Abstract
This study compared the levels of immunogenicity and safety of diphtheria–tetanus toxoid–five-component acellular pertussis (DTaP5), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib) (DTaP5-IPV-Hib) and DTaP3-IPV/Hib vaccines for study participants 3, 5, and 12 months of age. Post-dose 3 noninferiority criteria comparing DTaP5-IPV-Hib to DTaP3-IPV/Hib using rates of seroprotection were demonstrated against diphtheria, tetanus, and polio types 1 to 3, but not for polyribosylribitol phosphate (PRP). While PRP did not meet noninferiority criteria, the seroprotection rate and geometric mean concentration (GMC) were high, indicating a clinically robust immune response. GMCs or titers for other antigens (including pertussis) and the safety profiles were generally similar between groups. Fully liquid DTaP5-IPV-Hib can be administered using the 3-, 5-, and 12-month vaccination schedule. (This study has been registered at ClinicalTrials.gov under registration no. NCT00287092.)
INTRODUCTION
Diphtheria–tetanus toxoid–five-component acellular pertussis (DTaP5), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib) (DTaP5-IPV-Hib) vaccine (Pediacel; Sanofi Pasteur Limited, Toronto, Ontario, Canada) is a fully liquid combination vaccine for primary and booster vaccination of infants and toddlers against infectious diseases caused by Clostridium tetani, Corynebacterium diphtheriae, Bordetella pertussis, Hib, and poliovirus types 1, 2, and 3. This licensed pentavalent vaccine comprises a 5-component acellular pertussis vaccine, adsorbed diphtheria and tetanus toxoids, inactivated poliomyelitis vaccine (IPV) grown in Vero cells, and a purified polyribosylribitol phosphate (PRP) capsular polysaccharide of Hib conjugated to tetanus toxoid. As a fully liquid formulation, DTaP5-IPV-Hib prevents dosing errors that can occur during reconstitution of vaccine components and may be more convenient for health care providers.
The safety and immunogenicity of DTaP5-IPV-Hib using 3-dose primary vaccination schedules have previously been demonstrated in clinical studies in infants (1–8) and toddlers (4, 9–11). This is the first study to have evaluated the 3-, 5-, and 12-month schedule employed in some countries, particularly in Europe, and to have compared the safety and immunogenicity of DTaP5-IPV-Hib vaccine with those of a licensed pentavalent vaccine (DTaP3-IPV/Hib) containing 3-component acellular pertussis antigens.
(These data were presented in part at the 28th Annual Meeting of the European Society for Paediatric Infectious Diseases [ESPID], Nice, France, 4 to 8 May 2010.)
MATERIALS AND METHODS
This phase III, randomized, controlled, modified double-blind, multicenter study was conducted at 12 Finnish sites and 1 Swedish site (NCT ID:NCT00287092; EudraCT ID:2005-004133-17) from February 2006 to May 2007 (12). A modified double-blind design was utilized since the comparator vaccine (DTaP3-IPV/Hib) required reconstitution; an unblinded study team member prepared and administered study vaccines but was not involved in data collection. Parents/guardians were kept blinded to the identity of the study vaccine administered. The study complied with the Declaration of Helsinki. The study protocol and informed consent forms were approved by study site ethics committees. The participant parent(s) or legal guardian(s) provided written consent prior to study-specific procedures. The manuscript was prepared according to the Uniform Requirements for Manuscripts Submitted to Biomedical Journals guidelines.
Participants.
Participants were eligible for the study if they were 80 to 120 days old and born after a full-term pregnancy (>37 weeks). Other eligibility requirements can be found at ClinicalTrials.gov (12).
Vaccines.
