Abstract
Francisella tularensis is a Gram-negative facultative intracellular pathogen that causes an acute lethal respiratory disease in humans. The heightened virulence of the pathogen is linked to its unique ability to inhibit Toll-like receptor (TLR)-mediated inflammatory responses. The bacterial component and mechanism of this inhibition are unknown. Here we show that lipids isolated from virulent but not attenuated strains of F. tularensis are not detected by host cells, inhibit production of proinflammatory cytokines by primary macrophages in response to known TLR ligands, and suppress neutrophil recruitment in vivo. We further show that lipid-mediated inhibition of inflammation is dependent on TLR2, MyD88, and the nuclear hormone and fatty acid receptor peroxisome proliferator-activated receptor α (PPARα). Pathogen lipid-mediated interference with inflammatory responses through the engagement of TLR2 and PPARα represents a novel manipulation of host signaling pathways consistent with the ability of highly virulent F. tularensis to efficiently evade host immune responses.
INTRODUCTION
Francisella tularensis subsp. tularensis is the causative agent of tularemia. Tularemia is an acute, often lethal disease that develops following inhalation of very low numbers of F. tularensis organisms, i.e., 10 to 15 bacteria (1). There are two virulent subspecies of F. tularensis. F. tularensis subsp. holarctica can cause disease in humans but typically is not lethal (2). In contrast, F. tularensis subsp. tularensis causes more-severe infections and, when left untreated, has a mortality rate of approximately 30%. F. tularensis subsp. tularensis is a relatively promiscuous pathogen capable of infecting a wide variety of mammals, arthropods (including ticks and biting flies), and amoebae (2). The acute nature of infections mediated by F. tularensis subsp. tularensis, combined with the low numbers of bacteria capable of causing disease, provoked development of this bacterium as a biological weapon by both the United States and the Soviet Union and its eventual classification as a tier 1 select agent in the United States (3). Thus, there is renewed interest in defining and understanding virulence mechanisms employed by this bacterium during its interactions with mammalian hosts.
Unlike many Gram-negative bacterial pathogens and attenuated strains and subspecies of Francisella (e.g., F. tularensis subsp. holarctica live vaccine strain [LVS] and Francisella novicida), virulent F. tularensis fails to elicit a strong proinflammatory response in cells in vitro and in vivo. For example, following in vitro infection of primary human dendritic cells, F. tularensis subsp. tularensis strain SchuS4 does not elicit production of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), or the IL-12 p40 subunit (IL-12p40), whereas the LVS and F. novicida readily induce production of all three cytokines in human cells (4–6). Unlike attenuated F. novicida, which has been routinely used as a model for inflammasome activation, virulent SchuS4 fails to activate inflammasomes, resulting in an absence of secretion of two potent inflammatory cytokines, IL-1β and IL-18 (C. M. Bosio and T. J. Bauler, unpublished observations). Similarly, early inflammatory responses that are detected following intranasal inoculation of mice with F. novicida are largely absent during infection with SchuS4 (7, 8). Further, when inflammation after infection with SchuS4 does appear, it is not effective at controlling bacterial replication and, in some instances, has been suggested to contribute to death of the host (9, 10).
In addition to evading stimulation of proinflammatory responses, we have shown that SchuS4 interferes with the ability of host cells to mount inflammatory responses to other, unrelated, microbial ligands in primary human cells, in mouse cells, and in the lung following intranasal infection (4, 5, 7, 11, 12). Thus, the ability of SchuS4 both to evade and to inhibit protective host inflammatory responses is considered an important feature of its virulence. However, the bacterial ligands, host cell receptors, and signaling pathways modulated by these ligands remain largely unknown.
In this report, we demonstrate that lipids isolated from F. tularensis subsp. tularensis strain SchuS4 but not attenuated F. novicida represent an important virulence factor harbored by this organism. We show that SchuS4 lipids readily suppress the ability of primary mouse and human cells to respond to various secondary stimuli. Suppression of host cell responses by SchuS4 lipids in vitro and in vivo was dependent on the host cell membrane receptor Toll-like receptor 2 (TLR2) and the nuclear receptor peroxisome proliferator-activated receptor α (PPARα). These observations reveal an unappreciated role for Francisella lipids in modulating host immune responses and a novel role for two host receptors in executing these responses.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free, 6- to 8-week-old C57BL/6J (wild type [WT] for TLR2−/− and PPARα−/−), B6.129S1-Tlr2tm1kir/J (TLR2−/−), B6.129S4-PPARatm1Gonz (PPARα−/−), C3HeB/FeJ (WT for C3H/HeJ), and C3H/HeJ (TLR4−/−) mice (n = 4 or 5/group) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in sterile microisolator cages in the biosafety level 2 facility at the Rocky Mountain Laboratories. All mice were provided sterile water and food ad libitum. All research involving animals was conducted in accordance with animal care and use guidelines, and animal protocols were approved by the animal care and use committee at Rocky Mountain Laboratories.
Bacteria.
Virulent Francisella tularensis subsp. tularensis strain SchuS4 was provided by Jeannine Peterson (Centers for Disease Control and Prevention, Fort Collins, CO). F. novicida strain U112 was provided by Fran Nano (University of Victoria, Victoria, BC, Canada). Bacteria were cultured overnight in modified Mueller-Hinton broth at 37°C, with constant shaking, aliquoted into 1-ml samples, frozen at −80°C, and thawed just prior to use, as described previously (10).
Isolation of bacterial lipids.
Lipids were isolated from SchuS4 and F. novicida using the standard modified Folch method for isolation of bacterial lipids (13–16). Briefly, bacteria were thawed and plated onto 150-mm petri dishes containing modified Mueller-Hinton agar. Bacteria were incubated for 48 h at 37°C in 7% CO2, scraped from the agar plates, and added to high performance liquid chromatography (HPLC)-grade chloroform/methanol (2:1) (both from Sigma). The resulting mixture was stirred vigorously for 15 min at room temperature. Then, 20 ml of endotoxin-free water was added and the mixture was stirred for an additional 10 min. The mixture was centrifuged at 4,000 × g for 10 min at room temperature, to separate the organic and aqueous phases. The organic phase was pipetted into a separate container and dried under nitrogen. Dried organic samples were reconstituted in absolute ethanol (EtOH) (Warner-Graham) to 20 mg/ml. The average yield of lipids from Francisella was 80 mg/4 g (wet weight) of bacteria, representing approximately 2% of wet weight. Thus, 10 μg/ml of lipid is 0.00025% (wet weight) of bacteria. Lipid preparations were stored at 4°C for up to 2 months. An absence of proteins and carbohydrates present in the organic phase was confirmed by analysis of preparations on silver-stained SDS-PAGE gels, protein quantification using the Bradford assay (Sigma) according to the manufacturer's instructions, periodate staining of SDS-PAGE gels, and Western blotting for Francisella O antigen and capsule. No evidence for proteins or carbohydrates was observed in organic-phase preparations.
