experiments have shown that antibody-mediated killing of the S. Typhimurium isolate used to assess bactericidal activity in the Malawi study required unusually large amounts of complement (37). This characteristic may also contribute to the differences from our results.
The importance of serum bactericidal antibodies in protection against NTS sepsis in humans is supported by circumstantial evidence. Studies in the mouse model of infection indicate that both humoral and cell-mediated immunity contribute to defense against S. Typhimurium, although the role of T cells is generally considered to be more crucial (4). The strong correlation between the presence of serum bactericidal activity and resistance to NTS sepsis observed in the studies from Malawi certainly strengthens the idea that antibodies have an important role to play in protection against invasive S. Typhimurium infection (6–8). The age-dependent increase in bactericidal activity observed in our study is consistent with the results from Malawi and provides further support for this idea.
Only a single sample in our collection, NB, lacked bactericidal activity. This sample was able to abolish the killing of S. Typhimurium by the bactericidal sera, similar to the inhibitory effect observed with sera from HIV-infected African adults (8). However, our collection, including NB, was derived from healthy individuals without known acute or chronic illnesses. Moreover, NB did not have the hypergammaglobulinemia seen in the inhibitory sera from HIV-infected African adults (8). The IgM, IgG, and IgA concentrations of NB were 1.83 mg/ml, 3.04 mg/ml, and 1.87 mg/ml, respectively, which were comparable to the values for 7 randomly selected bactericidal samples from our collection (IgM, 2.11 ± 1.05 mg/ml; IgG, 5.28 ± 2.61 mg/ml; IgA, 2.19 ± 1.60 mg/ml) and were not above the normal range for adults (38). These findings suggest that the presence of the inhibitory activity may not be unique to the HIV-infected population and may simply represent a normal, albeit relatively rare, variation. Based on preabsorption experiments and the analysis of complement deposition on the bacterial surface, the inhibitory effect of our sample appears to be mediated by LPS-specific IgM antibodies that are unable to induce complement activation. These antibodies are probably directed against an LPS O-antigen epitope that is expressed specifically by S. Typhimurium, since they are removed by preabsorption with S. Typhimurium (Kaufmann-White O-antigen designation 1,4,5,12) but not S. Dublin (Kaufmann-White O-antigen designation 1,9,12) (23). However, we cannot definitively exclude the involvement of antibodies against S. Typhimurium-specific surface antigens other than LPS. Presumably, the binding of these inhibitory antibodies blocks the binding of the complement-activating bactericidal antibodies to the same region (if not the same epitope or overlapping epitopes). This presumptive mechanism of inhibition appears to be different from that operating in the sera of HIV-infected African adults, since complement activation and deposition on the Salmonella surface occurred normally in that population (8).
Since IgM is normally a robust activator of complement, it is not clear why the LPS-specific IgM in our nonbactericidal sample fails to activate complement. Glycosylation of the IgM heavy-chain constant region is known to affect complement activation (39–41), so it is possible that peculiarities of the oligosaccharides attached to the LPS-specific IgM in the nonbactericidal sample may be responsible for its failure to activate complement. Such variations, as well as the other differences in bactericidal activity revealed by our study and the studies from Africa, may contribute to interindividual and interpopulation differences in susceptibility to S. Typhimurium. Clarifying these issues will increase our understanding of the pathogenesis of NTS sepsis and facilitate the development of strategies to prevent this disease.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01AI089700 and R01AI095338 to B.J.C. and B.P.H., respectively, and by the Clinical Nutrition Research Center at Harvard (grant P30DK040561 to W. Allan Walker).
We thank the staff of the Partners Biorepository for assistance with collection of serum samples. We are also grateful to James Slauch, University of Illinois, Urbana, IL, for providing the JS93 and JS107 bacterial strains, and to Anastassia Mikhailova and Galit Alter of the Ragon Institute of Massachusetts General Hospital, Harvard University, and the Massachusetts Institute of Technology for assistance with purifying serum IgG. We thank Nanda Shanmugam and Hai Ning Shi for constructive comments on the manuscript.