The administered vaccines were DTaP5-IPV-Hib (lot C2314AA) and DTaP3-IPV/Hib (lot A20CA124A) (Infanrix-IPV+Hib; GlaxoSmithKline Biologicals, Rixensart, Belgium). DTaP5-IPV-Hib contains diphtheria (15 limits of flocculation [Lf]; ≥30 IU) and tetanus (5 Lf; ≥40 IU) toxoids and 5 pertussis antigens (20 μg pertussis toxoid [PT], 20 μg filamentous hemagglutinin [FHA], 3 μg pertactin [PRN], and 5 μg fimbria types 2 and 3 [FIM]) adsorbed to aluminum phosphate (1.5 mg; 0.33 mg Al), IPV (40 D antigen units poliovirus type 1 Mahoney, 8 D antigen units poliovirus type 2 mouse embryonic fibroblast 1 [MEF-1], and 32 D antigen units poliovirus type 3 Saukett), and 10 μg of H. influenzae type b capsular PRP conjugated to 20 μg of tetanus toxoid protein carrier/0.5-ml dose. DTaP3-IPV/Hib contains diphtheria toxoid (≥25 Lf; ≥30 IU), tetanus toxoid (≥10 Lf; ≥40 IU), and 3 pertussis antigens (25 μg each of PT and FHA and 8 μg PRN) adsorbed to aluminum hydroxide (0.95 mg; 0.5 mg Al) and IPV and PRP in amounts equivalent to those used for DTaP5-IPV-Hib in each 0.5-ml dose. Vaccines were stored in a temperature-monitored refrigerator at 2°C to 8°C. DTaP3-IPV/Hib was reconstituted immediately before administration. No other vaccines were to be coadministered during the study, consistent with country-specific vaccination schedules at the time.
Study design.
At study entry, participants were randomly assigned 1:1 to receive DTaP5-IPV-Hib (group A) or the control vaccine, DTaP3-IPV/Hib (group B). A single 0.5-ml dose of DTaP5-IPV-Hib or DTaP3-IPV/Hib was administered intramuscularly (i.m.) into the anterolateral thigh at 3 months of age (up to 28 days older [+28 days]) and 5 months of age (+28 days) and into the deltoid muscle at 12 months of age (+28 days).
Sera were obtained immediately prior to the first dose of vaccine (dose 1) to evaluate the pertussis antigen seroresponse to PT, FHA, PRN, and FIM; sera were also obtained 28 to 42 days after dose 2 and dose 3 to assess antibody responses to all vaccine antigens.
Endpoints.
The primary immunogenicity endpoints were the proportions of participants achieving established long-term seroprotective thresholds with respect to PRP (≥1.0 μg/ml), diphtheria toxoid (≥0.1 IU/ml), tetanus toxoid (≥0.1 IU/ml), and poliovirus types 1, 2, and 3 (≥1:8 dilution). In addition, the proportion of participants achieving seroresponse to pertussis antigens 1 month post-dose 3 was evaluated. Seroresponse was defined as the proportion of participants achieving antibody concentrations ≥ the assay lower limit of quantitation (LLOQ) (PT, PRN, and FIM ≥ 4 enzyme-linked immunosorbent assay [ELISA] units [EU]/ml; FHA ≥ 3 EU/ml) when baseline concentrations were less than the LLOQ or maintenance of baseline antibody concentrations in participants whose values were initially equal to or greater than the LLOQ. Given the lack of an established correlate of protection for pertussis, seroresponse was used to measure vaccine response as levels of maternal antibodies waned.
Secondary immunogenicity endpoints included the proportions of participants achieving established short-term seroprotection thresholds for PRP titers (≥0.15 μg/ml) and diphtheria and tetanus toxoids (≥0.01 IU/ml) 1 month post-dose 2 (seroprotection rates for poliovirus types 1, 2, and 3 [≥1:8 dilution] are also presented). For pertussis, the proportion of participants achieving ≥2-fold and ≥4-fold increases in dose 2 and dose 3 pertussis antigen antibody responses from prevaccination levels and dose 2 seroresponse rates were evaluated. Antibody geometric mean concentrations (GMCs) and geometric mean titers (GMTs) against all vaccine antigens after dose 2 and dose 3 were assessed.
Safety endpoints included frequency of solicited injection site reactions (tenderness, erythema, swelling) and solicited systemic reactions (fever, vomiting, abnormal crying, appetite lost, irritability) within 7 days after each vaccination. Solicited reaction intensity criteria are described elsewhere (4). Additional safety endpoints included frequency of unsolicited adverse events (AEs; reported within 28 days after each injection) and serious AEs (SAEs; reported during the study). Medical Dictionary for Regulatory Activities (MedDRA) version 9.0 terminology was applied to classify AEs. Data quantifying the use of antipyretics or analgesics on days 0 to 7 after vaccination were gathered.