Culture of bone marrow-derived and alveolar macrophages.
Alveolar macrophages were collected and immediately used in assays as described previously (7). Bone marrow-derived macrophages were generated as described previously (17), with the following modifications. Progenitor cells isolated from femurs from the indicated strains of mice were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 0.2 mM l-glutamine, 1 mM HEPES buffer, and 0.1 mM nonessential amino acids (all from Invitrogen, Carlsbad, CA) (complete DMEM [cDMEM]) plus 10 ng/ml macrophage colony-stimulating factor (M-CSF) (Peprotech), in 75-cm2 flasks. On day 2 of culture, nonadherent cells were collected and placed in fresh 75-cm2 flasks, and the cultures were replenished with fresh medium. Adherent cells were collected on day 5, resuspended at 2 × 105 cells/ml, and seeded in a 48-well tissue culture plate at 0.5 ml/well. All bone marrow-derived cells were used on day 6 of culture.
Generation of monocyte-derived human macrophages.
Human macrophages were differentiated from apheresis-prepared monocytes as described previously, with the following modifications (18). Human monocytes, enriched by apheresis, were obtained from peripheral blood provided by the Department for Transfusion Medicine and the NIH Clinical Center at the National Institutes of Health (Bethesda, MD), under a protocol approved by the NIH Clinical Center institutional review board. Signed informed consent was obtained from each donor, acknowledging that the donation would be used for research purposes by intramural investigators throughout the NIH. Monocytes were further enriched using Ficoll-Paque (GE Healthcare) and were differentiated into macrophages following culture in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 0.2 mM l-glutamine, 1 mM HEPES buffer, and 0.1 mM nonessential amino acids (all from Invitrogen) (complete RPMI 1640 medium [cRPMI]) plus either 100 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (for assessment of production of IL-12p40) or 100 ng/ml M-CSF (for assessment of production of IL-10) (both from Peprotech), in 75-cm2 tissue culture flasks. On day 3 of culture, one-half of the medium was replaced with cRPMI supplemented with 200 ng/ml of cytokine. The following day, medium was removed and adherent cells were released from the tissue culture flasks following incubation with 0.05% trypsin. Cells were washed, resuspended in reserved cRPMI, and placed in 48-well plates at 5 × 104 cells/well. The next day, one-half of the tissue culture medium was replaced with cRPMI supplemented with 200 ng/ml cytokine. All cells were used on day 6 of culture.
In vitro assessment of lipid activity.
Bacterial lipids were diluted in cDMEM, vortex-mixed, and added to macrophages at the indicated final concentrations. EtOH was diluted using the same scheme and was utilized as the vehicle control. In some experiments, macrophages were treated with the PPARγ antagonist GW9662 at 2.5 μM or the PPARα antagonist GW6471 at 2.0 μM (both from Tocris, Minneapolis, MN) 1 h prior to the addition of lipids. At the indicated time points after the addition of lipids, cells were stimulated with the following TLR agonists: 5 ng/ml ultrapure Escherichia coli K-12 lipopolysaccharide (LPS) (TLR4), 5 ng/ml Pam3Csk4 (TLR2), 0.25 μM CpG oligodinucleotide 2395 (TLR9), 2 ng/ml R848 (TLR8), and 100 μg/ml poly(I-C) (TLR3) (all from InvivoGen). Eighteen hours later, culture supernatants were collected and assessed for cytokines with enzyme-linked immunosorbent assays (ELISAs). The viability of macrophages following incubation with EtOH and lipids was assessed by staining cells with trypan blue (Invitrogen) and counting trypan blue-negative (viable) cells versus trypan blue-positive (dead/dying) cells. The percentage of viable cells was calculated using the following formula: number of trypan blue-negative cells/total cells counted × 100.
In vivo assessment of lipid activity.
Mice were anesthetized by intraperitoneal injection of 100 μl of 12.5 mg/ml ketamine plus 3.8 mg/ml xylazine. Mice were given 25 μg SchuS4 lipids in 25 μl phosphate-buffered saline (PBS) intranasally. Mice receiving EtOH alone served as negative controls for lipid-treated mice. Sixteen hours later, mice were anesthetized as described above and were given either ultrapure E. coli K-12 LPS (InvivoGen) or LPS derived from E. coli 0127:B8 (Sigma) (200 ng/25 μl). Similar results were observed regardless of which LPS type was administered to the animals. At the indicated time points, mice were euthanized. Tracheas were exposed and cannulated with disposable 18-gauge catheters. Airways were repeatedly flushed with 0.5 ml PBS. The first 0.5-ml wash from the bronchoalveolar lavage (BAL) was collected for assessment of cytokines, followed by 4 additional 0.5-ml washes to collect cells for analysis by flow cytometry.
Detection of secreted cytokines and chemokines.
TNF-α, IL-6, IL-12p40, IL-10, and transforming growth factor β (TGF-β) (all from BD Biosciences), prostaglandin E2 (PGE2) (Cayman Chemical), RANTES, and keratinocyte chemoattractant (KC) (R&D Systems) present in cell culture supernatants or bronchoalveolar lavage fluid were quantitated using commercially available ELISA kits, following the manufacturers' instructions.
Flow cytometry.
