Abstract
The standard opsonophagocytosis killing assay (OPKA) for antibodies to pneumococcal capsular polysaccharide was modified to permit an evaluation of the protection-mediating antibodies to pneumococcal surface protein A (PspA). We found that by increasing the incubation time with the complement and phagocytes from 45 min to 75 min, the protective activity was readily detected. In another modification, we used a capsule type 2 target strain that expressed PspA but not pneumococcal surface protein C (PspC). With these modifications separately or in combination, rabbit antisera to the recombinant α-helical or proline-rich domains of PspA mediated >50% killing of the target strain. The ability of normal human sera to mediate the killing of pneumococci in this modified OPKA correlated with their levels of antibodies to PspA and their ability to protect mice against fatal infection with a type 3 strain. Passive protection of mice against pneumococci and killing in the modified OPKA were lost when normal human sera were adsorbed with recombinant PspA (rPspA) on Sepharose, thus supporting the potential utility of the modified OPKA to detect protective antibodies to PspA. In the standard OPKA, monoclonal antibodies to PspA were strongly protective in the presence of subprotective amounts of anti-capsule. Thus, the currently established high-throughput OPKA for antibodies to capsule could be modified in one of two ways to permit an evaluation of the opsonic efficacy of antibodies to PspA.
INTRODUCTION
Pneumonia is the leading cause of death in children aged <5 years old worldwide, and Streptococcus pneumoniae is the leading etiology of serious pneumonia (1). Pneumococcal polysaccharide (PS) conjugate vaccines (PCVs) are highly efficacious against strains with capsular types that are present in the vaccine (2–4). PCV7, which covered about 83% of invasive pneumococcal disease in children when it was introduced, gradually resulted in an almost complete absence of carriage and disease caused by the original 7 capsular types (5–7). One of the largest studies reported that after 7 to 8 years of PCV7 usage, the incidence of invasive pneumococcal disease was reduced by 77% among children aged <5 years. However, in the same age group, meningitis and invasive pneumonia were reduced by only 64 and 49%, respectively, due to an increasing incidence of infections caused by non-PCV7 types (5). Thus, significant pneumococcal disease, especially pneumonia and meningitis, remained after the introduction of PCV7 (5, 6, 8–10).
To increase coverage, PCV7 was replaced in the United States in 2011 by PCV13, which includes 6 additional PSs. Unfortunately, PCV13 covered only 33 to 41% of the invasive pneumococcal disease (IPD) strains (>20 different capsular types) at the time of its approval (5, 6, 11). Total carriage was largely unaffected by PCV7, and 78% of carriage strains (30 different capsular types) in 2008 to 2009 were not covered by PCV13 (12). The diversity of carriage strains may be a harbinger of future invasive strains if even a minority of the nonvaccine serotypes develop genotypes that allow them to fill the niche created by PCV13.
These findings intensified interest in using protection-eliciting pneumococcal proteins as potential vaccine components (13–16). One of the leading candidates is pneumococcal surface protein A (PspA), which reduces complement deposition on pneumococci (17–19), minimizes complement-dependent phagocytosis (20), and protects pneumococci from being killed by cationic peptides released by apolactoferrin (21, 22). It is a surface-accessible (23, 24) choline-binding protein (25) expressed by virtually all pneumococci (26–28). Immunization with the α-helical region of PspA generates antibodies in humans, monkeys, rabbits, and mice that passively protect mice against infection (29–32). The proline-rich (PR) domain of PspA, as well as its nonproline block (NPB), elicits antibodies that passively protect mice against infection (33). Antibodies to PspA enhance complement deposition on pneumococci (34, 35) and phagocytosis of pneumococci in the presence of a complement (20). PspA-mediated clearance of pneumococci from the blood of mice is dependent on the complement (36). These properties of PspA make it likely that the mediation of phagocytosis in vivo is a major protective mechanism of immunity to PspA. Antibodies to PspA also enhance the killing of pneumococci by the antibacterial peptides of apolactoferrin (22, 37).
To efficiently evaluate PspA and other noncapsular vaccine components in clinical trials, quantitative in vitro surrogates of protection are needed to both bridge between phase II (immunogenicity) and phase III (efficacy) trials and provide better insight into whether expensive phase III trials may succeed.
The ability of PS conjugate vaccines to elicit protection against pneumococci in humans is estimated using a quantitative opsonophagocytosis killing assay (OPKA). This assay, along with the anti-PS enzyme-linked immunosorbent assay (ELISA), has been the basis for licensing new PS-protein conjugates. The OPKA uses baby rabbit complement (BRC) and human HL-60 cells differentiated with dimethylformamide (DMF) to quantitate antibody-mediated complement-dependent opsonophagocytosis and killing of pneumococci. The assay quantitates the protective capacity by determining the dilution of each patient serum sample that facilitates the killing of 50% of the target bacteria. This endpoint is important for the OPKA to be quantitative and reproducible (38–40).
The standard OPKA does not generally detect 50% killing with antibodies to PspA, even though these antibodies are able to provide strong passive protection in mice against sepsis (29, 31). Although early attempts to quantitatively measure PspA-mediated killing of pneumococci by phagocytes were not successful (41), one report describes a qualitative OPKA for PspA (42).
At this time, the only quantitative surrogate assay available for measuring human antibodies to PspA is to use serial dilutions of pre- and postimmune sera to passively protect mice against intravenous (i.v.) sepsis (29). This assay works well and can provide confidence regarding the potential efficacy of vaccine formulations containing PspA. Unfortunately, a well-controlled quantitative study to examine a single pair of pre- and postimmune sera at the necessary serum dilutions can require 70 or more mice (29) and cost about $3,000. A more rapid and much less expensive surrogate assay whose results strongly correlate with those of the passive protection assay is needed. It is considered likely that antibodies to PspA protect against i.v. sepsis primarily by enhancing the complement-dependent clearance of pneumococci by phagocytes (17, 20, 36, 43). If a standardized quantitative in vitro assay(s) for opsonic antibodies to PspA could be developed that used the existing OPKA platform, it would greatly facilitate the progress of PspA vaccine development, and possibly that of other noncapsular antigens that elicit opsonic antibodies, into phase II dose-response and phase III efficacy trials.
It seemed likely that although the levels of antibody to PspA that provide passive protection may be opsonic in vivo, they might not meet the threshold required to mediate 50% killing in the standard OPKA because of differences in the in vivo and in vitro environments. We expected that if the target pneumococci lacked pneumococcal surface protein C (PspC), antibodies to PspA might become more opsonic in vitro. This hypothesis was based on earlier observations that PspA and PspC (choline binding protein A [CbpA]) decrease complement deposition on pneumococci and that mutants lacking the two molecules are even less virulent and more susceptible to complement deposition (18, 19, 44–47). By binding factor H, PspC inhibits the alternative (amplification) pathway of the complement (48, 49). In this study, we examined the possibility that increasing the incubation time with complement and phagocytes would cause antibodies to PspA to more readily mediate killing in the OPKA. We also examined the possibility that using a target strain with a mutation in the pspC gene would cause antibodies to PspA to more readily mediate killing in an OPKA and that the bacterial killing observed would correlate with passive protection. We also investigated the possibility that antibodies to PspA might exhibit a protective effect in the standard OPKA in the presence of a subprotective level of antibodies to capsule.
MATERIALS AND METHODS
Bacterial strains and antisera to recombinant PspA proteins.
The capsule serotype 2 strain S. pneumoniae D39 and S. pneumoniae TRE118, a pspC mutant of D39 that fails to express PspC (44), were used as the OPKA target strains. The generation of clade-specific rabbit serum to clade 2 (family 1) PspA was previously described (50). The recombinant proline-rich domain from S. pneumoniae strain AC94 (capsule type 9L, PspA family 1, clade 1) (33, 51) was used to prepare a rabbit antiserum to the PspA proline-rich domain (50). This proline-rich domain (PR+NBP) contains a nonproline block (NPB) that is largely invariant and was shown to contribute to the ability of the proline-rich domain to elicit protective immunity in mice (33). The expression and isolation of the PR+NPB have been previously described (33).
