Abstract
Currently, the laboratory diagnosis of human fascioliasis is based on the parasitological examination of parasite eggs in stool specimens and serological detection of specific antibodies in serum samples, which are often unreliable diagnostic approaches. Ideally, a sensitive and specific diagnostic test for Fasciola infection should be based on the detection of circulating Fasciola antigen, which implies active infection. Here, a 27-kDa-molecular-mass antigen was identified in a Fasciola gigantica adult worm antigen preparation, excretory-secretory products, and sera from F. gigantica-infected individuals, and it was not detected in antigenic extracts of other parasites and sera from noninfected individuals. The target antigen was isolated and partially characterized as a protein. Immunoperoxidase staining located the target epitope within teguments and guts of F. gigantica adult worms. The performance characteristics of a newly developed enzyme-linked immunosorbent assay (ELISA) based on F. gigantica circulating antigen detection in serum (FgCA-27 ELISA) were investigated using sera of 120 parasitologically diagnosed F. gigantica-infected individuals and 80 noninfected individuals. The area under the receiving operating characteristic (ROC) curve (AUC) for ELISA was significantly high (AUC = 0.961, P < 0.0001) for discriminating Fasciola-infected and noninfected individuals. The developed assay showed high degrees of sensitivity, specificity, and efficiency (>93%), and a significant correlation (r = 0.715, P < 0.0001) between antigen level and parasite egg count was shown. In conclusion, a 27-kDa Fasciola antigen was identified in sera of F. gigantica-infected individuals. A highly sensitive and specific Fasciola antigen detection assay, FgCA-27 ELISA, was developed for laboratory diagnosis of human fascioliasis.
INTRODUCTION
Fascioliasis is a worldwide zoonotic infection caused by liver flukes of the genus Fasciola, of which F. hepatica and a larger species, F. gigantica, are the most common representatives. These two food-borne trematodes usually infect domestic ruminants and cause important economic losses of sheep, goats, and cattle in commercial herds (1). Today, fascioliasis is considered an important human disease, and several areas, e.g., tropical regions of Africa and Asia, have been described as areas of endemicity for the disease in humans, with estimates of 2.4 to 17 million people infected and 91.1 million at risk worldwide (2, 3). Egypt, especially the Nile Delta, is a hot spot for Fasciola infection, which has appeared to be endemic, with estimated prevalence varying between 2 and 17% (4–6). Furthermore, fascioliasis has been recognized as an emerging infection in international travelers and migrants, causing significant problems in diagnosis and therapy (7). Owing to the disease's high-level endemicity in some developing countries and its emergence in nature, the WHO classified it as a neglected parasitic infection and decided to launch a worldwide initiative against this infectious disease (2).
Diagnosis of human fascioliasis is based on clinical findings and laboratory tests. The most reliable means is the finding of parasite eggs in stool of an infected individual (8). However, parasitological diagnosis of human fascioliasis is time-consuming and usually lacks sensitivity and reproducibility, and it is often unreliable because parasite eggs are not found during the prepatent period, which lasts until approximately 3 to 4 months after infection (9). Moreover, once Fasciola worms have matured, diagnosis may still remain difficult, since eggs are frequently excreted at irregular intervals, and in most cases, repeated stool examinations are required to accurately diagnose (10). Anti-Fasciola antibodies can be detected as early as 2 weeks postinfection, and this can thus facilitate early diagnosis and chemotherapeutic intervention (11). Over the past years, several native and recombinant antigens were identified for the detection of serum-specific anti-Fasciola antibodies (12–19), and many antibody-based immunological techniques have been developed for diagnosis of Fasciola infection in a trial to replace the classical parasitological techniques (20). However, antibody tests do not distinguish between past, resolving, and current infections, and their results do not correlate with infection intensity (21).
In that sense, the direct detection of parasite antigens in stool (coproantigens) or serum (circulating antigens) of Fasciola-infected animals or humans has been reported as a new alternative approach with greater diagnostic accuracy (22–26). In our previous study, we identified a 26- to 28-kDa circulating antigen in sera of cattle infected with F. gigantica by using specific rabbit IgG antiserum (22). In the present study, we aimed to identify the target F. gigantica circulating antigen in sera of infected individuals and describe a novel enzyme-linked immunosorbent assay (ELISA) based on circulating antigen detection in serum for accurate laboratory diagnosis of human F. gigantica infection.
MATERIALS AND METHODS
Stool and blood samples from study subjects.
A total of 200 individuals (101 males and 99 females; age range, 6 to 65 years; mean, 24 years) admitted at the Tropical Medicine and Parasitology Department, Mansoura University Hospitals, Mansoura, Egypt, were included in the present study. Stool specimens were collected from each individual and examined at the day of collection using simple stool sedimentation by centrifugation. The Kato-Katz thick-smear technique was used for counting F. gigantica eggs in 3 to 5 slides, each containing 41.7 mg stool, and the egg count was expressed as eggs per gram of feces (EPG). A total of 120 individuals had F. gigantica eggs in their feces, 57 individuals had other parasitic infections, including 38 individuals infected with Entamoeba histolytica, 7 infected with Schistosoma mansoni, 5 infected with Ascaris lumbricoides, 4 infected with Hymenolepis nana, and 3 infected with Giardia lamblia, and 23 individuals were diagnosed as parasite-free, healthy individuals. According to the Kato-Katz technique results for individuals infected with F. gigantica, the intensity of infection was classified as light (<100 EPG) in 76 individuals, moderate (100 to 299 EPG) in 33 individuals, and severe (≥300 EPG) in 11 individuals. Blood samples were collected on the day of stool analysis. Sera were separated from blood, aliquoted, and stored at −20°C until used. The Ethical Committee of the Mansoura University Hospitals, Mansoura, Egypt, approved the present study. Informed consents were obtained from all participants, and all individuals were fully informed concerning the diagnostic procedures involved and nature of the disease.
F. gigantica and other parasite antigenic preparations.
F. gigantica soluble worm antigen preparation (FWAP) was prepared as described by Attallah et al. (22). The crude excretory/secretory (E/S) products of adult F. gigantica were prepared according to the description of Santiago de Weil and Hillyer (27). Adult A. lumbricoides worms, from the stools of infected individuals, were processed in exactly the same way as F. gigantica to prepare A. lumbricoides soluble worm antigenic preparation (AWAP), while S. mansoni soluble worm antigenic preparation (SWAP) was prepared as described by da Silva and Ferri (28). The protein content of a sample of each antigenic preparation was determined (29) before the rest of the preparation was split into aliquots and stored at −20°C until used.
SDS-PAGE and Western blot.
Various samples were subjected to analytical SDS-PAGE, at 50 μg/lane, using vertical slabs of 12% or 16% polyacrylamide (30). Prestained molecular mass standards (Sigma) were run in parallel. Samples separated on SDS-PAGE were electrotransferred onto nitrocellulose (NC) membrane (0.45 μm pore size) in a protein transfer unit (31). The NC membrane was blocked using 5% (wt/vol) nonfat dry milk dissolved in 0.05 M Tris-buffered saline (TBS) containing 200 mM NaCl (pH 7.4), rinsed in TBS, and incubated with anti-27-kDa Fasciola antigen IgG antibody, diluted (1:150) in the blocking buffer as described below, with constant shaking. The blots were washed three times (30 min each) in TBS and then incubated for 2 h with goat anti-rabbit IgG–alkaline phosphatase conjugate (Sigma) diluted 1:350 in TBS. After being washed three more times with TBS (15 min each), the blots were soaked in substrate. The color reaction was observed within 10 min, and the reaction was then stopped by dipping the blots in distilled water. To ensure that the 27-kDa protein purified from serum was a parasite molecule, the developed IgG rabbit antiserum (diluted 1:150 in blocking buffer) was absorbed with a proper concentration of the purified protein (200 ng/ml) for 2 h at 37°C. Then, the reactivity of rabbit antiserum was investigated on blots of FWAP and E/S products as described above.