Populations of cells in bronchoalveolar lavage fluid were assessed by flow cytometry as described previously (10). Briefly, the following antibodies in various combinations were used for flow cytometric analysis: PerCp/Cy5.5-CD11c, PECy7-CD11b, phycoerythrin (PE)-Ly6C, fluorescein isothiocyanate (FITC)-Ly6G, and antigen-presenting cell major histocompatibility complex II (MHC-II) (all from eBioscience, San Diego, CA). Neutrophils were characterized as Ly6G+ MHC-II−. Alveolar macrophages were characterized as CD11c+ Ly6G− Ly6C− MHC-II+/−. Staining was performed in fluorescence-activated cell sorting (FACS) buffer at room temperature. Following staining, cells were washed and fixed for 30 min in 1% paraformaldehyde at 4°C. Cells were washed a final time, resuspended in FACS buffer, and stored at 4°C until analysis. Samples were collected using a LSRII flow cytometer (BD Biosciences). Analysis gates were set on viable unstained cells and were designed to include all viable cell populations. Approximately 10,000 gated events were analyzed for each sample. Isotype control antibodies were included when analyses were first being performed, to ensure the specificity of staining, but were not routinely included in each experiment. Data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistical analysis.
Statistical differences between two groups were determined using an unpaired t test, with significance set at P < 0.05. For comparisons of three or more groups, analysis was done by one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparisons test, with significance determined at P < 0.05.
RESULTS
Lipids from virulent F. tularensis SchuS4 inhibit inflammatory responses in vitro.
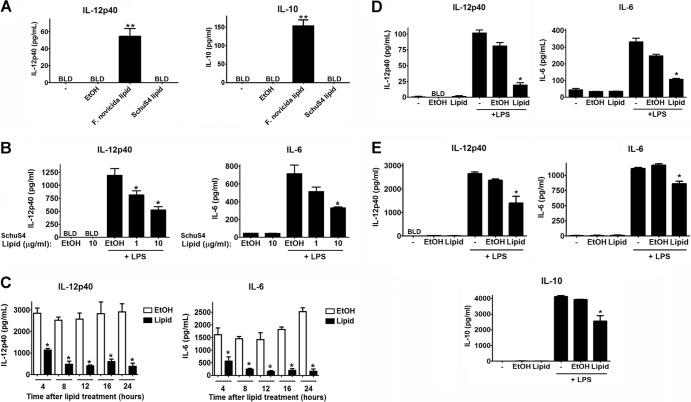
We established previously that, unlike more-attenuated subspecies, a primary mechanism of virulence embodied by virulent F. tularensis subsp. tularensis strain SchuS4 is its ability to evade and to suppress inflammatory responses (4, 5, 7, 11, 12). Our earlier work also suggested that a heat-stable component derived from SchuS4 was capable of inhibiting inflammation, similar to findings observed for cells infected with viable organisms (5, 12). Thus, we reasoned that lipids derived from SchuS4 may be acting as potent anti-inflammatory agonists. We first determined if lipids derived from virulent SchuS4 and attenuated F. novicida stimulated or inhibited inflammatory responses in primary macrophages. SchuS4-derived lipids did not induce secretion of detectable levels of IL-12p40, IL-6, TGF-β, IL-10, or PGE2 (Fig. 1A, B, D, and E and data not shown). In contrast, lipids isolated from F. novicida readily induced production of IL-12p40 and IL-10 (Fig. 1A). Stimulation of secretion of IL-12p40 and IL-10 from bone marrow-derived macrophages by lipids derived from F. novicida was similar to the production of these cytokines among cells infected with F. novicida (see Fig. S1 in the supplemental material). Thus, consistent with infection with viable organisms, lipids isolated from F. novicida induced an inflammatory response, whereas SchuS4 lipids did not.
Fig 1.
Lipids derived from virulent F. tularensis strain SchuS4 inhibit inflammatory responses in primary mouse and human macrophages. (A) SchuS4 lipids, F. novicida lipids, or EtOH (vehicle control) was added to bone marrow-derived macrophages at 10 μg/ml. Additional controls included untreated cells (−). Cells were incubated overnight. Culture supernatants were evaluated for IL-12p40 and IL-10. (B) SchuS4 lipids or EtOH (vehicle control) was added to primary bone marrow-derived macrophages at the indicated concentrations for 18 h, followed by stimulation of cells with E. coli LPS (5 ng/ml) or Pam3Csk4 (5 ng/ml) for an additional 18 h. Culture supernatants were evaluated for IL-12p40 and IL-6 by ELISAs. (C) Primary bone marrow macrophages were incubated with 10 μg/ml SchuS4 lipids for the indicated periods of time prior to the addition of E. coli LPS (5 ng/ml). Eighteen hours after the addition of LPS, supernatants were evaluated for IL-12p40 and IL-6. (D and E) Freshly isolated alveolar macrophages (D) and monocyte-derived human macrophages (E) were cultured with SchuS4 lipids (10 μg/ml) for 18 h, followed by stimulation of cells with E. coli LPS (10 ng/ml) for an additional 18 h. Culture supernatants were evaluated for IL-12p40, IL-6, and IL-10 by ELISAs. BLD, below the level of detection. Error bars, standard errors of the mean (SEMs). ∗, significantly less than the EtOH controls (P < 0.05); ∗∗, significantly greater than all other samples (P < 0.05). All conditions were assayed in triplicate. Data are representative of three experiments of similar design.
We then assessed the ability of SchuS4 lipids to inhibit inflammatory cytokines. SchuS4 lipids significantly inhibited the ability of bone marrow-derived macrophages to secrete IL-12p40 and IL-6 in response to E. coli LPS, Pam3Csk4, CpG oligonucleotides, or R848 (Fig. 1B; see also Fig. S2 in the supplemental material). SchuS4 lipid-mediated inhibition of secondary responses occurred rapidly after the addition of lipids to cells, with significant impairment of cytokine secretion being apparent within 4 h after the addition of SchuS4 lipids (Fig. 1C). In addition to macrophages derived in vitro, we determined the ability of SchuS4 lipids to inhibit the responsiveness of freshly isolated alveolar macrophages. Similar to our observations with bone marrow-derived macrophages, SchuS4 lipids did not induce secretion of proinflammatory cytokines and subsequently inhibited secretion of IL-12p40 and IL-6 by alveolar macrophages in response to LPS (Fig. 1D). Inhibition of cytokine production by lipids was not due to toxicity of the lipids for macrophages, as the viability was 96.7% for EtOH-treated cells and 96.8% for lipid-treated macrophages.