ELISA to detect Ab to PspA and PS.
Serum IgG antibody levels to PspA and capsular type 3 PSs were determined by ELISA (32, 52). Antibody levels to type 2 capsular PSs were determined by antibody binding to type 2 PS-coated latex beads compared with pneumococcal ELISA standard 89SF (modified from reference 53).
Human serum used in these studies.
Human blood serum pool 22 was made from two outdated blood plasma samples obtained from a blood bank and defibrinated using calcium and thromboplastin. Although the immunization history is unknown, this pool contains opsonic antibodies to all capsular types against which it was tested (38), including capsular type 2 (Fig. 1A). Normal human serum (J3) was obtained from a 41-year-old female volunteer who had several years of recent exposure to toddlers. This serum contained 83 μg/ml of antibodies that are reactive with family 2 PspA, <20 ng/ml antibodies to type 2 capsular PS, and 2.27 μg/ml of antibodies reactive with type 3 PS (54). Additional human serum samples were obtained from 19 volunteers (23 to 54 years of age) who also had not been immunized with any pneumococcal vaccine.
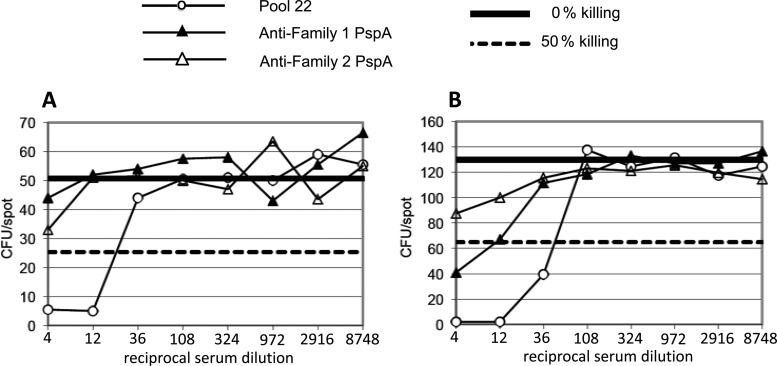
Fig 1.
Comparison of killing of wild-type S. pneumoniae D39 (A) and its pspC mutant TRE118 (B) in OPKA-1A. The sample dilutions start at 1:4 and extend through 3-fold dilutions to 1:8,748. The solid horizontal line in each panel represents 100% survival of the bacteria. The dashed horizontal line in each panel represents 50% killing. ○, human pool 22 (containing Abs to type 2 PS); ▲, rabbit antisera to family 1 PspA; △, rabbit antisera to family 2 PspA. The antiserum to family 2 was adsorbed with family 1 PspA to make it relatively specific for family 2 (46). The target strains express family 1 PspA.
OPKA-1A.
The OPKA-1A (Table 1) is a modification of the multiplexed opsonophagocytic killing assay (MOPA) used in the Nahm laboratory, which has been multiplexed to simultaneously detect protection by antibodies to four different capsular types in a single reaction (38, 39). In this assay, eight 3-fold dilutions are tested to determine the 50% killing endpoint. In this study, only one target bacterium was used in each experiment, and the CFU were counted by automation as previously described (38, 39). The OPKA-1A was used in the comparison studies shown in Fig. 1 and Table 2.
Table 1.
OPKA protocols used in this study
| OPKA typea | Type of assay | Colony enumeration method | No. of 3-fold serum dilutions tested | Assay buffer | E/Tb | Opsonizationc | Phagocytosisd | Reference |
|---|---|---|---|---|---|---|---|---|
| 1A | Endpoint dilution killing 50% of target bacteria | Automated counter | 8 | HBSS + 0.1% gelatin + 5% FBS | 400:1 | (Test serum + target bacteria) × 30 min, RT with shaking | (12.5% BRC + HL-60) × 45 min, 37°C, 5% CO2 with shaking | Modified from Burton and Nahm (38) |
| 1B | Endpoint dilution killing 50% of target bacteria | Automated counter | 8 | HBSS + 0.1% gelatin + 5% FBS | 400:1 | (Test serum + target bacteria) × 30 min, RT with shaking | 12.5% BRC × 30 min, 37°C, 5% CO2 with shaking; HL-60, × 45 min, 37°C, 5% CO2 with shaking | This study |
| 1C | Endpoint dilution killing 50% of target bacteria | Automated counter | 8 | HBSS + 0.1% gelatin + 5% FBS | 400:1 | (Test serum + target bacteria) × 30 min, RT with shaking | (12.5% BRC + HL-60) × 75 min, 37°C, 5% CO2 with shaking | This study |
| 2A | % killing of bacteria at indicated serum dilutions | Manual | 1–4 | HBSS + 0.1% gelatin + 5% FBS | 200:1 | (Test serum + target bacteria) × 30 min, 37°C, 5% CO2 with shaking | (7% BRC + HL-60) × 45 min, 37°C, 5% CO2 with shaking | This study |
| 2B | % killing of bacteria at indicated serum dilutions | Manual | 1–4 | HBSS + 0.1% gelatin + 5% FBS | 200:1 | (Test serum + target bacteria) × 30 min, 37°C, 5% CO2 with shaking | 10% BRC × 30 min, 37°C, 5% CO2 with shaking; HL-60, × 45 min, 37°C, 5% CO2 with shaking | This study |
The times of incubation with BRC and HL-60 cells are indicated. In OPKA-1B and OPKA-2B, bacteria are incubated with BRC and then incubated with HL-60 cells for the indicated time periods. In assays OPKA-1A and OPKA-1B, exactly the same total amount of BRC was used, but since the aliquot of phagocytes was present when the BRC was added in OPKA-1A, its concentration is less than in OPKA-1B, where the phagocytes were added after the initial incubation with BRC. OPKA-1B and OPKA-1C contain the indicated modifications of OPKA-1A, and OPKA-2B contains the indicated modifications of OPKA-2A.
E/T is the ratio of HL-60 cells to target bacteria.
RT, room temperature.
The concentrations of BRC used in the different assay conditions were tested to ensure functional complement activity at the concentrations used.
Table 2.
Sensitivity of OPKA-1A, OPKA-2A, and OPKA-3 methods for the detection of antibody-dependent killing
| Expt | OPKA variant | Target S. pneumoniae strain | Phagocytosis assay incubation conditions (°C, % CO2, time)a | OPKA titers |
|||
|---|---|---|---|---|---|---|---|
| Pool 22b | Anti-clade 2 PspAc | Anti-PR domainc | NRSd | ||||
| A | OPKA-1Be | TRE118 | BRC (37°C, 5% CO2, 30 min); HL-60 (37°C, 5% CO2, 45 min) | 1,962 | 352 | 133 | <4 |
| A | OPKA-1Cf | TRE118 | BRC + HL-60 (37°C, 5% CO2, 75 min) | 2,621 | 1,054 | 249 | <4 |
| B | OPKA-1Ag | D39 | BRC + HL-60 (37°C, 5% CO2, 45 min) | 720 | 8 | 41 | NTh |
| B | OPKA-1A | TRE118 | BRC + HL-60 (37°C, 5% CO2, 45 min) | 2,261 | 322 | 198 | NT |
| B | OPKA-1C | D39 | BRC + HL-60 (37°C, 5% CO2, 75 min) | 836 | 106 | 64 | NT |
| B | OPKA-1C | TRE118 | BRC + HL-60 (37°C, 5% CO2, 75 min) | 2,785 | 1,542 | 405 | NT |
In experiments A and B, the critical differences in assay conditions are incubation times and whether the BRC is incubated alone with the bacteria prior to addition of HL-60 cells (OPKA-1B) or if BRC and HL-60 cells are both added to the bacteria prior to a single incubation period.