Gel electroelution and purity of the 27-kDa Fasciola antigen.
In preparative slab gel electrophoresis, the SDS-PAGE running condition was adapted to reduce smear of proteins and to enable a considerably long migration distance between bands in the 27-kDa region according to the prestained molecular mass marker (22). In each run, 250 μl of diluted human serum sample from an infected individual per preparative gel was electrophoresed, and about 35 runs were completed to obtain 1 mg of the 27-kDa Fasciola antigen. The protein content of a sample of electroeluted antigen was determined before remainder was stored at −20°C.
Purity of the electroeluted Fasciola antigen.
The purity of the electroeluted 27-kDa Fasciola antigen was assessed using gel silver staining (32) and capillary zone electrophoresis (CZE) with a modification of the method described by Gordon et al. (33) using an autosampler (model 1-LIFT; Prince Technologies, Emmen, The Netherlands), a variable UV-visible detector (Lambda 1010; Metrhom, Herisau, Switzerland), and WinPrince software (version 5; Prince Technologies). The signals were analyzed using Dax software (version 5; Prince Technologies).
Production of specific anti-27-kDa Fasciola antigen IgG antibody.
Specific IgG antibodies were produced in 4 New Zealand White rabbits immunized subcutaneously in three different inoculation sites with the purified 27-kDa Fasciola antigen according to the method described by Attallah et al. (22). In brief, equal volumes (500 μl) of the antigen (500 μg/ml) and complete Freund's adjuvant (CFA) or incomplete Freund's adjuvant (IFA) were homogenized together using two Luer-lock syringes connected to a three-way stainless steel valve. Each rabbit was immunized subcutaneously three times, once with antigen in CFA (on day 0) and twice with antigen in IFA (on days 15 and 28), before being sacrificed on day 32. Blood samples were collected from all rabbits 0, 28 and 32 days after immunization. Sera were separated and its immunoreactivity was tested using ELISA (see below) against Fasciola antigenic preparations, and the purified 27-kDa antigen. The specificity of the developed sera was tested using ELISA against adult S. mansoni and A. lumbricoides antigenic preparations. Sera of nonimmunized rabbits were tested in parallel. The highly reactive rabbit sera were then aliquoted and stored at −20°C until used.
Biochemical treatments.
To determine some of the target Fasciola antigen biochemical characteristics, samples of the purified 27-kDa antigen were treated with protease and sodium meta-periodate being tested in ELISA, to see if these treatments affected the reactive epitope. A periodate oxidation of purified antigen (25 μg/ml) was carried out overnight with 20 mM sodium meta-periodate at room temperature, and the reaction was then inhibited by adding an equal volume of 130 mM glycerol. In the test with protease, purified antigen was incubated at 37°C with pepsin (1 mg/ml; Sigma) for 5, 10, 15, or 20 min. Sera of a Fasciola-infected patient and a healthy individual were tested in parallel, as positive and negative controls, respectively.
Immunohistochemical detection of native Fasciola antigen by using indirect immunoperoxidase.
A paraffin section (4 μm) of adult worm tissues was deparaffinized and rehydrated through descending grades of alcohols in water. The slides were washed in TBS 3 times for 5 min each. Excess liquid from around the section was wiped, and the slides were laid flat. Four to six drops of 3% hydrogen peroxide (H2O2) were applied for each slide, and slides were incubated for 5 min in a dark chamber. Normal goat serum was applied with a dilution of 1:5 with 3% bovine serum albumin (BSA) in TBS for 60 min. Then, the developed rabbit anti-27-kDa Fasciola antigen IgG antibody diluted 1:100 in TBS was applied and incubated for 30 min. After the slides were washed, horseradish peroxidase-conjugated goat antibody to rabbit immunoglobulins diluted 1:250 in 1% BSA-TBS was applied and incubated for 60 min. The slides were washed, and then tissue reacted with 3-amino-9-ethyl carbazole–H2O2 solution for 30 min in the dark. The reaction was stopped with distilled water. The sections were counterstained with Mayer's hematoxylin for 2 min and washed in running tap water for 3 min to the desired intensity of blue color. The slides were dried out and applied onto coverslips using glycerol. All washings and incubations were performed at room temperature.
Development of F. gigantica circulating antigen detection assay (FgCA-27 ELISA).
Serial dilutions (1:25 to 1:3,200) of selected serum samples from patients with no parasite eggs (n = 4) and low (n = 4, <100 EPG), moderate (n = 4, 100 to 299 EPG), and high (n = 4, ≥300 EPG) parasite egg counts, in duplicate, were coated with carbonate-bicarbonate buffer, pH 9.6, and were investigated to get the proper saturation of the polystyrene solid phase with the target antigen. Checkerboard titrations of the specific anti-27-kDa IgG antibody as well as alkaline phosphatase conjugate were also performed. After optimization of assay conditions, polystyrene flat-bottom microtiter plates (Costar, Acton, MA) were coated with 1:200-diluted human serum samples or serial concentrations (1,024 to 0 ng/ml) of the purified 27-kDa Fasciola antigen diluted in normal human serum. In parallel and at 2.5 μg/ml, FWAP and E/S products were tested as positive controls and SWAP and EWAP were tested as negative controls for the reactivity of the developed rabbit antibody. After the plate was blocked with 0.3% BSA, 50 μl/well of rabbit anti-27-kDa antigen IgG antibody at a 1:400 dilution, in PBS with 0.05% (vol/vol) Tween 20 (PBS-T20), was added and the plates were incubated at 37°C for 2 h. After the plate was washed, 50 μl/well of anti-rabbit IgG–alkaline phosphatase conjugate (The Binding Site, Birmingham, United Kingdom) diluted 1:600 in 0.2% (wt/vol) BSA in PBS-T20 was added and the plate was incubated at 37°C for 1 h. After the plate was washed, the substrate (p-nitrophenyl phosphate in 0.1 M glycine buffer; pH 10.4) was added and the plates incubated for 30 min at 37°C.
Optical densities (OD) were read at 405 nm using a microplate autoreader (Σ960; Metertech Inc., Taipei, Taiwan). The cutoff level of the FgCA-27 ELISA above or below which the tested sample is considered positive or negative was calculated as the mean OD for the FgCA-27 ELISA (range, 0.093 to 0.227) from a control group of 32 serum samples from healthy individuals with no parasitic infection plus 3 standard deviations (SDs) (i.e., cutoff level = 0.131 + [3 × 0.053] = 0.29). The mean OD value of a group of 16 sera from parasitologically diagnosed F. gigantica-infected individuals and investigated in parallel was 0.755 (range, 0.394 to 1.985). The mean OD of another group of 16 serum samples of individuals parasitologically diagnosed as being not infected with Fasciola but having other parasitic infections, including schistosomiasis mansoni (n = 3), ascariasis (n = 3), hymenolepiasis (n = 3), giardiasis (n = 3), and entamoebiasis (n = 4), and investigated in parallel was 0.181 (range, 0.105 to 0.261). To establish the dose-response curve, serial concentrations (1 to 1,024 ng/ml) of the target 27-kDa antigen were mixed with diluted serum samples showing FgCA-27 ELISA-negative results for antigen detection and with no detected antibody levels (n = 3, OD < 0.25; cutoff level of an in-house ELISA based on specific anti-27-kDa IgG antibodies in human serum) and tested in duplicate as well as with selected serum samples with low (n = 3, OD = 0.25 to 0.49), moderate (n = 3, OD = 0.5 to 1.5), and high (n = 3, OD > 1.5) levels of specific anti-27-kDa IgG antibodies.