Footnotes
Published ahead of print 26 June 2013
REFERENCES
- 1.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM, International Collaboration on Enteric Disease ‘Burden of Illness' Studies 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882–889 [DOI] [PubMed] [Google Scholar]
- 2.Graham SM. 2010. Nontyphoidal salmonellosis in Africa. Curr. Opin. Infect. Dis. 23:409–414 [DOI] [PubMed] [Google Scholar]
- 3.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive nontyphoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin AJ, McSorley SJ. 2011. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 4:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank MM, Joiner K, Hammer C. 1987. The function of antibody and complement in the lysis of bacteria. Rev. Infect. Dis. 9:S537–S545 [DOI] [PubMed] [Google Scholar]
- 6.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Gougan G, Hart CA, Molyneux ME, Drayson MT. 2008. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 118:1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Matroeni P, Drayson MT, MacLennan CA. 2010. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc. Natl. Acad. Sci. U. S. A. 107:3070–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, Kingsley RA, van Oosterhout JJ, Msefula CL, Mandala WL, Leyton DL, Marshall JL, Gondwe EN, Bobat S, Lopez-Macias C, Doffinger R, Henderson IR, Zijlstra EE, Dougan G, Drayson MT, MacLennan IC, Molyneux ME. 2010. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 328:508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Shaughnessy CM, Micoli F, Gavini M, Goodall M, Cobold M, Saul A, MacLennan CA. 2013. Purification of antibodies to O antigen of Salmonella Typhimurium from human serum by affinity chromatography. J. Immunol. Methods 387:199–210 [DOI] [PubMed] [Google Scholar]
- 10.Heffernan EJ, Reed S, Hackett J, Fierer J, Roudier C, Guiney D. 1992. Mechanism of resistance to complement-mediated killing of bacteria encoded by the Salmonella Typhimurium virulence plasmid gene rck. J. Clin. Invest. 90:953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hand WL, King NL. 1977. Deficiency of serum bactericidal activity against Salmonella Typhimurium in sickle cell anemia. Clin. Exp. Immunol. 30:262–270 [PMC free article] [PubMed] [Google Scholar]
- 12.Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, Cieslak PR, Deneen VC, Tauxe RV, Emerging Infections Program FoodNet Working Group 2004. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin. Infect. Dis. 38(Suppl 3):S127–S134 [DOI] [PubMed] [Google Scholar]
- 13.Wray C, Sojka WJ. 1978. Experimental Salmonella Typhimurium infection in calves. Res. Vet. Sci. 25:139–143 [PubMed] [Google Scholar]
- 14.Hoiseth SK, Stocker BAD. 1981. Aromatic-dependent Salmonella Typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239 [DOI] [PubMed] [Google Scholar]
- 15.Bakshi CS, Singh VP, Wood MW, Jones PW, Wallis TS, Galyov EE. 2000. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol. 182:2341–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes SJ, Eschmann M, Mantis NJ. 2008. Inhibition of Salmonella enterica serovar Typhimurium motility and entry into epithelial cells by a protective anti-lipopolysaccharide monoclonal immunoglobulin A antibody. Infect. Immun. 76:4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood MW, Rosqvist R, Mullan PB, Edwards MH, Galyov EE. 1996. SopE, a secreted protein of Salmonella Dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol. 22:327–338 [DOI] [PubMed] [Google Scholar]
- 18.Baggesen DL, Sandvang D, Aarestrup FM. 2000. Characterization of Salmonella enterica serovar Typhimurium DT104 isolated from Denmark and comparison with isolates from Europe and the United States. J. Clin. Microbiol. 38:1581–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CC, Young JD. 1988. A semi-automated microassay for complement activity. J. Immunol. Methods 114:33–39 [DOI] [PubMed] [Google Scholar]
- 20.Harrington L, Srikanth CV, Antony R, Rhee SJ, Mellor AL, Shi HN, Cherayil BJ. 2008. Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infect. Immun. 76:3045–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BD, Nichols WA, Gibson BW, Sunshine MG, Apicella MA. 1997. Study of the role of the htrB gene in Salmonella Typhimurium virulence. Infect. Immun. 65:4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raetz CRH. 1996. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles, p 1035–1063 In Neidhardt FC, Curtis R, III, Ingraham JL. (ed), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, DC [Google Scholar]