Pool 22 (38) is a pool of normal human serum that contains antibodies to most capsular polysaccharides, including capsular type 2.
Rabbit antisera to the α-helical regions of clade 2 (50) PspA and to a proline-rich (PR) domain of PspA, including the nonproline block (33).
NRS, nonimmune normal rabbit serum.
In OPKA-2B, the opsonized target cells are incubated first with BRC for 30 min and then HL-60 cells are added and incubated for another 45 min.
OPKA-2C is a modification of the OPKA-2A, where the incubation time with complement and HL-60 cells was extended from 45 to 75 min.
Standard UAB OPKA, where the opsonized target cells are incubated with BRC and HL-60 cells for 45 min.
NT, not tested.
OPKA-2A.
The OPKA-2A (Table 1) is a modification of the OPKA-1A and was used in the experiments described in Fig. 2 to 5. Its primary variation from OPKA-1A is that OPKA-2A was run with fewer different serial dilutions of serum, and the analysis of the data was focused on the percentage of CFU killed at each dilution rather than the dilution needed to obtain 50% killing. Briefly, 2,000 pneumococci were suspended in 80 μl of Hanks balanced salt solution (Invitrogen, Life Technologies, Grand Island, NY) with 1% gelatin (HBSS+G) and opsonized by the addition of antibody dilutions in 9 μl of HBSS+G for 30 min at room temperature and with shaking at 700 rpm. Ten microliters of undiluted BRC (Pel-Freez, Rogers, AR) was added to the mixture along with 40 μl of HBSS containing 400,000 HL-60 cells (200:1 phagocyte-to-bacterium ratio) that had been stimulated for 5 to 6 days with dimethylformamide (DMF) in HBSS+G containing 5% heat-inactivated (30 min, 56°C) fetal bovine serum (FBS) (FetalClone I; HyClone, Logan, UT). The resultant mixture was incubated for 45 min at 37°C in 5% CO2 with shaking at 700 rpm. The reaction mixture was then cooled to 0°C for 20 min to stop phagocyte activity, serially diluted 1:3, and plated to determine the number of CFU per 25 μl. Each sample was run in triplicate. The control wells contained immune serum and HL-60 cells but used heat-inactivated (56°C for 30 min) BRC. These wells provided a control for any bacterial growth during the incubation steps. The average CFU per 25 μl from samples with active BRC was divided by the average CFU per 25 μl from reactions with heat-inactivated BRC to determine the percentage of CFU that survived. The percentage of bacteria killed was determined by subtracting the percent that survived from 100%. In the OPKA-1B, either D39 or TRE118 was used as the target bacterial strain, as indicated. This assay was always run in triplicate except for the data shown in Fig. 4B, for which the assay was run in sextuplicate.
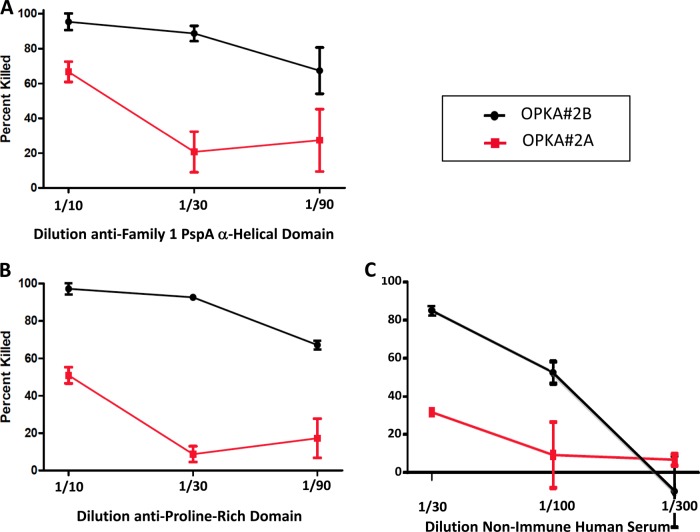
Fig 2.
Comparisons of combined 45-min versus sequential (30 plus 45 min) incubations on killing of the antibody-coated pspC mutant, TRE118, in OPKA-2A and OPKA-2B. In the OPKA-2A, antibody-coated bacteria were incubated with BRC (complement) and HL-60 cells for 45 min. In OPKA-2B, the antibody-coated bacteria had separate sequential incubations with BRC for 30 min and HL-60 cells for 30 min. Rabbit serum specific for the family 1 PspA α-helical domain (A) and rabbit antiserum specific for the proline-rich domain containing a nonproline block (B) were diluted 1:10, 1:30, or 1:90 and evaluated for killing in OPKA-2A and OPKA-2B. A human serum sample J3 from a nonimmunized adult (C) was diluted 1:30, 1:100, and 1:300 and tested in OPKA-2A and OPKA-2B. In each panel, the percentages of the target bacteria killed in OPKA-2A are shown in black; the results for OPKA-2B are shown in red. The greater killing in OPKA-2B than in OPKA-2A was statistically significant at P values of 0.0009, 0.0004, and 0.035 for panels A, B, and C, respectively. The means and standard errors are shown for each data set. To calculate statistical significance between the treatments with each serum, we first ranked all 6 data points (three from the OPKA-2A and three from the OPKA-2B) at each dilution. Next (A and B), the ranked data points all for all three dilutions were grouped into two groups of 9 according to assay type. Next, the 9 data points for OPKA-2A were compared with the 9 for OPKA-2B by the Mann-Whitney two-sample rank test. With the human serum (C), the highest dilution showed no killing with either treatment. For this serum, only data from the 1:30 and 1:100 dilutions were used for statistical analysis.
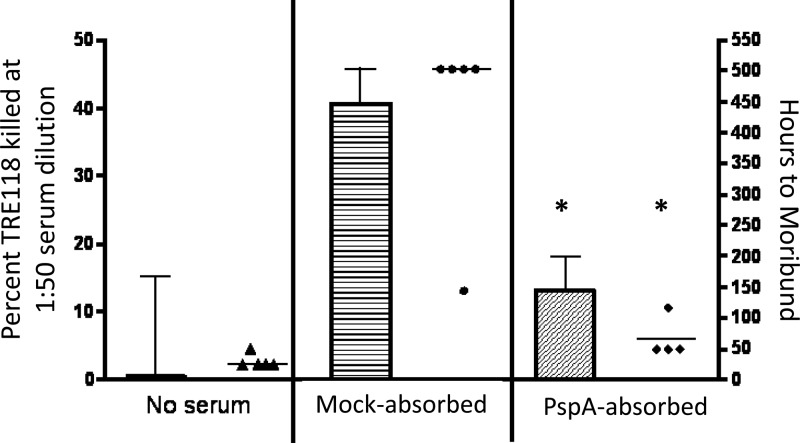
Fig 5.