Statistical analyses.
Descriptive results were expressed as means ± SDs and ranges or numbers of patients with a condition. Differences in continuous variables were assessed using Student's t test or analysis of variance (ANOVA). All tests were two-tailed, and statistical significance was assessed at the 0.05 level. The diagnostic accuracy of the Fasciola antigen detection test was assessed by the area under the receiver operating characteristic (ROC) curve (AUC). An AUC equal to 1.0 is characteristic of an ideal test, whereas 0.5 indicates a test of no diagnostic value. The diagnostic accuracy was calculated by sensitivity, specificity, and efficiency, and the values are expressed as percentages. The reproducibility and repeatability of the developed assay, calculated as intra-assay and interassay coefficients of variation (CV%), were assessed using 10 serum samples with different concentrations of Fasciola circulating antigen (17 to 1,200 ng/ml) tested in quadruplicate and undertaken along 4 consecutive weeks. All statistical analyses were done by a statistical software package (SPSS 15.0 for Microsoft Windows; SPSS Inc., Chicago, IL, USA).
RESULTS
Identification of the target Fasciola antigen among F. gigantic antigenic extracts and human sera from infected individuals.
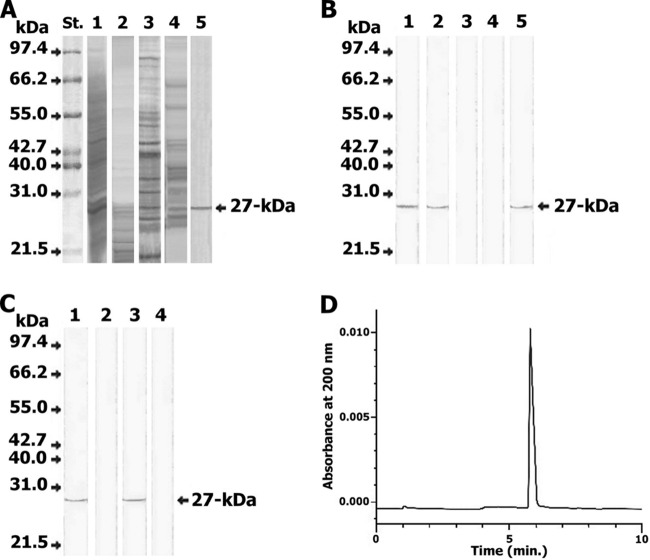
FWAP and E/S products of F. gigantica, SWAP of S. mansoni, and AWAP of A. lumbricoides were subjected to SDS-PAGE (Fig. 1A), and Western blotting was carried out to detect the target epitope of Fasciola antigen. It was found that a polypeptide band of 27 kDa in the FWAP and E/S product of Fasciola reacted with anti-27-kDa antigen IgG antibody. This antibody did not recognize any antigens in the Ascaris and Schistosoma antigenic extracts (Fig. 1B). No reaction against E/S products or FWAP using the saturated rabbit antiserum with of the 27-kDa protein was shown (Fig. 1C). The electroeluted 27-kDa antigen gave a single polypeptide band when investigated by SDS-PAGE and silver staining, and the purity of the eluted 27-kDa polypeptide was confirmed using CZE; only a single peak (5.9 min) was observed (Fig. 1D). A sharp band was observed at 27 kDa in sera from individuals infected with F. gigantica, but no reaction was observed in sera of uninfected individuals (Fig. 2). Partial biochemical characterization of the reactive epitope confirmed its protein moiety. The reactivity of the anti-27-kDa antibody toward the purified antigen was maintained after periodate oxidization (i.e., a positive result using FgCA-27 ELISA). However, antibody reactivity toward the purified 27-kDa antigen decreased when the incubation time of the antigen with pepsin enzyme was increased, and it was completely lost at 20 min (i.e., a negative result using FgCA-27 ELISA).
Fig 1.
Identification and purification of the target Fasciola antigen. (A) Silver-stained SDS-PAGE. (B) Western blot for different antigenic sources of F. gigantica, S. mansoni, and A. lumbricoides using rabbit anti-27-kDa Fasciola IgG antibody. (A and B) Lane 1, F. gigantica adult worm antigen preparation (FWAP); lane 2, excretory/secretory (E/S) products from F. gigantica; lane 3, S. mansoni adult worm antigenic preparation (SWAP); lane 4, A. lumbricoides adult worm antigenic preparation (AWAP); lane 5, the purified 27-kDa antigen. The developed anti-Fasciola antibody identified its target epitope at 27 kDa in FWAP, E/S products, and the purified antigen (lanes 1, 2, and 5) but not in SWAP and AWAP (lanes 3 and 4). (C) Inhibition Western blot for different antigenic sources of F. gigantica by using rabbit anti-27-kDa Fasciola IgG antibody saturated with purified 27-kDa antigen. Lane 1, FWAP immunostained with the anti-Fasciola antibody; lane 2, FWAP immunostained with the saturated antibody; lane 3, E/S products immunostained with the anti-Fasciola antibody; lane 4, E/S products immunostained with saturated antibody. The saturated anti-Fasciola antibody did not identify its target epitope at 27 kDa in FWAP and E/S products (lanes 2 and 4). Molecular mass standards (St.) included were phosphorylase B (97.4 kDa), bovine serum albumin (66.2 kDa), glutamate dehydrogenase (55 kDa), ovalbumin (42.7 kDa), aldolase (40 kDa), carbonic anhydrase (31 kDa), and soybean trypsin inhibitor (21.5 kDa). (D) The 27-kDa antigen purified from serum of an F. gigantica-infected individual showing a single peak, at an absorbance of 200 nm, at 5.9 min, using CZE.
Fig 2.
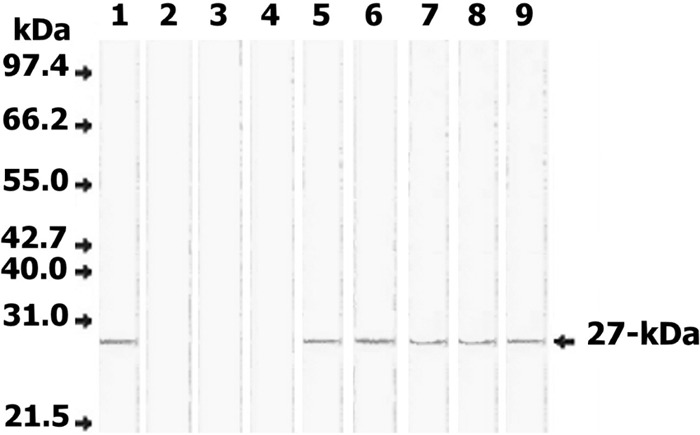
Western blot of serum samples from Fasciola-infected and noninfected individuals for the detection of circulating Fasciola antigen. Lane 1, FWAP; lanes 2 to 4, serum samples from 3 noninfected individuals; lanes 5 to 9, serum samples from 5 individuals infected with F. gigantica. Fifty micrograms/lane of each sample was separated on 12% acrylamide gels, transferred to an NC sheet, and reacted with 100 μg/ml rabbit anti-27-kDa IgG antibody. Anti-rabbit IgG-alkaline phosphatase and BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium (NBT) substrate were used to visualize the reaction products. The developed rabbit antibody identified a 27-kDa antigen in all sera of infected individuals (lanes 5 to 9) but not in sera of noninfected individuals (lanes 2 to 4). Molecular mass standards included were phosphorylase B (97.4 kDa), bovine serum albumin (66.2 kDa), glutamate dehydrogenase (55 kDa), ovalbumin (42.7 kDa), aldolase (40 kDa), carbonic anhydrase (31 kDa), and soybean trypsin inhibitor (21.5 kDa).