For a human pathogen, it was also critical to determine if SchuS4 lipids were effective at limiting inflammatory responses in primary human cells. Thus, we also assessed the activity of SchuS4 lipids in primary human macrophages. As in mouse cells, SchuS4 lipids failed to induce secretion of TNF-α, IL-6, IL-12p40, IL-10, TGF-β, or PGE2 from human macrophages (Fig. 1E and data not shown). Upon secondary stimulation with LPS, SchuS4 lipids significantly impaired secretion of IL-12p40, IL-10, and IL-6 by human macrophages. Importantly, these data also confirmed that the suppressive activity of SchuS4 lipids in macrophages was not due to induction of IL-10 production and, in fact, lipids suppressed production of IL-10 by macrophages. Thus, SchuS4 lipids represent important components of virulent F. tularensis that are capable of broadly inhibiting inflammatory responses in primary mouse and human cells.
SchuS4 lipids inhibit secretion of chemokines.
In addition to proinflammatory cytokines, induction of chemokines is essential for initiating and sustaining inflammation in the host. Thus, we extended our studies to determine if SchuS4 lipids also had negative effects on the production of chemokines associated with induction of inflammatory responses. SchuS4 lipids did not induce significant production of RANTES or KC by macrophages (Fig. 2). Furthermore, SchuS4 lipids also significantly reduced the secretion of RANTES and KC by macrophages stimulated with LPS (Fig. 2). Thus, SchuS4 lipids appear to broadly dampen induction of inflammatory responses in primary macrophages.
Fig 2.
SchuS4 lipids inhibit production of chemokines. SchuS4 lipids or EtOH (vehicle control) was added to primary bone marrow-derived macrophages (10 μg/ml) for 18 h, followed by stimulation of cells with Pam3Csk4 (5 ng/ml) for an additional 18 h. Culture supernatants were evaluated for KC and RANTES by ELISAs. ∗, significantly less than the EtOH-treated controls (P < 0.05). All conditions were assayed in triplicate. Data are representative of three experiments of similar design.
SchuS4 lipid modulation of inflammatory responses is dependent on TLR2 and MyD88.
The ability of SchuS4 lipids to rapidly inhibit production of inflammatory cytokines (Fig. 1C) initially suggested that lipids may engage receptors on the host cell surface. It has been suggested that Francisella subspecies possess both TLR2 and TLR4 ligands. However, studies have been largely restricted to attenuated subspecies and point toward a stimulatory, rather than inhibitory, function. Recent reports have pointed toward an alternative role for TLR2 in mediating anti-inflammatory responses following engagement of specific ligands (19). Thus, we tested whether suppression of cytokine production by SchuS4 lipids was dependent on TLR2 or TLR4. The absence of TLR4 on macrophages had no effect on the ability of SchuS4 lipids to inhibit secretion of IL-12p40 in response to Pam3Csk4 (Fig. 3B). In contrast, the absence of TLR2 reversed the inhibition of host cell responses by SchuS4 lipids (Fig. 3A). Both TLR2 and TLR4 require the adaptor MyD88 to signal responses in host cells. Thus, we next determined if SchuS4 lipid modulation of host cell responses was dependent on MyD88. Since cells lacking MyD88 do not respond to TLR2 or TLR4 agonists, we utilized poly(I-C) (TLR3 agonist) as the secondary stimulus following exposure of cells to SchuS4 lipids. Surprisingly, SchuS4 lipids enhanced macrophage responsiveness to poly(I-C) in wild-type cells (Fig. 3C). However, this enhancement was nullified in MyD88-deficient cells (Fig. 3C). Together, these data show that TLR2 and MyD88 are required for SchuS4 lipid modulation of host cell responses to microbial ligands.
Fig 3.

SchuS4 lipid-mediated modulation of inflammatory responses in macrophages is dependent on TLR2 and MyD88. Primary bone marrow-derived macrophages from wild-type (A to C), TLR2−/− (A), TLR4−/− (B), or MyD88−/− mice were treated with 10 μg/ml SchuS4 lipids for 18 h, followed by stimulation of cells with E. coli LPS (5 ng/ml) (A), Pam3Csk4 (5 ng/ml) (B), or poly(I-C) (100 μg/ml) (C) for an additional 18 h. Culture supernatants were evaluated for IL-12p40 by ELISA. BLD, below the level of detection; ns, not significantly different. Error bars, SEMs. ∗, P < 0.05, compared to EtOH control; **, significantly greater than untreated and EtOH-treated controls (P < 0.05). All conditions were assayed in triplicate. Data are representative of three experiments of similar design.
PPARα is required for SchuS4 lipid-mediated suppression of cytokine responses in vitro.
PPARs are important nuclear immunoregulatory receptors that can specifically modulate inflammatory responses emanating from TLRs (20). Further, PPARs are activated following ligation of lipids (21). Recent reports have shown that the TLR2 ligand lipoteichoic acid (LTA) inhibits neutrophil recruitment in vivo and this inhibition is dependent on TLR2 and the nuclear receptor PPARγ (19). Additionally, others have shown that activation of PPARγ by other microbial ligands induces suppression of host inflammatory responses in vitro and in vivo (22). The presence of PPARα is required for modulation of inflammation following exposure to LPS or sepsis in vivo (23–26). Therefore, we hypothesized that suppression of inflammatory responses mediated by SchuS4 lipids in macrophages may require either PPARγ or PPARα. Since deletion of PPARγ is lethal in mice, we utilized PPAR-specific antagonists to block signaling through these receptors. GW9662 specifically antagonizes PPARγ, while GW6471 antagonizes PPARα. Addition of GW9662 to macrophages had no effect on lipid-mediated inhibition of IL-12p40 or IL-6 secretion in response to LPS (Fig. 4). In contrast, inhibition of LPS-driven secretion of IL-12p40 and IL-6 cells by SchuS4 lipids was reversed in cells incubated with GW6471 (Fig. 4). Thus, SchuS4 lipids also require PPARα, but not PPARγ, to mediate inhibitory responses in vitro.
Fig 4.
SchuS4 lipid-mediated suppression of inflammatory responses in macrophages is dependent on PPARα. Primary bone marrow-derived macrophages were pretreated with 2.5 μM GW9962 (PPARγ antagonist) or 2.0 μM GW6471 (PPARα antagonist) for 2 h prior to the addition of 10 μg/ml SchuS4 lipids or EtOH (vehicle control). Cells were incubated for 18 h, followed by stimulation of cells with E. coli LPS (5 ng/ml) for an additional 18 h. Culture supernatants were evaluated for IL-12p40 and IL-6 by ELISAs. BLD, below the level of detection; ns, not significantly different. Error bars, SEMs. ∗, P < 0.05, compared to controls treated with EtOH or the PPAR antagonist alone. All conditions were assayed in triplicate. Data are representative of three experiments of similar design.