Adsorption of normal human serum with rPspA Sepharose removes its antipneumococcal activity in OPKA-2B against TRE118 target strains and in passive protection against fatal infection. The normal human serum sample J3, also depicted in Fig. 2C, was adsorbed with rPspA Sepharose 4B. The adsorption also resulted in a dilution that reduced the serum concentration to 61% of its original concentration. Correcting for this dilution, the adsorption removed 97.3% of the antibodies to PspA and none of the IgG antibodies to type 3 PS (data not shown). A mock adsorption with BSA-conjugated Sepharose 4B resulted in the same dilution but did not remove Abs to PspA (data not shown). Shown is the mean percent killing and standard error of TRE118 in the OPKA-2B for the no-serum control (Ringer's diluent), the mock-adsorbed serum, and the PspA-adsorbed serum in the bar graphs. A passive protection study was also conducted with Ringer's lactate, mock-adsorbed serum, and PspA-adsorbed serum using 5 mice per group. Each mouse was given 100 μl of a 1:10 dilution of the indicated serum intraperitoneally (i.p.) 1 h prior to i.v. injection of CBA/N mice with 500 CFU of strain A66.1 type 3 S. pneumoniae. The time to moribund is depicted for each mouse as individual data points. The study was terminated at 510 h postchallenge, and all surviving mice are plotted at 510 h. The postadsorption sera showed significantly (*) less killing in the modified OPKA (P = 0.019) and in passive protection (P = 0.02) than did the serum adsorbed with BSA Sepharose.
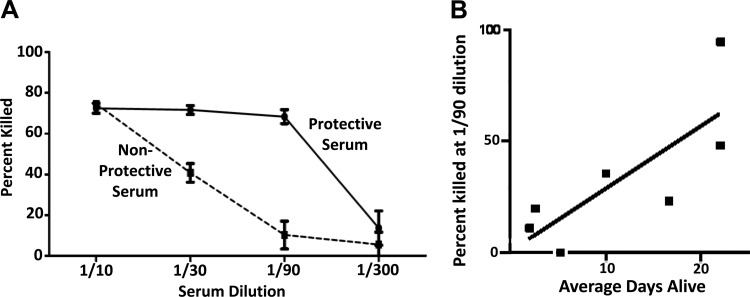
Fig 4.
Correlation of killing activity in the OPKA-2B against the TRE118 target strain with passive protection against type 3 infection. (A) A human serum sample selected from the 19 examined in Fig. 3 that was highly protective in mice and a poorly protective human serum sample were examined in the OPKA-2B (at 6 replicates per data point) for their ability to kill strain TRE118. The poorly protective serum showed less killing of TRE118 at all dilutions than the highly protective serum. At 1:30 and 1:90, the two sera differed at a P value of <0.0001 by Student's t test. (B) Seven serum samples were selected from the 19 sera in Fig. 3 (open symbols) that covered the range of activity in the passive protection and anti-PspA assays. All 7 sera were tested at a 1:90 dilution in the OPKA-2B against strain TRE118, with 6 replicates per data point; the mean percentage of OPKA killing is shown. The means and standard errors for each data set are shown. The mean percentage of TRE118 pneumococci killed at a 1:90 dilution showed a significant correlation with passive protection (r = 0.85, P = 0.024) by Pearson's linear regression correlation.
OPKA-1B and OPKA-2B.
The OPKA-1B and OPKA-2B (Table 1) are modifications of the OPKA-1A and OPKA-2A, respectively. Incubations of opsonized bacteria with BRC and phagocytes were separated into two incubations. The first (30-min incubation) was after the addition of the BRC to the opsonized bacteria, and the second (45-min incubation) followed the subsequent addition of HL-60 cells. As with OPKA-1A and OPKA-2A, all incubations with BRC and phagocytes were carried out at 37°C in 5% CO2 with shaking at 700 rpm (Table 1). The target bacterial strain used was either D39 or TRE118, as indicated in the descriptions of individual experiments in Results.
OPKA-1C.
The OPKA-1C (Table 1) was identical to the OPKA-1A except that the combined incubation of the antibody-coated bacteria with BRC and HL-60 cells lasted for 75 min rather than 45 min. The OPKA-1C target bacterial strain used was D39 or TRE118.
Passive protection against pneumococcal infection.
For passive protection studies, CBA/CaHN-Btkxid/J (CBA/N) mice were injected intraperitoneally with 100 μl of a 1:10 lactated Ringer's solution or 100 μl of 1:10 normal human serum sample from unimmunized volunteers diluted in lactated Ringer's 1 h before intravenous (i.v.) challenge with 500 CFU of capsular serotype 3 S. pneumoniae strain A66.1 (PspA family 1) (29, 55) or capsular serotype 6A S. pneumoniae strain BG7322 (PspA family 1) (29). Although originally reported as a 6B (29), a recent retyping of BG7322 using monoclonal antibodies (53) showed it to be a 6A. These two pneumococcal strains have PspAs that are from PspA family 1 and have been used in published passive protection models (29). The mice were monitored for 21 days. Mice that became moribund (unresponsive to touch or with a surface temperature of ≤25°C) were scored as moribund. At the end of 21 days, the surviving mice were sacrificed and recorded as having survived >21 days. The surviving mice and moribund mice were euthanized with CO2 narcosis and then cervical dislocation. All animal protocols were conducted in accordance with Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (IACUC).
Adsorption of human and rabbit sera to remove protective antibodies.
Activated Sepharose beads were coated with recombinant PspA (rPspA) of Rx1 by a published procedure (56). A column made from these beads and a control column to which bovine serum albumin (BSA) was conjugated to the Sepharose beads were used to adsorb human serum samples.
Examination of synergy between monoclonal antibodies to PspA and type 3 PS.
Some experiments used OKPA-1A assay conditions, monoclonal antibodies (MAbs) to PspA, and amounts of MAb to type 3 capsule that were too low to give significant killing so that it would be possible to see if synergy existed between MAbs to PspA and MAbs to type 3 capsule. The MAbs used were XiR278 (specific for PspA family 1 of strain D39 [31]), Hyp3M6 (specific for capsular type 3 PS [53]), and Hyp6BG8 (specific for type 6B PS [57]). The anti-type 3 MAbs were tested alone or in conjunction with MAb Hyp6BG8 or MAb XiR278. The target strain in these assays was capsular serotype 3 strain S. pneumoniae OREP3, an optochin-resistant variant of S. pneumoniae strain Wu2 (38).
Statistics.
The statistical tests used in each study are indicated in either the figure legends or the text. To compare the times to death for mice and, in some cases, killing in the OPKA, the Mann-Whitney two-sample rank test was used. For comparisons of the OPKA percent killing data, the Student t test was used. To examine the correlations between antibodies to PspA and passive protection or comparisons of the OPKA and passive protection, a parametric Pearson's linear regression correlation was used.
RESULTS
Antibodies to PspA support the killing of PspC− pneumococci in a modified OPKA.
Using the OPKA-1A protocol, we observed >50% killing with human serum pool 22 (which contains Abs to type 2 PS) at dilutions of 1:4 and 1:12 but only 37% killing with the highest test concentration of the rabbit anti-PspA family 1 serum (1:4) (Fig. 1A). In contrast, when TRE118 (the pspC mutant of type 2 strain D39) was used as the target strain, a 1:4 dilution of rabbit antiserum to PspA gave 67% killing, and a 1:12 dilution gave 50% killing (Fig. 1B). This rabbit antiserum to PspA lacked a detectable Ab response to type 2 PS. Thus, the use of TRE118 as the target strain provided a significant improvement in the assay for detecting opsonic antibodies to PspA and permitted protective levels of antibodies to be expressed as the dilution, which killed 50% of the target strain. Most of the OPKA studies described in this paper used target strain TRE118. Exceptions are shown in the results depicted in Table 2, Fig. 1A, and Fig. 6.
Fig 6.