Anatomic localization of the native target antigen in an F. gigantica adult worm.
The antigenic determinant of the developed IgG antibody was located in the gut and tegumental surface of an adult F. gigantica worm. A strong immunoperoxidase reaction was found within the tegument, the muscularis, and the gut cells (Fig. 3A). No peroxidase reaction was shown within tegument, muscularis, and gut cells of a Fasciola adult worm using normal rabbit serum as a negative control (Fig. 3B).
Fig 3.
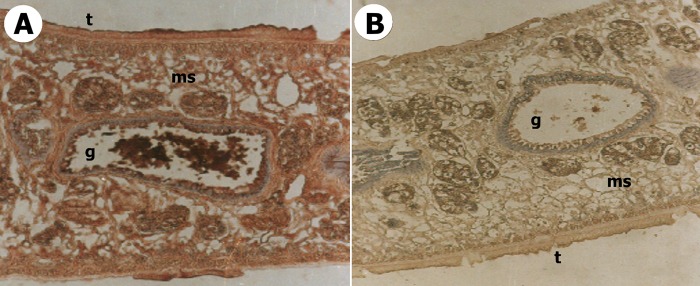
Localization of the target 27-kDa Fasciola antigen within an F. gigantica adult worm by using specific rabbit antisera and indirect immunoperoxidase staining. (A) Section of an F. gigantica adult worm showing a positive immunoperoxidase reaction (red color within tegument [t], muscularis [ms], and gut cells [g]) (×100). (B) Section of an F. gigantica adult worm showing no immunoperoxidase reaction (×100).
Detection of Fasciola circulating antigen in human serum samples by using FgCA-27 ELISA.
From the established calibration curve (Fig. 4A), the developed FgCA-27 ELISA successfully detected the 27-kDa Fasciola antigen serially diluted in normal human serum up to 1 ng/ml; the point did not overlap the concentration corresponding to 3 SDs above the mean OD of the zero calibrator (n = 4). However, the detection limit of the target circulating antigen in human serum samples at 8 ng/ml corresponds to the cutoff level (OD = 0.29) of the developed assay (n = 32). The presence of different levels of specific anti-27-kDa IgG antibodies in serum decreased the antigen levels but did not reach a significant level of difference (P > 0.05) in comparison with normal human serum, i.e., negative for specific IgG antibodies to Fasciola (data not shown). Serum samples from 120 individuals with fascioliasis and 80 noninfected individuals were tested using FgCA-27 ELISA for the detection of circulating Fasciola antigen. The antigen concentrations (ng/ml) in serum samples are shown in Fig. 4B. The AUC for the Fasciola antigen detection assay was significantly high (AUC = 0.961, P < 0.0001) for discriminating between individuals infected with Fasciola and noninfected individuals (Fig. 5A). The developed assay had a sensitivity of 93.3% and had false-positive results for 4 of 80 noninfected individuals, with a specificity of 95% in detecting human fascioliasis (Table 1). Cross-reactivity with other parasitic infections was studied based on microscopic stool examinations of 80 noninfected individuals. The FgCA-27 ELISA showed specificities of 100% with S. mansoni (7 cases), 100% with A. lumbricoides (5 cases), 100% with H. nana (4 cases), 100% with G. lamblia (3 cases), 92% with E. histolytica (3 out of 38 cases showing false-positive result), and 96% with parasite-free healthy individuals (1 out of 23 cases showing a false-positive result) (Table 1). All serum samples showing false-negative and false-positive results by FgCA-27 ELISA were investigated using the more-sensitive Western blot analysis. The 27-kDa antigen was identified in all 8 sera showing false-negative ELISA results. Among the four sera showing false-positive ELISA results, only one sample from a parasite-free healthy individual showed a sharp band at 27-kDa, and the remaining 3 sera of E. histolytica-infected individuals showed no bands in their blots. Moreover, antigen detection rates (percent positivity) increased with increasing severity of infection (expressed as parasite egg count [EPG]) (Table 1), and a highly significant correlation (Spearman r = 0.715, P < 0.0001) between circulating Fasciola antigen level in serum (ng/ml) and EPG was shown (Fig. 5B). The percent intra-assay and interassay coefficients of variation (CV%) in antigen concentrations were 3.3% and 5.8%, respectively.
Fig 4.
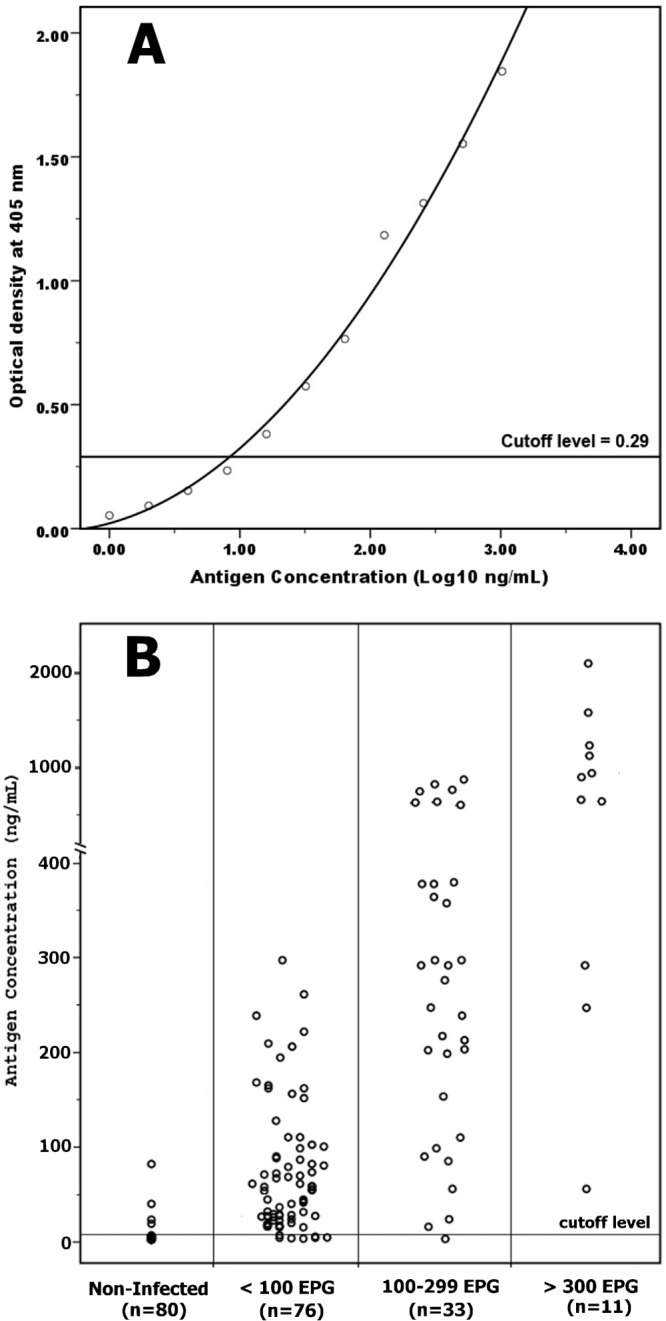
Quantification of the 27-kDa Fasciola gigantica antigen in human serum by using the developed FgCA-27 ELISA. (A) Calibration curve of the 27-kDa Fasciola antigen. The purified 27-kDa antigen (ng/ml) was serially diluted in normal human serum and tested using the developed ELISA. The optical densities (OD) were measured at 405 nm, and the cutoff level was set at an OD of 0.29. (B) Scattergram showing Fasciola antigen levels (expressed as ng/ml) in sera of 120 parasitologically diagnosed infected individuals whose infections were classified as light (<100 EPG; n = 76), moderate (100 to 299 EPG; n = 33), and severe (≥300 EPG; n = 11) in comparison with antigen levels in sera of 80 noninfected individuals. The cutoff level of the developed ELISA was set at 8 ng/ml.