- 23.Fierer J, Guiney DG. 2001. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Invest. 107:775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slauch JM, Mahan MJ, Michetti P, Neutra MR, Mekalanos JJ. 1995. Acetylation (O-factor 5) affects the structural and immunological properties of Salmonella Typhimurium lipopolysaccharide. Infect. Immun. 63:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris JR, Neil KP, Behravesh CB, Sotir MJ, Angulo FJ. 2010. Recent multistate outbreaks of human Salmonella infections acquired from turtles: a continuing public health challenge. Clin. Infect. Dis. 50:554–559 [DOI] [PubMed] [Google Scholar]
- 26.Wells EV, Boulton M, Hall W, Bidol SA. 2004. Reptile-associated salmonellosis in pre-school children in Michigan, January 2001–June 2003. Clin. Infect. Dis. 39:687–691 [DOI] [PubMed] [Google Scholar]
- 27.Jertborn M, Haglind P, Iwarson S, Svennerholm M. 1990. Estimation of symptomatic and asymptomatic Salmonella infections. Scand. J. Infect. Dis. 22:451–455 [DOI] [PubMed] [Google Scholar]
- 28.Haeusler GM, Curtis N. 2013. Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv. Exp. Med. Biol. 764:13–26 [DOI] [PubMed] [Google Scholar]
- 29.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E, Allergy and Endotoxin Study Team 2002. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 347:869–877 [DOI] [PubMed] [Google Scholar]
- 30.Lozupone CA, Stombaugh JI, Gordon JI, Jannson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas A, Zimmermann K, Graw F, Slack E, Rusert P, Ledergerber B, Bossart W, Weber R, Thurnheer MC, Battegay M, Hirschel B, Vernazza P, Patuto N, Macpherson AJ, Gunthard HF, Oxenius A, Swiss HIV Cohort Study 2011. Systemic antibody responses to gut commensal bacteria during chronic HIV-1 infection. Gut 60:1506–1519 [DOI] [PubMed] [Google Scholar]
- 32.Schneerson R, Robbins JB. 1975. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli O75:K100:H5. N. Engl. J. Med. 292:1093–1096 [DOI] [PubMed] [Google Scholar]
- 33.Anderson P. 1975. Editorial: fecal flora and resistance to invasive bacteria. N. Engl. J. Med. 292:1124–1125 [DOI] [PubMed] [Google Scholar]
- 34.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G. 2009. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 19:2279–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, Wain J, Heyderman RS, Obaro S, Alonso PL, Mandomando I, MacClennan CA, Tapia MD, Levine MM, Tennant SM, Parkhill J, Dougan G. 2012. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat. Genet. 44:1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker CT, Liebana E, Henzler DJ, Guard-Petter J. 2001. Lipopolysaccharide O-chain microheterogeneity of Salmonella serotypes Enteritidis and Typhimurium. Environ. Microbiol. 3:332–342 [DOI] [PubMed] [Google Scholar]
- 37.Goh YS, MacLennan CA. 2013. Invasive African nontyphoidal Salmonella requires high levels of complement for cell-free antibody-dependent killing. J. Immunol. Methods 387:121–129 [DOI] [PubMed] [Google Scholar]
- 38.Massachusetts General Hospital Department of Pathology 2011. Reference intervals—MGH clinical laboratories. MGH laboratory handbook Massachusetts General Hospital, Boston, MA: http://mghlabtest.partners.org/MGH_Reference_Intervals_August_2011.pdf [Google Scholar]
- 39.Shulman MJ, Pennell N, Collins C, Hozumi N. 1986. Activation of complement by immunoglobulin M is impaired by the substitution serine 406-asparigine in the immunoglobulin mu heavy chain. Proc. Natl. Acad. Sci. U. S. A. 83:7678–7682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muraoka S, Shulman MJ. 1989. Structural requirements for IgM assembly and cytolytic activity. Effects of mutations in the oligosaccharide acceptor site at Asn402. J. Immunol. 142:695–701 [PubMed] [Google Scholar]
- 41.Wright JF, Shulman MJ, Isenman DE, Painter RH. 1990. C1 binding by mouse IgM. The effect of abnormal glycosylation at position 402 resulting from a serine to asparagine exchange at residue 406 of the mu-chain. J. Biol. Chem. 265:10506–10513 [PubMed] [Google Scholar]