SchuS4 lipids inhibit pulmonary inflammation.
Given the potent anti-inflammatory effects of SchuS4 lipids on macrophages in vitro, we next determined if SchuS4 lipids could provoke or inhibit inflammatory responses in vivo. Mice were given either SchuS4 lipids, EtOH (vehicle control), or E. coli LPS intranasally, and cellular infiltration and cytokine and chemokine production in the airways were monitored over time. As shown previously (27), LPS induced infiltration of statistically significant numbers of neutrophils and production of TNF-α and IL-6 as early as 4 h after inoculation (Fig. 5B and C). Neither SchuS4 lipids nor EtOH induced significant changes in neutrophil or macrophage populations, compared to untreated controls, throughout the time course of the experiment (Fig. 5B). Furthermore, neither EtOH nor SchuS4 lipids elicited detectable concentrations of TNF-α or IL-6 in the BAL fluid (Fig. 5C). Interestingly, neither LPS, EtOH, nor SchuS4 lipids reproducibly provoked statistically significant increases in IL-12p40 levels in the airways (data not shown). Finally, lipid treatment did not alter the expression of MHC-II on airway cells (data not shown). We next assessed the ability of SchuS4 lipids to inhibit pulmonary responsiveness to LPS. Mice exposed to SchuS4 lipids had significantly fewer neutrophils in their airways in response to LPS than did EtOH-treated controls (Fig. 6A). However, neutrophils that were recruited to the airways of SchuS4 lipid-treated mice expressed levels of CD11b similar to those observed in EtOH-treated controls. We did not observe changes in MHC-II expression on macrophages from any mice treated with LPS at the time point assessed. Exposure to SchuS4 lipids also significantly reduced the amounts of TNF-α, IL-6, and KC detected in the airways of mice following exposure to LPS, compared to EtOH-treated controls (Fig. 6A and B). RANTES levels were not significantly increased above levels in untreated controls for any group (data not shown). Together, these data suggest that, while SchuS4 lipids fail to induce inflammatory responses in the lung, these bacterial components significantly attenuate secondary pulmonary inflammation.
Fig 5.
SchuS4 lipids do not provoke pulmonary inflammation. Mice (n = 4/group for each time point) were intranasally inoculated with 25 μg/25 μl SchuS4 lipids, 25 μl diluted EtOH, or 200 ng/25 μl E. coli LPS. At the indicated time points, mice were euthanized and fluid and cells from the airways were collected by bronchoalveolar lavage. (A) Representative dot plots and the gating strategy used to identify alveolar macrophages and infiltrating neutrophils are shown. (B) Infiltration of neutrophils and emigration of macrophages into the airways were evaluated by flow cytometry. (C) BAL fluid was assessed for TNF-α and IL-6 by ELISAs. Error bars represent SEMs. ∗, P < 0.05, compared to lipid- and EtOH-treated controls. FSC, forward scatter; SSC, side scatter; Un, unmanipulated. Data are representative of two experiments of similar design.
Fig 6.
SchuS4 lipids inhibit pulmonary inflammation. Mice (n = 5/group) were intranasally inoculated with 25 μg/25 μl SchuS4 lipids or 25 μl diluted EtOH. Eighteen hours later, mice were treated with 200 ng/25 μl E. coli LPS. Five hours after administration of LPS, mice were euthanized and fluid and cells from the airways were collected by bronchoalveolar lavage. Completely unmanipulated (−) mice served as negative controls. (A) Infiltration of neutrophils was evaluated by flow cytometry. (B) BAL fluid was assessed for TNF-α, IL-6, and KC by ELISAs. Error bars, SEMs. ∗, P < 0.05, compared to EtOH-treated controls. Data are representative of two experiments of similar design.
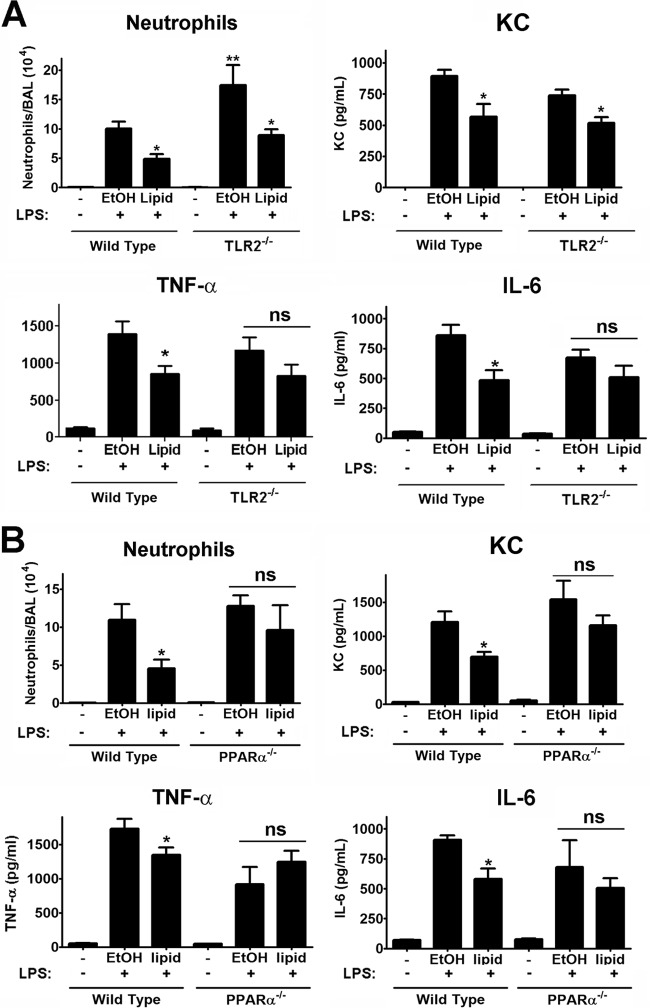
SchuS4 lipid-mediated suppression of pulmonary inflammation is dependent on TLR2 and PPARα.