Fig 5.
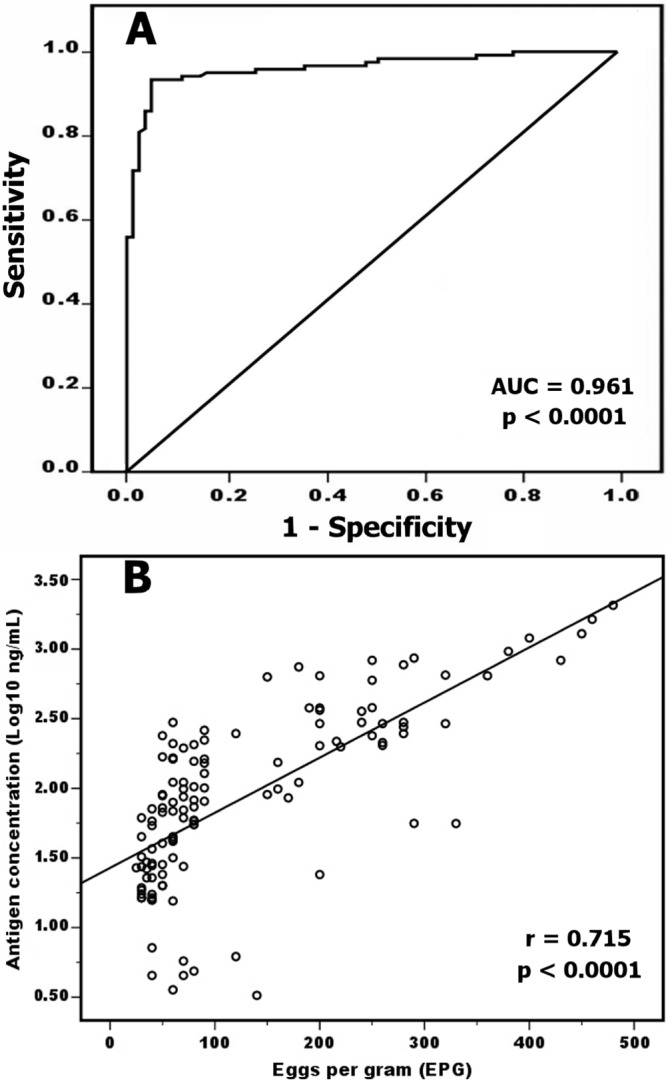
Diagnostic accuracy of the developed FgCA-27 ELISA. (A) Receiver operating characteristic (ROC) curve of circulating Fasciola antigen detection using ELISA for discriminating F. gigantica-infected individuals and noninfected individuals. The true-positive rate (sensitivity) is plotted as a function of the false-positive rate (1 − specificity). Each point on the ROC curve represents a sensitivity/specificity pair corresponding to a particular decision threshold. The area under the curve (AUC) value represents the combined effects of both sensitivity and specificity of circulating Fasciola antigen in diagnosing individuals infected with F. gigantica (AUC = 0.961, P < 0.0001). (B) Correlation between the levels of Fasciola circulating antigen measured in human sera by ELISA (expressed in log10 ng/ml) and the egg count measured by Kato-Katz technique (expressed in EPG). The results of both assays for 120 serum samples were statistically analyzed and showed a highly significant correlation (Spearman r = 0.715, P < 0.0001).
Table 1.
Performance characteristics of a newly developed ELISA based on Fasciola circulating antigen detection in sera of 120 parasitologically diagnosed F. gigantica-infected individuals and 80 noninfected individuals
| Parasitological diagnosis | No. of individuals | No. of individuals with FgCA-27 ELISA resulta |
% positivity | |
|---|---|---|---|---|
| Positive | Negative | |||
| F. gigantica infected | TP | FN | ||
| Light infection (<100 EPG) | 76 | 69 | 7 | 90.8 |
| Moderate infection (100–299 EPG) | 33 | 32 | 1 | 96.9 |
| Severe infection (≥300 EPG) | 11 | 11 | 0 | 100 |
| Total | 120 | 112 | 8 | 93.3 |
| Not infected with F. gigantica | FP | TN | ||
| Other parasitic infection | 57 | 3 | 54 | 5.2 |
| No parasitic infection | 23 | 1 | 22 | 4.3 |
| Total | 80 | 4 | 76 | 5.0 |
Abbreviations: TP, true positive (a serum sample from an individual with confirmed Fasciola infection showing a positive result by the ELISA); FP, false positive (a serum sample from an individual with confirmed absence of Fasciola infection showing a positive result by the ELISA); TN, true negative (a serum sample from an individual with confirmed absence of Fasciola infection showing a negative result by the ELISA); FN, false negative (a serum sample from a patient with confirmed Fasciola infection showing a negative result by the ELISA). Sensitivity (%) = TP/(TP + FN) = 112/(112 + 8) × 100% = 93.3%; specificity (%) = TN/(TN + FP) = 76/(76 + 4) × 100% = 95.0%; efficiency of the test (%) = (TP + TN)/total = (112 + 76)/200 × 100% = 94.0%.
DISCUSSION
During the last 2 decades, a major focus of research has been directed toward the identification of Fasciola antigens during human infection as a step toward the development of an efficient diagnostic assay (34). Fasciola antigens are mostly released from the gut and the tegument into the blood circulation and excreted in stools of infected animals and humans (35–39). Several Fasciola antigens have been detected as circulating antigens in serum or as coproantigens in feces and were successfully used in immunodiagnosis of human fascioliasis (8, 11, 16, 34, 40). Of these, one antigenic component with an approximate molecular mass of 27 kDa was found to give a consistent reaction with human fascioliasis sera (14, 18, 41–43). Of note, all serologic tests based on the 27-kDa antigen were developed and optimized with an emphasis on the detection of antibodies to F. hepatica (12, 13, 44, 45). However, the direct detection of Fasciola antigens secreted by the living flukes has apparent advantages over antibody detection tests, in that antigenemia implies active infection, and this approach has the ability to assess efficacy of chemotherapy and determine the effectiveness of future vaccines (24, 46–48).
In our previous study (22), we identified a wide band of 26 to 28 kDa in adult worm and E/S antigenic preparations of F. gigantica and also in sera of cattle infected with F. gigantica by using specific IgG antiserum developed in rabbit to the target 26- to 28-kDa antigen purified from FWAP. This antibody did not recognize any target epitopes in the Ascaris and Schistosoma antigenic extracts, and its antigenic determinant was located in the guts and teguments of adult F. gigantica worms. This enhanced our efforts to identify the target antigen of the specific IgG antibody to the 26- to 28-kDa Fasciola antigen in sera of individuals infected with F. gigantica; we fortunately observed a sharp narrow band at 27 kDa in selected sera from individuals infected with F. gigantica, and no specific reaction was observed in sera of uninfected individuals via Western blot analysis (unpublished data).
In the present study, the target 27-kDa circulating antigen was isolated and purified from serum samples from F. gigantica-infected humans, and a highly reactive IgG antibody to the purified 27-kDa antigen was developed in rabbit. The newly developed IgG antibody identified a polypeptide band of 27 kDa in adult worm and E/S antigenic preparations of F. gigantica but did not recognize any target epitopes in the Ascaris and Schistosoma antigenic extracts, and its antigenic determinant was located in the guts and teguments of adult F. gigantica worms. Moreover, the target circulating antigen was identified at 27 kDa in sera from individuals infected with F. gigantica, and no specific reaction was observed in sera of uninfected individuals using Western blotting or ELISA. An inhibition-Western blot assay was performed to confirm that the 27-kDa protein isolated from serum is a parasite molecule. Our antigen is similar in molecular mass to an F. gigantica antigen designated FG27 (42, 49). The partial biochemical characterization of our purified antigen confirmed its protein moiety. However, further molecular characterization of our 27-kDa target antigen, including peptide mapping followed by mass spectrometry (MS) amino acid sequence will be performed in collaboration with a well-equipped laboratory to clarify its identity.