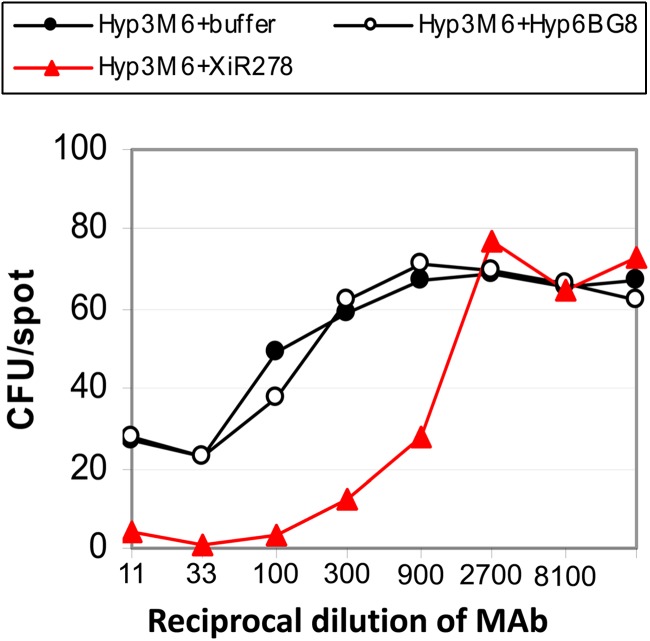
Synergy between MAbs to capsule and MAbs to PspA in the standard UAB OPKA. For this study, the Hyp3M6 MAb to type 3 PS was purposefully used at a concentration that only weakly mediated killing of the type 3 target strain (●). The anti-PspA MAb XiR278 was used as an ascites fluid without prior dilution. When XiR278 was used by itself, it mediated no (<5%) measurable killing at any dilution (data not shown). Red line, the result when XiR278 is mixed with the anti-type 3 MAb. Open symbols, the results when the anti-type 3 MAb was mixed with an anti-type 6B MAb, which was anticipated not to react with the type 3 PS target strain.
Longer total time of incubation increased assay sensitivity of protection mediated by antibodies to PspA.
This experiment compared the killing of TRE118 in assay protocols the OPKA-2A and OPKA-2B. In the OPKA-2A, the target bacteria are incubated with antibodies for 30 min followed by incubation with BRC and phagocytes for 45 min prior to plating to determine the CFU count (38, 39). In the OPKA-2B, the antibody-coated bacteria were incubated for 30 min after the addition of BRC and then were incubated for 45 additional min after the addition of HL-60 cells. We examined a rabbit antiserum to PspA family 1, which detects the α-helical domain of PspA (Fig. 2A), a rabbit antiserum to the proline-rich domain of PspA (Fig. 2B), and serum from nonimmunized human J3, which appeared to lack Abs to the type 2 PS of the target strain but contained antibodies to PspA. Figure 2 shows that with each of these three sera, higher percentages of killing were observed with the sequential incubations with BRC and HL-60 phagocytes. The percentage of bacteria killed was approximately doubled with the sequential incubation technique (P values, 0.0004 to 0.035), and the sensitivities of the assays (based on the amount of each serum required for 50% killing) were increased by approximately 10-fold. At a 1:90 dilution, all three sera showed ≥50% killing in the OPKA-2B, compared to 20 to 30% killing in the OPKA-2A.
Passive protection of mice by human sera correlated with the level of antibodies to PspA.
It is well established that most normal children >2 years of age and normal adults have readily detectable levels of antibodies to PspA in their blood serum (58–60). Normal human serum samples from 19 adult volunteers who were not previously immunized with any pneumococcal vaccine were evaluated for their ability to passively protect mice against i.v. challenge with the serotype 3 pneumococcal strain A66.1 expressing family 1 PspA. The ability of these human serum samples to passively protect CBA/N mice was determined by giving the serum intraperitoneally 1 h prior to i.v. injection of live A66.1. This protocol has been previously shown to provide a robust passive protection assay for evaluating human antibodies to family 1 PspA (29). The protection of mice correlated with the amount of IgG serum antibodies to PspA as determined by ELISA (r = 0.85, P = 0.0007) but not with the negligible levels of serum antibodies to the type 3 capsular PS (r = 0.39, P = 0.85) (Fig. 3). It is well established that anti-type 3 antibodies can passively protect mice from infection (41, 61). Thus, the lack of a correlation between the level of anti-type 3 antibodies and protection was probably because of the virtual absence (Fig. 3) of antibodies to the type 3 PS in most of these serum samples.
Fig 3.
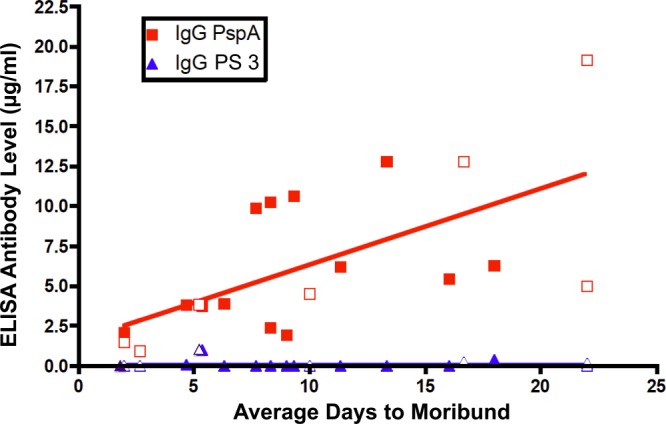
Analysis of 19 normal human serum samples to look for correlations between levels of antibody to PspA or capsule (y axis) and the ability of the sera to protect mice from fatal pneumococcal infection with capsular serotype 3 strain A66.1 (x axis). The correlation between IgG antibody levels to PspA and passive protection was highly significant by Pearson's linear regression correlation (r = 0.71, P = 0.0007). However, there was no correlation between IgG antibody levels to type 3 capsule and passive protection (r = 0.39, P = 0.11). Seven of these sera (open symbols) were also evaluated in the experiment shown in Fig. 4B.
These same human serum samples were also used to passively protect mice from fatal i.v. sepsis with strain BG7322 (type 6A PS, PspA family 1). As with the type 3 challenge strain, a statistically significant correlation was observed between the anti-family 1 PspA levels in these serum samples and the time to death (r = 0.67, P = 0.0016; data not shown).
Killing of pspC mutant TRE118 in OPKA-2B correlates with the levels of antibodies to PspA in passive protection of mice by human serum.
We next examined two of the 19 human serum samples, one that provided strong protection when given passively to mice and one that was not able to passively protect mice. These two serum samples were examined in the OPKA-2B at dilutions of 1:10, 1:30, 1:90, and 1:300 against the target strain TRE118. The highly protective serum demonstrated better killing in the OPKA-2B than the nonprotective serum at the 1:30 and 1:90 dilutions. At both dilutions, the differences were statistically significant (P < 0.001) (Fig. 4A), but the difference was the largest at the 1:90 dilution. The protective serum resulted in 87% killing, whereas the nonprotective serum resulted in only 14.6% killing. Two other human serum samples, which were highly protective in mice, were also examined in the OPKA-2B. Each showed the greatest differences versus the nonprotective serum at the 1:90 dilution (data not shown).
We next used the OPKA-2B to examine 7 serum samples (Fig. 3, open squares) from the original 19 that represented the overall diversity of the 19 samples in terms of passive protection in mice and antibody levels to PspA. The ability of these 7 serum samples to kill TRE118 (a pspC mutant of D39) pneumococci at a 1:90 dilution in the OPKA-2B had a statistically significant correlation (r = 0.85, P = 0.0238) with the ability of the same samples to passively protect mice from type 3 pneumococci expressing clade 2, family 1 PspA (Fig. 4B). For the same 7 samples, the association of the percent killing in the OPKA-2B with antibody levels to PspA was almost significant (r = 0.75, P = 0.05; data not shown).
Protective human serum lost its ability to protect mice and mediate killing in the OPKA-2B when its antibodies to PspA were adsorbed.