We established that suppression of inflammatory responses mediated by SchuS4 lipids among macrophages in vitro required both TLR2 and PPARα (Fig. 3 and 4). Thus, we next determined if inhibition of inflammation required these two receptors in vivo. SchuS4 lipids mediated significant inhibition of neutrophil recruitment into the airways of wild-type and TLR2−/− mice in response to LPS (Fig. 7A). Consistent with inhibition of neutrophil recruitment, lipid treatment also suppressed production of the neutrophil chemoattractant KC in WT and TLR2−/− animals (Fig. 7A). However, in contrast to wild-type mice, there was no significant difference in TNF-α and IL-6 levels detected in the airways of TLR2−/− mice following exposure to LPS (Fig. 7A). This suggested that, while SchuS4 lipid-mediated inhibition of neutrophil recruitment was not dependent on TLR2, this host receptor was critical for lipids to limit production of proinflammatory cytokines in vivo. We also examined the requirement for the host nuclear receptor PPARα in vivo. Unlike in TLR2−/− mice, PPARα was required for lipid-mediated suppression of neutrophil recruitment and production of TNF-α, IL-6, and KC in the airways of mice in response to LPS (Fig. 7B). Therefore, TLR2 was required for selective inhibition of cytokine responses, while PPARα was required for broad suppression of cytokines, chemokines, and neutrophil recruitment in the lung.
Fig 7.
SchuS4 lipid-mediated suppression of pulmonary inflammation is dependent on TLR2 and PPARα in vivo. Wild-type (A and B), TLR2−/− (A), or PPARα−/− (B) mice (n = 5/group) were intranasally inoculated with 25 μg/25 μl SchuS4 lipids or 25 μl similarly diluted EtOH. Eighteen hours later, mice were treated with 200 ng/25 μl E. coli LPS. Five hours after the administration of LPS, mice were euthanized and fluid and cells from the airways were collected by bronchoalveolar lavage. Completely unmanipulated (−) mice served as negative controls. Infiltration of neutrophils was evaluated by flow cytometry. BAL fluid was assessed for TNF-α, IL-6, and KC by ELISAs. ns, not significantly different. Error bars, SEMs. ∗, P < 0.05, compared to EtOH-treated controls. Data are representative of two experiments of similar design.
DISCUSSION
The data presented here established that lipids isolated from virulent but not attenuated F. tularensis strain SchuS4 are potent anti-inflammatory agonists that suppress antimicrobial responses in vitro and in vivo. Remarkably, and in contrast to other bacterium-associated antagonists, SchuS4 lipids potently suppressed inflammation in the absence of initiation of any detectable inflammatory response. Therefore, our data identified SchuS4 lipids as important virulence factors embodied by F. tularensis, and they provided evidence for a completely novel bacterial component capable of profoundly disabling host immune responses. Although the specific nature of the SchuS4 lipids responsible for inhibiting host inflammatory responses was not fully defined, our data suggest that interaction of SchuS4 lipids with a host cell surface receptor typically required for defense against invading pathogens, i.e., TLR2, is an important step in pathogenesis mediated by these microbial ligands. In addition to engagement with TLR2, SchuS4 lipids mediated their suppressive activity via the nuclear receptor PPARα following either direct interaction or induction of endogenous PPARα ligands.
The immunomodulatory nature of SchuS4 lipids is unique in that these lipids are TLR2 agonists that fail to first elicit the requisite inflammatory response common to all other naturally occurring TLR2 agonists associated with pathogens. Although previous reports have suggested that other bacterial lipoglycans and lipopeptides that interact with TLR2 may suppress inflammatory responses, there are several important features of these bacterial components that distinguish them from SchuS4 lipids. First, modifications of many of these TLR2 agonists that allow the provocation of anti-inflammatory responses result in a mechanism that is no longer dependent on TLR2. For example, lipomannans (LMs) isolated from mycobacteria are well known TLR2 agonists capable of provoking strong inflammatory responses (28). When LMs are altered from tetra- or triacylated forms to di- or monoacylated species, they both fail to provoke an inflammatory response and inhibit the ability of cells to respond to secondary stimuli (28–30). However, the inhibition of inflammation mediated by diacylated LM is independent of TLR2, suggesting that this molecule is no longer a TLR2 agonist (29). This feature effectively dissociates the suppressive activity of modified LM from the TLR2 receptor. Second, inhibition of inflammation mediated by modified LM has routinely been shown to be associated with IL-10 production (31). This is in contrast to our findings that SchuS4 lipids both failed to induce IL-10 production and inhibited the secretion of this cytokine (Fig. 1A and E).
In addition to LMs, there are other examples of TLR2 agonists that are capable of interfering with inflammatory responses. However, the functions of these agonists diverge from those of SchuS4 lipids in that they act directly on neutrophils, do not affect the production of cytokines, and function only in vivo. One such bacterial lipid mediating these effects is lipoteichoic acid (LTA). LTA is a surface component present in Gram-positive bacteria and a well-known TLR2 agonist with the ability to induce proinflammatory responses that is considered an important virulence factor (32). However, when purified and delivered in vivo, LTA inhibited neutrophil recruitment independent of its ability to induce proinflammatory cytokine production (19, 33). LTA-mediated interference with neutrophil recruitment was tissue dependent, in that it did not inhibit neutrophil recruitment when delivered to the lung (34). Thus, SchuS4 lipids are unique, compared to previously described bacterial components capable of modulating inflammation, in that they broadly interfered with induction of inflammatory responses via inhibition of proinflammatory cytokines and chemokines and suppressed neutrophil recruitment in vitro and in vivo.
There are several possibilities that could explain how SchuS4 lipids are able to mediate an anti-inflammatory signal through TLR2. A simple explanation would be that SchuS4 lipids alter the expression of TLRs within cells. However, we have not observed differences in TLR expression among lipid-treated mouse macrophages (C. M. Bosio, unpublished observation). Provocation of a strong inflammatory signal through TLRs is dependent on the ability of ligands to interact with specific sites in the receptors. For example, signaling through TLR4 is dependent on the ability of the TLR4 ligand LPS to interact with specific sites on the TLR and with the coreceptor MD-2 (35). Modifications of LPS that alter these interactions result in dampened or absent proinflammatory responses and/or inhibition of the ability of nonmodified LPS to stimulate responses (36). It is possible that the structure of SchuS4 lipids prevents the full interactions with TLR2 that are required for elicitation of proinflammatory responses. Alternatively, SchuS4 lipids may interact with different binding sites within TLR2 that initiate anti-inflammatory responses in host cells. Another possibility is that TLR2 acts as a “carrier” receptor. It has been proposed that the requirement for TLR2 in LTA-mediated anti-inflammatory responses in vivo involves TLR2 acting to transport LTA into and through the intracellular environment, delivering it to PPARγ. Thus, the anti-inflammatory signal is dependent on TLR2 but does not emanate from this receptor. Rather, TLR2 delivers LTA to the receptor (PPARγ), which initiates the anti-inflammatory response (19). SchuS4 lipid-mediated suppression of inflammatory responses was dependent on both TLR2 and PPARα. Thus, TLR2 may be acting to deliver SchuS4 lipids to PPARα in a manner similar to that observed for LTA and PPARγ.