The identification of the Fasciola antigens in different FWAPs and E/S products and in human serum is of crucial importance for reliable diagnosis of active Fasciola infection. Several sensitive and specific methods based on Fasciola antigen detection in serum and stool using specific antibodies were applied to F. hepatica and F. gigantica infections in animals (8, 25, 26, 47). The antigen tests can detect experimental infection a few days after inoculation (23). However, few antigen detection assays have been developed for detection of F. gigantica in human fluids, and these have varied ranges of sensitivities and specificities (21). In the present study, we have developed FgCA-27 ELISA, which successfully detected the 27-kDa Fasciola antigen with a detection limit of 8 ng/ml in human serum samples. The presence of Fasciola antigens in free form in the circulation of infected individuals may be attributed to continuous shedding and release of circulating antigens as a possible escape mechanism by the parasites, to reinfections, and to exposure to maternal antigens that may lead to developing tolerance rather than immunity to free antigens of natural infections (50). Moreover, the presence of high titers of specific IgG antibodies in the sera of infected individuals does not indicate a complete elimination of the pathogen; such high titers of antibodies may not neutralize all of the circulating antigens, i.e., via immune complex formation, and also, the specific IgM antibody response will not be stimulated for new infections. The presence of different levels of specific anti-27 kDa IgG antibodies in serum samples did not affect the detection of different concentrations of the target Fasciola antigen using the optimized FgCA-27 ELISA. A nonsignificant decrease (P > 0.05) in the antigen concentrations with the increase of anti-27-kDa IgG antibody levels in serum was shown (data not shown). This indicates that the 27-kDa antigen could attach to the solid phase in the presence of specific IgG antibodies or other serum proteins.
The performance characteristics of the developed assay were investigated using sera of 200 parasitologically diagnosed individuals. The developed assay gave high degrees of sensitivity, specificity, and efficiency (>93%). The accuracy of the developed ELISA, calculated by the area under the ROC curve (AUC), yielded a 0.961 value, indicating a high performance of the assay that can significantly (P < 0.0001) discriminate F. gigantica-infected and noninfected individuals. Moreover, for the precision of the test, the calculated intra-assay and interassay coefficients of variation were 3.3% and 5.8%, respectively, indicating also high performance in reproducibility and repeatability of the assay. Interestingly, our developed assay shows results that are superior or at least similar to those of the previously reported antigen tests for diagnosis of human fascioliasis (37, 51, 52), and the differences may be due to the different natures of antigens used. A recent study evaluated an antigen capture ELISA using a pair of monoclonal antibodies raised against F. gigantica E/S antigens in human serum and stools at a lower detection limit of 3 ng/ml (25). The sensitivity (94%) and specificity (94.6%) of the capture ELISA in serum were similar to those shown in our study. Another more recent study showed a similar degree of sensitivity (94.5%) but a lower specificity (84.6%) using sandwich ELISA based on monospecific rabbit IgG antibody to 14.5-kDa protein antigen obtained from F. gigantica adult worms (26). Protein-based tests (e.g., protein microarrays) as well as DNA-based molecular tests (including PCR) can be used for clinical detections as well as field screening (53). Further investigations comparing the immunodiagnostic performances of our FgCA-27 ELISA with such tests will be performed in collaboration with a well-equipped laboratory.
In the present study, all serum samples showing false-negative ELISA results, except for one (which was of a moderate parasite burden [i.e., egg count of 100 to 299 EPG]), corresponded to light infection (i.e., egg count < 100 EPG); however, the target 27-kDa antigen was identified in all these samples by using the more sensitive Western blot technique. This result may support the idea of immune complex formation in the samples showing false-negative ELISA results. Therefore, the absence of the target 27-kDa antigen in 8 sera of our infected individuals by ELISA may be due to undetectable levels of circulating antigen in these serum samples as a result of immune complex formation with host antibodies that tend to decrease the potential rate of circulating antigen (47, 54). Consequently, the developed assay conditions will be optimized to detect 100% of positive samples by using sample pretreatment with a dissociating buffer for immune complexes. The use of specific capturing of the 27-kDa antigen by using specific rabbit IgG polyclonal antibody coating of the solid phase will be also investigated. Moreover, the identification and detection of the target 27-kDa antigen in stool samples will be investigated to overcome the drawback of immune complex formation.
Cross-reactivity with other parasites represents a major problem in specificity of immunodiagnostic assays like ELISA, especially in areas of disease endemicity (13). Moreover, Fasciola has antigens that are cross-reactive with antigens of some parasites, such as Schistosoma and Echinococcus (12, 54). Regarding the specificity of the developed FgCA-27 ELISA, there were no major problems related to cross-reactivity when common protozoans or helminthes were present. The developed assay had false-positive results for only 4 of 80 noninfected individuals, with a high degree of specificity of 95% in detecting human fascioliasis. This is not surprising, because the specificity of the FgCA-27 ELISA depends mainly on the recognition of a single Fasciola antigen (27 kDa) by monospecific IgG antibody. However, the ELISA positivity of 4 individuals showing false-positive ELISA results was investigated using Western blot analysis. Only one sample of a parasite-free healthy individual showed a sharp band at 27 kDa, and consequently, this individual may be infected with Fasciola but not identified by stool analysis due to the variable sensitivity of that technique; as well, the eggs might be present in small numbers at irregular intervals (55) and in most cases, repeated stool examinations are required to accurately diagnose (10). Regarding the remaining 3 sera of E. histolytica-infected individuals, no bands were identified in their blots. The ELISA positivity of these sera may attributed to cross-reactivity with some epitopes on antigens of other parasites that were not identified in our study (26, 55) or may be due to further purification steps of the developed anti-27-kDa IgG polyclonal antibody that are required to improve the assay specificity (41). However, a further study using a large number of individuals with other common parasitic infections is required to draw a final conclusion. Also, it will be necessary to carry out new studies in countries where other types of human trematodosis occur to confirm the usefulness of the FgCA-27 ELISA in countries where these parasitic diseases are endemic.
Of note, a highly significant correlation (Spearman r = 0.715, P < 0.0001) between circulating Fasciola antigen level in serum (ng/ml) and parasite egg count (EPG) was shown. Significant correlations of Fasciola antigens with parasite burden in animals (23, 56) and also with egg counts in human fascioliasis (25, 51) have been demonstrated. In contrast, Ubeira et al. (24) reported that there was no correlation between egg counts and antigens levels in stool as measured by ELISA, as the egg excretion is probably more erratic in patients with chronic infections. However, the presence of a highly significant correlation between circulating Fasciola antigen levels and parasite egg count which is presumably dependent on the number of flukes in the host is of crucial importance to the use of the developed assay to monitor the efficiency of flukicide treatment in Fasciola-infected individuals and to assess potential new vaccine efficacy.
In conclusion, a 27-kDa Fasciola antigen was identified in sera of F. gigantica-infected individuals. A highly sensitive and specific Fasciola circulating antigen detection assay, FgCA-27 ELISA, was developed for reliable laboratory diagnosis of human fascioliasis. Further characterization of the target antigen will be performed, and further optimization and field studies are necessary to draw a final conclusion regarding the use of FgCA-27 ELISA for diagnosis and follow-up for Fasciola-infected individuals.