The normal human serum J3, which mediated strong killing activity in the OPKA-2B against strain TRE118 (Fig. 2C), was adsorbed with PspA-coated Sepharose beads to remove antibodies to PspA. Before adsorption, this serum contained 84 μg/ml of IgG antibodies to PspA. After adsorption, 97.3% of this antibody was removed compared to the control adsorption with BSA-conjugated Sepharose beads. The ability of this serum to mediate passive protection of mice against type 3 challenge and killing of TRE118 in the OPKA-2B were also largely removed (Fig. 5). This serum contained <20 ng/ml of antibodies to type 2 capsular PS and 2.3 μg/ml of anti-type 3 capsular PS. Since only 100 μl of a 1:10 dilution of the adsorbed serum was injected into the mice, the amount of passive antibodies to type 3 PS/ml in the recipient's serum would have been about 15 ng/ml, well below the 350 ng/ml of antibody to PS shown to be required for protection against pneumococci in children (62). These data support the conclusion that the antibodies to PspA in this serum were required for the serum's activity in the modified OPKA and for the passive protection of mice.
Further refinement of the modified OPKA.
After the above studies were completed, we conducted additional studies with some of these sera in an effort to both improve and simplify the assay (Table 2). These studies were conducted using a 50% killing endpoint and compared the results obtained using the OPKA-1A, OPKA-1B, and OPKA-1C. The latter two assays used longer incubation times than did the OPKA-1A (Table 1). The most important result from this group of studies was that incubating the antibody-opsonized TRE118 bacteria simultaneously with BRC and phagocytes for 75 min (OPKA-1C) rather than separate 30- and 45-min incubations after the addition of BRC and HL-60 cells, respectively (OPKA-1B), yielded a 2- to 3-fold increase in the killing titer for antibodies to the α-helical and proline-rich domains of PspA (Table 2, experiment A). Thus, the killing by OPKA-1B that is mediated by antibodies to PspA (Fig. 2, 4, and 5) was probably an underestimation of the potential for antibodies to PspA to mediate the killing of pneumococci in the modified OPKA. The 75-min versus the 30- plus 45-min incubations resulted in only about 30% greater killing by antibodies to capsule. The failure of nonimmune rabbit sera to mediate killing in OPKA opsonic activity with nonimmune rabbit serum (Table 2, experiment A) further supported the conclusion that the protection seen with the two rabbit antisera was dependent on their antibodies to PspA.
In the studies in experiment B (Table 2), BRC and phagocytes were always mixed together prior to incubation with the antibody-opsonized bacteria at 37°C. The results from the incubations of antibody-coated D39 or TRE118 for 45 and 75 min with a mixture of BRC and phagocytes were compared. Regardless of the time of incubation or source of antibody, a severalfold-higher killing titer of TRE118 than D39 was observed (Table 2, experiment B) using protocol OPKA-1C than OPKA-1A. It was also observed, however, that the killing titers for antibodies to the α-helical and proline-rich domains were 2- to 5-fold higher when the incubation time was increased from 45 to 75 min. In contrast, the killing titer of the anti-capsular antibody (pool 22) was only slightly increased (Table 2, experiment B). Thus, the increase in killing titer resulting from the use of a pspC mutant target strain affected the titers with both antibodies to PspA and antibodies to capsule, whereas the increase in 50% killing titer due to increased incubation was quite specific for antibodies to PspA but not antibodies to capsule. In summary, using optimal conditions (TRE118 target strain and 75-min incubation with BRC and phagocytes) in experiments A and B, the OPKA titer of the antiserum to α-helical PspA ranged from 1,000 to 1,500, and the titer of the antiserum to the proline-rich domain ranged from 250 to 400 (Table 2). However, by increasing the incubation time, it was possible to detect 50% killing of wild-type D39 at reciprocal dilutions of 106 and 64, respectively, for the rabbit antisera to the α-helical and proline-rich domains of PspA, respectively.
Synergy between MAbs to PspA and MAbs to type 3 PS.
We also investigated the possibility that an in vitro assay to detect potentially protective antibodies to PspA might also be developed by using OPKA-1A, the standard UAB OPKA, in the presence of levels of anti-type 3 MAb that are too low to elicit protection on their own. As anticipated from the data with immune sera to PspA in Fig. 1, we observed that MAb XiR278 to PspA was unable to mediate significant levels of killing in the standard OPKA-1A even when undiluted XiR278 ascites fluid was used (data not shown). MAb to type 3 capsular PS was mixed 1:1 with MAb XiR278, and the mixture was diluted out in the OPKA-1A format using a capsular type 3 target (whose PspA is recognized by XiR278). The mixture (Fig. 6, red line) gave much more than 50% killing at a dilution of 1:900, while the same dilution of the anti-type 3 MAb by itself showed no killing (black line). Also depicted in Fig. 6 is a control experiment, showing that mixing an anti-type 6B MAb with the anti-type 3 MAb failed to result in synergistic killing of the type 3 target strain.
DISCUSSION
With some modification, the standard OPKA may be used to quantitatively evaluate the protective efficacy of antibodies generated to PspA. Rabbit sera generated by immunization with rPspA fragments of the two major immunogenic regions of PspA, the α-helical (29, 31, 63) and the PR (33) domains, could be diluted to 1:200 or higher and kill the encapsulated target strains that expressed PspA and carried the pspC mutation. We also showed that the ability of normal human sera to passively protect mice from infection correlated with the level of antibody to PspA in the sera and with the ability of the sera to support killing in our modified OPKA. Using a human serum sample with a high antibody level to PspA and a negligible antibody level to capsular PS, we observed that adsorption with PspA-Sepharose removed antibodies to PspA and eliminated both the passive protection of the serum and killing in the modified OPKA. These observations indicate that (i) antibodies to PspA were able to mediate killing in the modified OPKA and (ii) the modified OPKA appeared to act as an in vitro surrogate for the ability of these same sera to passively protect mice from infection. Thus, although the modified OPKA used a mutant target strain, it detected antibodies to PspA that might protect mice from otherwise fatal infection with fully virulent pneumococci.
In our initial OPKA studies summarized above, pspC mutant pneumococci were opsonized with antibodies to PspA and then incubated for 30 min with BRC followed by a 45-min incubation with HL-60 phagocytes. Using this procedure, we observed that dilutions of about 1:100 to 1:300 of rabbit antisera to PspA facilitated the killing of the pspC mutant pneumococci by HL-60 cells. In a further modification, the antibody-opsonized bacteria were incubated simultaneously with BRC and HL-60 cells for 75 min. With this procedure, immune rabbit serum to PspA supported the killing of >50% of the target pneumococci at dilutions of 1:1,000 to 1:1,500 (Table 2). The increased sensitivity of anti-PspA-mediated killing with the 75-min versus the 45-min incubation was probably because the longer incubation gave the HL-60 cells more time to phagocytize and kill the pneumococci opsonized by antibodies to PspA and BRC. Moreover, using the combined 75-min incubation rather than sequential 30- and 45-min incubations makes the assay amenable to the type of large-scale studies that would be needed in vaccine trials. The OPKA modifications we have described permit the ready detection of the protective effects of antibodies to PspA and also increase the OPKA titers of anti-capsular antibodies. However, such modifications are not recommended for the anti-capsule assay since they would be expected to reduce its specificity for antibodies to capsule by allowing antibodies to PspA and possibly other antigens to be more readily detected.
Passive protection of mice is the current standard for evaluating human immune responses to immunization with PspA (29). Our present findings suggest that it may be possible to use OPKAs similar to the ones described here as in vitro surrogates to detect protective immunity to PspA in serum from immunized humans. Such assays might greatly facilitate further human trials of vaccines containing portions of PspA.
It has been shown that antibodies to PspA can activate complement deposition (20, 35). Prior studies also indicated that the absence of PspC facilitates the alternative pathway following a classical pathway trigger (18, 19, 47, 48). As a result, we expected the antibodies to PspA to result in greater complement deposition and enhanced in vitro phagocytosis and killing of pneumococci if PspC were absent. The pspC mutant did turn out to be a much more sensitive target for detecting opsonization by antibodies to PspA, although we do not yet know if the mechanisms we proposed are the correct ones. Mutations in other genes that interfere with complement deposition might also enhance the protective effects of antibodies to PspA in the OPKA. Dalia and Weiser (64) and Dalia et al. (65) have recently shown that longer chain lengths of pneumococci and mutations in exoglycosidases can enhance complement deposition and phagocytosis by HL-60 cells.