PPARs are nuclear receptors that sense and bind both endogenous and exogenous fatty acids. The critical role PPARs play in regular maintenance of host glucose, cholesterol, and lipid metabolism is well established (37). However, over the past 15 years there has been a growing understanding of how these receptors function to suppress and to downmodulate inflammation. Among the PPARs, PPARγ has been routinely implicated in bacterium-mediated suppression of inflammation (38). This suppression can occur via direct interactions with PPARγ or following induction of host ligands that bind to this receptor (38). PPARγ mediates its inhibitory activity via trans repression of NF-κB or the N-CoR transcription complex and has been noted to inhibit cytokine production by inhibiting the binding of interferon regulatory factor 3 (IRF3) to interferon signaling response elements (ISREs) in specific genes (39). PPARγ is also closely associated with induction of alternative activation of macrophages, a state of macrophage activation associated with downmodulation of Th1-type inflammatory responses (40). We did not observe an overt role for PPARγ in SchuS4 lipid-mediated inhibition of inflammation (Fig. 4). Moreover, SchuS4 lipids had no effect on the activation and translocation of IRF3 following exposure of cells to LPS (41). Finally, neither SchuS4 lipids nor intact viable SchuS4 induced alternative activation of macrophages in vitro or in vivo (C. M. Bosio and A. J. Griffin, unpublished observations). Thus, with our data demonstrating that addition of a PPARγ antagonist had no effect on SchuS4 lipid-mediated suppression (Fig. 4), it is unlikely that PPARγ plays a role in the interference with inflammatory responses we observed with SchuS4 lipids.
In contrast, we found that PPARα was required for the interference with inflammatory responses mediated by SchuS4 lipids in vitro and in vivo (Fig. 4 and 7). Ligands for PPARα include endogenously produced leukotriene B4 (LTB4) and, like other PPARs, saturated and unsaturated fatty acids. Several synthetic ligands have also been generated, including fibrates, phthalates, and derivatives of trichloroethylene (38, 42). It is unlikely that the endogenous ligand leukotriene B4 is the active PPARα agonist at work in our study, since the first function of LTB4 is to promote inflammation. Induction of inflammatory responses was not noted in cells treated with SchuS4 lipids, arguing against LTB4 as the PPARα ligand. Preliminary data suggest that the “active” lipid species present in our preparations of SchuS4 lipids are fatty acids (G. Nardone, R. Ireland, and C. M. Bosio, unpublished observations). As described above, fatty acids represent important ligands for all PPARs (38). Fatty acids have been implicated as TLR agonists capable of both positive and negative induction of inflammatory responses (43, 44). Thus, it is possible that one or more of the fatty acids enriched in SchuS4 lipid preparations interact directly with TLR2 followed by PPARα to provoke anti-inflammatory responses in host cells. Alternatively, SchuS4 lipids may induce increased production of host fatty acids (e.g., via oxidation of arachidonic acid) that act as efficient agonists for PPARα.
There is a growing body of literature detailing the importance of lipids and lipid metabolism in the function of the immune system. For example, synthetic PPAR agonists have been, and continue to be, developed to stem the destructive inflammation associated with diabetes and other metabolic disorders (45). It is also appreciated that bacterial lipids play an integral role in the pathogenesis of infections. However, this role is typically one in which excessive inflammation caused by the lipids promotes pathogenesis and death (46). We provide evidence herein that virulent F. tularensis SchuS4 possesses a naturally occurring, purely anti-inflammatory lipid. We also demonstrate that, unlike lipids derived from other bacteria, SchuS4 lipids broadly inhibit multiple facets of inflammation, similar to findings observed following infection with intact organisms (7). Thus, we have identified a critical bacterial component and host cell receptors utilized by this component that contribute to the unique virulence of F. tularensis. Finally, given the broad anti-inflammatory activity of these lipids, they may represent novel therapeutic agents for use against a variety of inflammation-driven diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Harlan Caldwell and Alan Sher for helpful discussions and critiques of the work presented herein. We also thank Tom Wynn and Brian Kelsall for their thoughtful insights with regard to our studies.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print 7 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00319-13.