ACKNOWLEDGMENTS
We thank W. A. Ragab, M. J. Saadh, and M. El-Bendary at Biotechnology Research Center, New Damietta City, Egypt, for their kind help during the study.
Footnotes
Published ahead of print 14 August 2013
REFERENCES
- 1.Soares MP, da Silva SS, Nizoli LQ, Felix SR, Schild AL. 2007. Chronic fascioliasis in farmed and wild greater rheas (Rhea Americana). Vet. Parasitol. 145:168–171 [DOI] [PubMed] [Google Scholar]
- 2.WHO 2007. Fact sheet on fascioliasis, p 8 In Action against worms, issue 10. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.Mas-Coma S, Valero MA, Bargues MD. 2009. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 69:41–146 [DOI] [PubMed] [Google Scholar]
- 4.el-Shazly AM, Soliman M, Gabr A, Haseeb AN, Morsy AT, Arafa MA, Morsy TA. 2001. Clinico-epidemiological study of human fascioliasis in an endemic focus in Dakahlia Governorate, Egypt. J. Egypt. Soc. Parasitol. 31:725–736 [PubMed] [Google Scholar]
- 5.Curtale F, Abd El-Wahab Hassanein Y, El Wakeel A, Mas-Coma S, Montresore A. 2003. Distribution of human fascioliasis by age and gender among rural population in the Nile Delta, Egypt. J. Trop. Pediatr. 49:264–268 [DOI] [PubMed] [Google Scholar]
- 6.Soliman MF. 2008. Epidemiological review of human and animal fascioliasis in Egypt. J. Infect. Dev. Ctries. 2:182–189 [DOI] [PubMed] [Google Scholar]
- 7.Fica A, Dabanch J, Farias C, Castro M, Jercic MI, Weitze T. 2012. Acute fascioliasis: clinical and epidemiological features of four patients in Chile. Clin. Microbiol. Infect. 18:91–96 [DOI] [PubMed] [Google Scholar]
- 8.Hillyer GV. 1999. Immunodiagnosis of human and animal fasciolosis, p 435–447 In Dalton JP. (ed), Fasciolosis. CABI Publishing, Oxon, Wallingford, United Kingdom [Google Scholar]
- 9.Mas-Coma S. 2005. Epidemiology of fascioliasis in human endemic areas. J. Helminthol. 79:207–216 [DOI] [PubMed] [Google Scholar]
- 10.Valero MA, Perez-Crespo I, Periago MV, Khoubbane M, Mas-Coma S. 2009. Fluke egg characteristics for the diagnosis of human and animal fascioliasis by Fasciola hepatica and F. gigantica. Acta Trop. 111:150–159 [DOI] [PubMed] [Google Scholar]
- 11.Cordova M, Herrera P, Nopo L, Bellatin J, Naquira C, Guerra H, Espinoza JR. 1997. Fasciola hepatica cysteine proteinases: immunodominant antigens in human fascioliasis. Am. J. Trop. Med. Hyg. 57:660–666 [DOI] [PubMed] [Google Scholar]
- 12.O'Neill SM, Parkinson M, Dowd AJ, Strauss W, Angles R, Dalton JP. 1999. Immunodiagnosis of human fascioliasis using recombinant Fasciola hepatica cathepsin L1 cysteine proteinase. Am. J. Trop. Med. Hyg. 60:749–751 [DOI] [PubMed] [Google Scholar]
- 13.Carnevale S, Rodriguez MI, Guarnera EA, Carmona C, Tanos T, Angel SO. 2001. Immunodiagnosis of fasciolosis using recombinant procathepsin-L cysteine proteinase. Diagn. Microbiol. Infect. Dis. 41:43–49 [DOI] [PubMed] [Google Scholar]
- 14.Intapan PM, Maleewong W, Nateeworanart S, Wongkham C, Pipitgool V, Sukolapong V, Sangmaneedet S. 2003. Immunodiagnosis of human fascioliasis using an antigen of Fasciola gigantica adult worm with the molecular mass of 27-kDa by a dot-ELISA. Southeast Asian J. Trop. Med. Public Health 34:713–714 [PubMed] [Google Scholar]
- 15.Velusamy R, Singh BP, Sharma RL, Chanda D. 2004. Detection of circulating 54 kDa antigen in sera of bovine calves experimentally infected with F. gigantica. Vet. Parasitol. 119:187–195 [DOI] [PubMed] [Google Scholar]
- 16.Espinoza JR, Timoteo O, Herrera-Velit P. 2005. Fas2-ELISA in the detection of human infection by Fasciola hepatica. J. Helminthol. 79:235–240 [DOI] [PubMed] [Google Scholar]
- 17.Marcilla A, De la Rubia JE, Sotillo J, Bernal D, Carmona C, Villavicencio Z, Acosta D, Tort J, Bornay FJ, Esteban JG, Toledo R. 2008. Leucine aminopeptidase is an immunodominant antigen of Fasciola hepatica excretory and secretory products in human infections. Clin. Vaccine Immunol. 15:95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TGT, Le TH, De NV, Doan TT, Dao THT, Vercruysse J, Dorny P. 2010. Assessment of a 27-kDa antigen in enzyme-linked immunosorbent assay for the diagnosis of fasciolosis in Vietnamese patients. Trop. Med. Int. Health 15:462–467 [DOI] [PubMed] [Google Scholar]
- 19.Valero MA, Periago MV, Pérez-Crespo I, Rodríguez E, Perteguer JM, Gárate T, González-Barbera EM, Mas-Coma S. 2012. Assessing the validity of an ELISA test for the serological diagnosis of human fascioliasis in different epidemiological situations. Trop. Med. Int. Health 17:630–636 [DOI] [PubMed] [Google Scholar]
- 20.Robinson MW, Dalton JP. 2009. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:2763–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabada MM, White AC., Jr 2012. New developments in epidemiology, diagnosis, and treatment of fascioliasis. Curr. Opin. Infect. Dis. 25:518–522 [DOI] [PubMed] [Google Scholar]
- 22.Attallah AM, Karawia EA, Ismail H, Tabll AA, Nawar AA, Ragab WA, Abdel Aziz MM, El-Dosoky I. 2002. Identification and characterization of a 26- to 28-kDa circulating antigen of Fasciola gigantica. Ann. Trop. Med. Parasitol. 96:271–282 [DOI] [PubMed] [Google Scholar]
- 23.Anuracpreeda P, Wanichanon C, Chawengkirtikul R, Chaithirayanon K, Sobhon P. 2009. Fasciola gigantica: immunodiagnosis of fasciolosis by detection of circulating 28.5 kDa tegumental antigen. Exp. Parasitol. 123:334–340 [DOI] [PubMed] [Google Scholar]
- 24.Ubeira FM, Muiño L, Adela Valero M, Victoria Periago M, Pérez-Crespo I, Mezo M, González-Warleta M, Romarís F, Paniagua E, Cortizo S, Llovo J, Más-Coma S. 2009. MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. Am. J. Trop. Med. Hyg. 81:156–162 [PubMed] [Google Scholar]
- 25.Demerdash ZA, Diab TM, Aly IR, Mohamed SH, Mahmoud FS, Zoheiry MK, Mansour WA, Attia ME, El-Bassiouny AE. 2011. Diagnostic efficacy of monoclonal antibody based sandwich enzyme linked immunosorbent assay (ELISA) for detection of Fasciola gigantica excretory/secretory antigens in both serum and stool. Parasit. Vectors 4:176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allam G, Bauomy IR, Hemyeda ZM, Sakran TF. 2012. Evaluation of a 14.5 kDa-Fasciola gigantica fatty acid binding protein as a diagnostic antigen for human fascioliasis. Parasitol. Res. 110:1863–1871 [DOI] [PubMed] [Google Scholar]