In a different set of experiments, we observed that in the standard OPKA, the levels of monoclonal antibodies to capsule and to PspA that were too low to mediate detectable killing were able to synergize and mediate >50% killing. In view of this finding, it is possible that minute amounts of Abs to capsule or other pneumococcal antigens may have contributed to the killing in the PspA OPKAs described in this paper; also, the OPKA values for capsule in general may be affected in some cases by the presence of antibodies to PspA or other pneumococcal antigens in the immune sera examined. The synergy finding has the potential to be developed into a useful surrogate assay to detect protective antibodies to PspA in human or animal serum. Such an assay can be carried out using a constant nonprotective level of antibodies to capsular PS, to which decreasing amounts of antibodies to PspA would be added, and would be most applicable using target strains, such as type 3, for which anti-capsular antibodies can be rare.
As PspA and other surface proteins move closer to being used in human vaccines, the need for quantitative in vitro assays to measure the protective effects of anti-PspA in pre- and postimmune sera becomes more critical. We have developed potential surrogate assays on the platform of the current very successful UAB OPKA, which measures antibodies to capsular PS. The modified OPKAs described in this paper should be able to form the basis for the development of validated assays to detect protective responses to PspA that are elicited by PspA-containing vaccines. Such assays can be run with the same equipment and computer software that are now used in the UAB OPKA for anti-capsular assays. The assays described here might also be applicable to other pneumococcal proteins (or antigens of other bacteria) that also elicit opsonizing antibody. To better understand whether these modified OPKAs can be used for the detection of protective antibodies, studies will be needed where human pre- and postimmune sera are tested in these in vitro assays and also in passive protection in mice. To know if the assays are true surrogates of protection in people, they will need to be used in prospective studies of invasive infection or in vaccine efficacy trials.
ACKNOWLEDGMENTS
We acknowledge Susan K. Hollingshead for her interest and encouragement during this project and Christina M. Croney and Kristopher R. Genschmer for their insights regarding the manuscript and its submission.
We acknowledge support from NIH grant no. AI021458 (to D.E.B.), the Epitope Recognition Core P30AR48311, contract no. AI-30021 (to M.H.N.), and KFDA no. 11172-360 (to K.H.K.).
Footnotes
Published ahead of print 7 August 2013
REFERENCES
- 1.Wardlaw T, Johannson EW, Hodge M, World Health Organization 2006. Pneumonia: the forgotten killer of children. The United Nations Children's Fund (UNICEF)/World Health Organization (WHO), Geneva, Switzerland: http://whqlibdoc.who.int/publications/2006/9280640489_eng.pdf [Google Scholar]
- 2.Haber M, Barskey A, Baughman W, Barker L, Whitney CG, Shaw KM, Orenstein W, Stephens DS. 2007. Herd immunity and pneumococcal conjugate vaccine: a quantitative model. Vaccine 25:5390–5398 [DOI] [PubMed] [Google Scholar]
- 3.Hammitt LL, Bruden DL, Butler JC, Baggett HC, Hurlburt DA, Reasonover A, Hennessy TW. 2006. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J. Infect. Dis. 193:1487–1494 [DOI] [PubMed] [Google Scholar]
- 4.Scott JR, Millar EV, Lipsitch M, Moulton LH, Weatherholtz R, Perilla MJ, Jackson DM, Beall B, Craig MJ, Reid R, Santosham M, O'Brien KL. 2012. Impact of more than a decade of pneumococcal conjugate vaccine use on carriage and invasive potential in Native American communities. J. Infect. Dis. 205:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR, Active Bacterial Core Surveillance/Emerging Infections Program Network 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 6.Croney CM, Coats MT, Nahm MH, Briles DE, Crain MJ. 2012. PspA family distribution, unlike capsular serotype, remained unaltered following introduction of the heptavalent-pneumococcal conjugate vaccine. Clin. Vaccine Immunol. 19:891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black S, Shinefield H, Cohen R, Floret D, Gaudelus J, Olivier C, Reinert P. 2004. Clinical effectiveness of seven-valent pneumococcal conjugate vaccine (Prevenar) against invasive pneumococcal diseases: prospects for children in France. Arch. Pediatr. 11:843–853 (In French.) [DOI] [PubMed] [Google Scholar]
- 8.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, Butler JC, Rudolph K, Parkinson A. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297:1784–1792 [DOI] [PubMed] [Google Scholar]
- 9.Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, Craig AS, Farley MM, Jorgensen JH, Lexau CA, Petit S, Reingold A, Schaffner W, Thomas A, Whitney CG, Harrison LH. 2009. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 360:244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ampofo K, Pavia AT, Chris S, Hersh AL, Bender JM, Blaschke AJ, Weng HY, Korgenski KE, Daly J, Mason EO, Byington CL. 2012. The changing epidemiology of invasive pneumococcal disease at a tertiary children's hospital through the 7-valent pneumococcal conjugate vaccine era: a case for continuous surveillance. Pediatr. Infect. Dis. J. 31:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wroe PC, Lee GM, Finkelstein JA, Pelton SI, Hanage WP, Lipsitch M, Stevenson AE, Rifas-Shiman SL, Kleinman K, Dutta-Linn MM, Hinrichsen VL, Lakoma M, Huang SS. 2012. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Pediatr. Infect. Dis. J. 31:249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AlonsoDeVelasco E, Verheul AF, Verhoef J, Snippe H. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 59:591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barocchi MA, Censini S, Rappuoli R. 2007. Vaccines in the era of genomics: the pneumococcal challenge. Vaccine 25:2963–2973 [DOI] [PubMed] [Google Scholar]
- 15.Briles DE, Paton JC, Hollingsehad SK, Boslego JW. 2010. Pneumococcal common proteins and other vaccine strategies, p 482–488 In Levine MM, Dougan G, Good MF, Liu MA, Nabel GJ, Nataro NP, Rappouli R. (ed), New generation vaccines, 4th ed. Informa Healthcare USA, Inc., New York, NY [Google Scholar]
- 16.Miyaji EN, Oliveira MLS, Carvalho E, Ho PL. 2012. Serotype-independent pneumococcal vaccines. Cell. Mol. Life Sci. 10.1007/s00018-012-1234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu A-HT, Fulgham RL, McCory MA, Briles DE, Szalai AJ. 1999. Pneumococcal surface protein A (PspA) inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Glover DT, Szalai AJ, Hollingshead SK, Briles DE. 2007. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 75:5877–5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukerji R, Mirza S, Roche AM, Widener RW, Croney CM, Rhee DK, Weiser JN, Szalai AJ, Briles DE. 2012. Pneumococcal surface protein A inhibits complement deposition on the pneumococcal surface by competing with the binding of C-reactive protein to cell-surface phosphocholine. J. Immunol. 189:5327–5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren B, Li J, Genschmer K, Hollingshead SK, Briles DE. 2012. The absence of PspA or presence of antibody to PspA facilitates the complement-dependent phagocytosis of pneumococci in vitro. Clin. Vaccine Immunol. 19:1574–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza S, Wilson L, Benjamin WH, Jr, Novak J, Barnes S, Hollingshead SK, Briles DE. 2011. Serine protease PrtA from Streptococcus pneumoniae plays a role in the killing of S. pneumoniae by apolactoferrin. Infect. Immun. 79:2440–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaper M, Hollingshead SK, Benjamin WH, Jr, Briles DE. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect. Immun. 72:5031–5040 (Erratum, 72:7379.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels CC, Briles TC, Mirza S, Hakansson AP, Briles DE. 2006. Capsule does not block antibody binding to PspA, a surface virulence protein of Streptococcus pneumoniae. Microb. Pathog. 40:228–233 [DOI] [PubMed] [Google Scholar]