REFERENCES
- 1.McCrumb FJ, Snyder MJ, Woodward TE. 1957. Studies on human infection with Pasteurella tularensis: comparison of streptomycin and chloramphenicol in the prophylaxis of clinical disease. Trans. Assoc. Am. Physicians 70:74–79 [PubMed] [Google Scholar]
- 2.Sjostedt A. 2005. Francisella, p 200–210 In Garrity G, Brenner DJ, Krieg NR, Staley JR. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 The proteobacteria, part B: the gammaproteobacteria. Springer, New York, NY [Google Scholar]
- 3.Harris S. 1992. Japanese biological warfare research on humans: a case study of microbiology and ethics. Ann. N. Y. Acad. Sci. 666:21–52 [DOI] [PubMed] [Google Scholar]
- 4.Chase JC, Bosio CM. 2010. The presence of CD14 overcomes evasion of innate immune responses by virulent Francisella tularensis in human dendritic cells in vitro and pulmonary cells in vivo. Infect. Immun. 78:154–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase JC, Celli J, Bosio CM. 2009. Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4. Infect. Immun. 77:180–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsa KV, Ganesan LP, Rajaram MV, Gavrilin MA, Balagopal A, Mohapatra NP, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S. 2006. Macrophage pro-inflammatory response to Francisella novicida infection is regulated by SHIP. PLoS Pathog. 2:e71. 10.1371/journal.ppat.0020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 178:4538–4547 [DOI] [PubMed] [Google Scholar]
- 8.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 76:5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlan JW, Zhao X, Harris G, Shen H, Bolanowski M, Rietz C, Sjostedt A, Chen W. 2008. Molecular immunology of experimental primary tularemia in mice infected by respiratory or intradermal routes with type A Francisella tularensis. Mol. Immunol. 45:2962–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crane DD, Scott DP, Bosio CM. 2012. Generation of a convalescent model of virulent Francisella tularensis infection for assessment of host requirements for survival of tularemia. PLoS One 7:e33349. 10.1371/journal.pone.0033349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauler TJ, Chase JC, Bosio CM. 2011. IFN-β mediates suppression of IL-12p40 in human dendritic cells following infection with virulent Francisella tularensis. J. Immunol. 187:1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crane DD, Warner SL, Bosio CM. 2009. A novel role for plasmin-mediated degradation of opsonizing antibody in the evasion of host immunity by virulent, but not attenuated, Francisella tularensis. J. Immunol. 183:4593–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497–509 [PubMed] [Google Scholar]
- 14.Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1:235–247 [DOI] [PubMed] [Google Scholar]
- 15.Dunnick JK, O'Leary WM. 1970. Correlation of bacteria lipid composition with antibiotic resistance. J. Bacteriol. 101:892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Curtiss R., III 2012. Thermorecovery of cyanobacterial fatty acids at elevated temperatures. J. Biotechnol. 161:445–449 [DOI] [PubMed] [Google Scholar]
- 17.Bosio CM, Elkins KL. 2001. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect. Immun. 69:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ireland R, Olivares-Zavaleta N, Warawa JM, Gherardini FC, Jarrett C, Hinnebusch BJ, Belisle JT, Fairman J, Bosio CM. 2010. Effective, broad spectrum control of virulent bacterial infections using cationic DNA liposome complexes combined with bacterial antigens. PLoS Pathog. 6:e1000921. 10.1371/journal.ppat.1000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long EM, Klimowicz AC, Paula-Neto HA, Millen B, McCafferty DM, Kubes P, Robbins SM. 2011. A subclass of acylated anti-inflammatory mediators usurp Toll-like receptor 2 to inhibit neutrophil recruitment through peroxisome proliferator-activated receptor gamma. Proc. Natl. Acad. Sci. U. S. A. 108:16357–16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. 2005. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moraes LA, Piqueras L, Bishop-Bailey D. 2006. Peroxisome proliferator-activated receptors and inflammation. Pharmacol. Ther. 110:371–385 [DOI] [PubMed] [Google Scholar]
- 22.Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. 2010. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J. Immunol. 185:929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzzocrea S, Bruscoli S, Mazzon E, Crisafulli C, Donato V, Di Paola R, Velardi E, Esposito E, Nocentini G, Riccardi C. 2008. Peroxisome proliferator-activated receptor-α contributes to the anti-inflammatory activity of glucocorticoids. Mol. Pharmacol. 73:323–337 [DOI] [PubMed] [Google Scholar]
- 24.Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N, Pons F. 2005. PPARα downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir. Res. 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. 1999. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 274:32048–32054 [DOI] [PubMed] [Google Scholar]
- 26.Standage SW, Caldwell CC, Zingarelli B, Wong HR. 2012. Reduced peroxisome proliferator-activated receptor alpha expression is associated with decreased survival and increased tissue bacterial load in sepsis. Shock 37:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reutershan J, Basit A, Galkina EV, Ley K. 2005. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L807–L815 [DOI] [PubMed] [Google Scholar]
- 28.Gilleron M, Nigou J, Nicolle D, Quesniaux V, Puzo G. 2006. The acylation state of mycobacterial lipomannans modulates innate immunity response through Toll-like receptor 2. Chem. Biol. 13:39–47 [DOI] [PubMed] [Google Scholar]
- 29.Doz E, Rose S, Nigou J, Gilleron M, Puzo G, Erard F, Ryffel B, Quesniaux VF. 2007. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J. Biol. Chem. 282:26014–26025 [DOI] [PubMed] [Google Scholar]
- 30.Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. 2004. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172:4425–4434 [DOI] [PubMed] [Google Scholar]
- 31.Barnes PF, Chatterjee D, Abrams JS, Lu S, Wang E, Yamamura M, Brennan PJ, Modlin RL. 1992. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan: relationship to chemical structure. J. Immunol. 149:541–547 [PubMed] [Google Scholar]
- 32.Ginsburg I. 2002. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2:171–179 [DOI] [PubMed] [Google Scholar]
- 33.Alves-Filho JC, Freitas A, Souto FO, Spiller F, Paula-Neto H, Silva JS, Gazzinelli RT, Teixeira MM, Ferreira SH, Cunha FQ. 2009. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. U. S. A. 106:4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knapp S, von Aulock S, Leendertse M, Haslinger I, Draing C, Golenbock DT, van der Poll T. 2008. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J. Immunol. 180:3478–3484 [DOI] [PubMed] [Google Scholar]
- 35.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195 [DOI] [PubMed] [Google Scholar]
- 36.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. 2007. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130:906–917 [DOI] [PubMed] [Google Scholar]
- 37.Wang YX. 2010. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 20:124–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straus DS, Glass CK. 2007. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28:551–558 [DOI] [PubMed] [Google Scholar]
- 39.Zhao W, Wang L, Zhang M, Wang P, Zhang L, Yuan C, Qi J, Qiao Y, Kuo PC, Gao C. 2011. Peroxisome proliferator-activated receptor γ negatively regulates IFN-β production in Toll-like receptor (TLR) 3- and TLR4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-β promoter. J. Biol. Chem. 286:5519–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. 2007. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ireland R, Wang R, Alinger JB, Small P, Bosio CM. 2013. Francisella tularensis SchuS4 and SchuS4 lipids inhibit IL-12p40 in primary human dendritic cells by inhibition of IRF1 and IRF8. J. Immunol. 191:1276–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. 2001. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat. Med. 7:48–52 [DOI] [PubMed] [Google Scholar]
- 43.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. 2012. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 53:2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. 2003. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 44:479–486 [DOI] [PubMed] [Google Scholar]
- 45.Bensinger SJ, Tontonoz P. 2008. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454:470–477 [DOI] [PubMed] [Google Scholar]
- 46.Morrison DC, Ryan JL. 1987. Endotoxins and disease mechanisms. Annu. Rev. Med. 38:417–432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.