- 27.Santiago de Weil N, Hillyer GV. 1986. Isolation of potential serodiagnostics F. hepatica antigens by electroelution from polyacrylamide gels. Am. J. Trop. Med. Hyg. 35:1210–1217 [DOI] [PubMed] [Google Scholar]
- 28.da Silva LC, Ferri RG. 1968. Schistosoma mansoni homogenate for active immunization of mice. Am. J. Trop. Med. Hyg. 17:369–371 [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosenbrough NJ, Farr AL, Randael RJ. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 30.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 31.Towbin H, Staehlin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrissey JH. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307–310 [DOI] [PubMed] [Google Scholar]
- 33.Gordon MJ, Lee KJ, Arias AA, Zare RN. 1991. Protocol for resolving protein mixtures in capillary zone electrophoresis. Anal. Chem. 63:69–72 [DOI] [PubMed] [Google Scholar]
- 34.Raina OK, Yadav SC, Sriveny D, Gupta SC. 2006. Immuno-diagnosis of bubaline fasciolosis with Fasciola gigantica cathepsin-l and recombinant cathepsin l 1-d proteases. Acta Trop. 98:145–151 [DOI] [PubMed] [Google Scholar]
- 35.Knobloch J. 1985. Human fascioliasis in Cajamarca/Peru. II. Humoral antibody response and antigenaemia. Trop. Med. Parasitol. 36:91–93 [PubMed] [Google Scholar]
- 36.Langley RJ, Hillyer GV. 1989. Detection of circulating parasite antigen in murine fascioliasis by two-site enzyme linked immunosorbent assay. Am. J. Trop. Med. Hyg. 41:472–478 [DOI] [PubMed] [Google Scholar]
- 37.Espino AM, Marcet R, Finlay CM. 1990. Detection of circulating excretory-secretory antigens in human fascioliasis by sandwich enzyme-linked immunosorbent assay. J. Clin. Microbiol. 28:2637–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viyanant V, Krailas D, Sobhon P, Upatham ES, Kusamran T, Chompocochan T, Thammasart S, Prastittirat P. 1997. Diagnosis of cattle fasciolosis by the detection of a circulating antigen using monoclonal antibody. Asian Pac. J. Allerg. Immunol. 15:153–159 [PubMed] [Google Scholar]
- 39.Anuracpreeda P, Wanichanon C, Chaithirayanon K, Preyavichyapugdee N, Sobhon P. 2006. Distribution of 28.5 kDa antigen in the tegument of adult Fasciola gigantica. Acta Trop. 100:31–40 [DOI] [PubMed] [Google Scholar]
- 40.Díaz A, Espino AM, Marcet C, Otero O, Torres D, Finlay CM, Sarracent J. 1998. Partial characterization of the epitope on excretory-secretory products of Fasciola hepatica recognized by monoclonal antibody ES78. J. Parasitol. 84:55–61 [PubMed] [Google Scholar]
- 41.Maleewong W, Wongkham C, Intapan PM, Pipitgool V. 1999. Fasciola gigantica-specific antigens: purification by a continuous-elution method and its evaluation for the diagnosis of human fascioliasis. Am. J. Trop. Med. Hyg. 61:648–651 [DOI] [PubMed] [Google Scholar]
- 42.Tantrawatpan C, Maleewong W, Wongkham C, Wongkham S, Intapan PM, Nakashima K. 2003. Characterisation of Fasciola gigantica adult 27-kDa excretory-secretory antigen in human fascioliasis. Parasitol. Res. 91:325–327 [DOI] [PubMed] [Google Scholar]
- 43.Dixit AK, Dixit P, Sharma RL. 2008. Immunodiagnostic/protective role of cathepsin L cysteine proteinases secreted by Fasciola species. Vet. Parasitol. 154:177–184 [DOI] [PubMed] [Google Scholar]
- 44.Strauss W, O'Neill SM, Parkinson M, Angles R, Dalton JP. 1999. Diagnosis of human fascioliasis: detection of anti-cathepsin L antibodies in blood samples collected on filter paper. Am. J. Trop. Med. Hyg. 60:746–748 [DOI] [PubMed] [Google Scholar]
- 45.Cornelissen JB, Gaasenbeek CP, Borgsteede FH, Holland WG, Harmsen MM, Boersma WJ. 2001. Early immunodiagnosis of fasciolosis in ruminants using recombinant Fasciola hepatica cathepsin L-like protease. Int. J. Parasitol. 31:728–737 [DOI] [PubMed] [Google Scholar]
- 46.Fagbemi BO, Obarisiagbon IO, Mbuh JV. 1995. Detection of circulating antigen in sera of Fasciola gigantica infected cattle with antibodies reactive with a Fasciola specific 88 kDa antigen. Vet. Parasitol. 58:235–246 [DOI] [PubMed] [Google Scholar]
- 47.Mezo M, González-Warleta M, Carro C, Ubeira FM. 2004. An ultrasensitive capture ELISA for detection of Fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (MM3). J. Parasitol. 90:845–852 [DOI] [PubMed] [Google Scholar]
- 48.Kueakhai P, Changklungmoa N, Chaithirayanon K, Songkoomkrong S, Riengrojpitak S, Sobhon P. 2013. Production and characterization of a monoclonal antibody against recombinant saposin-like protein 2 of Fasciola gigantica. Acta Trop. 125:157–162 [DOI] [PubMed] [Google Scholar]
- 49.Tantrawatpan C, Maleewong W, Wongkham C, Wonngkham S, Intapan PM, Nakashima K. 2005. Serodiagnosis of human fascioliasis by a cystatin capture enzyme linked immunosorbent assay with recombinant Fasciola gigantica cathepsin L antigen. Am. J. Trop. Med. Hyg. 72:82–86 [PubMed] [Google Scholar]
- 50.Attallah AM, Ghanem GE, Ismail H, El-Waseef AM. 2003. Placental and oral delivery of Schistosoma mansoni antigen from infected mothers to their newborns and children. Am. J. Trop. Med. Hyg. 68:647–651 [PubMed] [Google Scholar]
- 51.Espino A, Finlay C. 1994. Sandwich enzyme-linked immunosorbent assay for detection of excretory/secretory antigens in humans with fascioliasis. J. Clin. Microbiol. 32:190–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arafa MS, Abaza SM, El-Shewy KA, Mohareb EW, El-Moamly AA. 1999. Detection of Fasciola-specific excretory/secretory (E/S) protein fraction band (49.5 kDa) and its utilization in diagnosis of early fascioliasis using different diagnostic techniques. J. Egypt. Soc. Parasitol. 29:911–926 [PubMed] [Google Scholar]
- 53.Chen J-X, Chen M-X, Ai L, Chen J-H, Chen S-H, Zhang Y-N, Cai YC, Zhu X-Q, Zhou X-N. 2012. A protein microarray for the rapid screening of patients suspected of infection with various food-borne helminthiases. PLoS Negl. Trop. Dis. 6:e1899. 10.1371/journal.pntd.0001899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espino AM, Diaz A, Perez A, Finlay CM. 1998. Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J. Clin. Microbiol. 36:2723–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haseeb AN, El-Shazly AM, Arafa MA, Morsy AT. 2002. A review on fascioliasis in Egypt. J. Egypt. Soc. Parasitol. 32:317–354 [PubMed] [Google Scholar]
- 56.Duménigo B, Espino A, Finlay C. 1996. Detection of Fasciola hepatica antigens in cattle feces by a monoclonal antibody-based sandwich immunoassay. Res. Vet. Sci. 60:278–279 [DOI] [PubMed] [Google Scholar]