- 24.McDaniel LS, Scott G, Widenhofer K, Carroll J, Briles DE. 1986. Analysis of a surface protein of Streptococcus pneumoniae recognised by protective monoclonal antibodies. Microb. Pathog. 1:519–531 [DOI] [PubMed] [Google Scholar]
- 25.Yother J, White JM. 1994. Novel surface attachment mechanism for the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crain MJ, Waltman WD, Jr, Turner JS, Yother J, Talkington DF, McDaniel LS, Gray BM, Briles DE. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollingshead SK, Baril L, Ferro S, King J, Coan P, Briles DE, Pneumococcal Proteins Epi Study Group 2006. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 55:215–221 [DOI] [PubMed] [Google Scholar]
- 28.Brandileone MC, Andrade AL, Teles EM, Zanella RC, Yara TI, Di Fabio JL, Hollingshead SK. 2004. Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine 22:3890–3896 [DOI] [PubMed] [Google Scholar]
- 29.Briles DE, Hollingshead SK, King J, Swift A, Braun PA, Park MK, Ferguson LM, Nahm MH, Nabors GS. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694–1701 [DOI] [PubMed] [Google Scholar]
- 30.Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, Schilling M, Gravenstein S, Braun P, King J, Swift A. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707–1711 [DOI] [PubMed] [Google Scholar]
- 31.McDaniel LS, Ralph BA, McDaniel DO, Briles DE. 1994. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17:323–337 [DOI] [PubMed] [Google Scholar]
- 32.Nabors GS, Braun PA, Herrmann DJ, Heise ML, Pyle DJ, Gravenstein S, Schilling M, Ferguson LM, Hollingshead SK, Briles DE, Becker RS. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743–1754 [DOI] [PubMed] [Google Scholar]
- 33.Daniels CC, Coan P, King J, Hale J, Benton KA, Briles DE, Hollingshead SK. 2010. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect. Immun. 78:2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochs MM, Bartlett W, Briles DE, Hicks B, Jurkuvenas A, Lau P, Ren B, Millar A. 2008. Vaccine-induced human antibodies to PspA augment complement C3 deposition on Streptococcus pneumoniae. Microb. Pathog. 44:204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren B, Szalai AJ, Hollingshead SK, Briles DE. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briles DE, Tart RC, Wu HY, Ralph BA, Russell MW, McDaniel LS. 1996. Systemic and mucosal protective immunity to pneumococcal surface protein A. Ann. N. Y. Acad. Sci. 797:118–126 [DOI] [PubMed] [Google Scholar]
- 37.Bitsaktsis C, Iglesias BV, Li Y, Colino J, Snapper CM, Hollingshead SK, Pham G, Gosselin DR, Gosselin EJ. 2012. Mucosal immunization with an unadjuvanted vaccine that targets Streptococcus pneumoniae PspA to human Fcγ receptor type I protects against pneumococcal infection through complement- and lactoferrin-mediated bactericidal activity. Infect. Immun. 80:1166–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton RL, Nahm MH. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13:1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton RL, Nahm MH. 2012. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin. Vaccine Immunol. 19:835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briles DE, Forman C, Horowitz JC, Volanakis JE, Benjamin WH, Jr, McDaniel LS, Eldridge J, Brooks J. 1989. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect. Immun. 57:1457–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, Lundberg U, Senn BM, Schunn M, Habel A, Henriques-Normark B, Ortqvist A, Kalin M, von Gabain A, Nagy E. 2008. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 205:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren B, McCrory MA, Pass C, Bullard DC, Ballantyne CM, Xu Y, Briles DE, Szalai AJ. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 173:7506–7512 [DOI] [PubMed] [Google Scholar]
- 44.Balachandran P, Brooks-Walter A, Virolainen-Julkunen A, Hollingshead SK, Briles DE. 2002. The role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogunniyi AD, LeMessurier KS, Graham RM, Watt JM, Briles DE, Stroeher UH, Paton JC. 2007. Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect. Immun. 75:1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quin LR, Moore QC, III, McDaniel LS. 2007. Pneumolysin, PspA, and PspC contribute to pneumococcal evasion of early innate immune responses during bacteremia in mice. Infect. Immun. 75:2067–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarva H, Janulczyk R, Hellwage J, Zipfel PF, Björck L, Meri S. 2002. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8–11 of factor H. J. Immunol. 168:1886–1894 [DOI] [PubMed] [Google Scholar]
- 49.Janulczyk R, Iannelli F, Sjoholm AG, Pozzi G, Bjorck L. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257–37263 [DOI] [PubMed] [Google Scholar]
- 50.Coral MCV, Fonseca N, Castañeda E, Di Fabio JL, Hollingshead SK, Briles DE. 2001. Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from Colombian children. Emerg. Infect. Dis. 7:832–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollingshead SK, Becker RS, Briles DE. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee H, Nahm MH, Kim KH. 2010. The effect of age on the response to the pneumococcal polysaccharide vaccine. BMC Infect. Dis. 10:60. 10.1186/1471-2334-10-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu J, Lin J, Benjamin WH, Jr, Waites KB, Lee CH, Nahm MH. 2005. Rapid multiplex assay for serotyping pneumococci with monoclonal and polyclonal antibodies. J. Clin. Microbiol. 43:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nahm MH, Olander JV, Magyarlaki M. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698–703 [DOI] [PubMed] [Google Scholar]
- 55.Avery OT, MacLeod CM, McCarty M. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wofsy L, Burr B. 1969. The use of affinity chromatography for the specific purification of antibodies and antigens. J. Immunol. 103:380–382 [PubMed] [Google Scholar]
- 57.Sun Y, Hwang Y, Nahm MH. 2001. Avidity, potency, and cross-reactivity of monoclonal antibodies to pneumococcal capsular polysaccharide serotype 6B. Infect. Immun. 69:336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baril L, Briles DE, Crozier P, King J, Punar M, Hollingshead SK, McCormick JB. 2004. Characterization of antibodies to PspA and PsaA in adults over 50 years of age with invasive pneumococcal disease. Vaccine 23:789–793 [DOI] [PubMed] [Google Scholar]
- 59.Rapola S, Jäntti V, Haikala R, Syrjänen R, Carlone GM, Sampson JS, Briles DE, Paton JC, Takala AK, Kilpi TM, Käyhty H. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146–1152 [DOI] [PubMed] [Google Scholar]
- 60.Virolainen A, Russell W, Crain MJ, Rapola S, Käyhty H, Briles DE. 2000. Human antibodies to pneumococcal surface protein A in health and disease. Pediatr. Infect. Dis. J. 19:134–138 [DOI] [PubMed] [Google Scholar]
- 61.Austrian R. 1979. Pneumococcal vaccine: development and prospects. Am. J. Med. 67:547–549 [DOI] [PubMed] [Google Scholar]
- 62.Siber GR, Chang I, Baker S, Fernsten P, O'Brien KL, Santosham M, Klugman KP, Madhi SA, Paradiso P, Kohberger R. 2007. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 25:3816–3826 [DOI] [PubMed] [Google Scholar]
- 63.Darrieux M, Miyaji EN, Ferreira DM, Lopes LM, Lopes AP, Ren B, Briles DE, Hollingshead SK, Leite LC. 2007. Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 75:5930–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalia AB, Weiser JN. 2011. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe 10:486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalia AB, Standish AJ, Weiser JN. 2010. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect. Immun. 78:2108–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]