Serologic evaluations.
Antibodies to PRP were assessed using a Farr-type radioimmunoassay. Levels of antibodies to diphtheria toxin and poliovirus antigens were measured by seroneutralization assays. Levels of antibodies to tetanus toxoid and pertussis antigens (PT, FHA, PRN, FIM) were assessed by ELISA. These validated assays were performed at Sanofi Pasteur Inc., Swiftwater, PA.
Statistical analysis.
The study was powered to test the primary hypotheses in a per-protocol analysis set using the primary endpoints and a prespecified noninferiority margin. The expected seroprotection rates for diphtheria, tetanus, polio and PRP were ≥95%; therefore, a noninferiority margin of 5% was selected. No primary hypothesis was tested for pertussis given the lack of an established correlate of protection and the differing antigen formulations in the study vaccines. With 334 evaluable participants per group, the overall power was approximately 80%. Assuming an attrition rate of 16%, 400 participants per group were to be enrolled.
The primary hypotheses tested whether the proportion of participants achieving post-dose 3 seroprotection levels after DTaP5-IPV-Hib vaccination was noninferior to that seen after DTaP3-IPV/Hib vaccination. The difference in proportions between group A and group B and the 2-sided 95% confidence interval (CI) based on the Wilson Score method without continuity correction were computed (13). When the lower limit of the CI was greater than −0.05 for PRP, diphtheria, tetanus, and all poliovirus antigens, DTaP5-IPV-Hib was considered noninferior to DTaP3-IPV/Hib. The proportions of participants achieving a pertussis seroresponse post-dose 3 were provided for each group, with 2-sided 95% CIs calculated by the exact method using the F-distribution of Collett (14).
A secondary hypothesis was that the PRP GMCs after dose 3 of DTaP5-IPV-Hib were noninferior to those seen with DTaP3-IPV/Hib. The GMC ratio (group A/group B) was calculated, and the associated 2-sided 95% CI was computed by assuming the log normality of concentrations and using the t distribution and the pooled sample variance. If the lower limit of the GMC ratio 95% CI was >0.67, then the DTaP5-IPV-Hib PRP response was considered noninferior to that of DTaP3-IPV/Hib. If noninferiority was demonstrated and the lower limit of the 95% CI of the GMC ratio was >1, then the DTaP5-IPV-Hib PRP response was considered superior to the DTaP3-IPV/Hib PRP response.
Statistical summaries were provided for each group for secondary endpoints based on the methods described above. Data were analyzed as observed, with no imputation for missing values.
The safety analysis set comprised all participants receiving ≥1 dose of study vaccine and having any safety assessment. The per-protocol analysis set was used for the primary immunogenicity endpoints, including randomized participants who had received the study vaccine as randomized, had a post-dose 3 blood draw within 28 to 42 days, and had no protocol violation potentially affecting primary endpoint immunogenicity assessments. For the immunogenicity endpoints, the full analysis set included all randomized participants who received ≥1 dose of vaccine and provided any blood sample after vaccination. The full analysis set results were consistent with the per-protocol analysis set and are not presented.
RESULTS
Participants.
Randomized participants (n = 807) were allocated to receive DTaP5-IPV-Hib (n = 402, group A) and DTaP3-IPV/Hib (n = 405; group B) as shown in Fig. 1; 773 participants (95.8%) completed the study. Slightly more participants in group A (5.5%) did not complete the study, largely because of more SAE withdrawals unrelated to vaccination. The safety analysis set comprised 400 (99.5%) participants in group A (2 participants did not report safety data) and 405 (100%) participants in group B; for the per-protocol analysis, 325/402 (80.8%) and 341/405 (84.2%) were included, respectively. For both groups, most protocol violations were for vaccinations given outside the specified time window (>28 days after the schedule-specified time), blood draws outside the specified time window (>28 days and <42 days postvaccination), or no post-dose 3 blood sample drawn: 13.2% (53/402) for group A and 11.1% (45/405) for group B.
Fig 1.
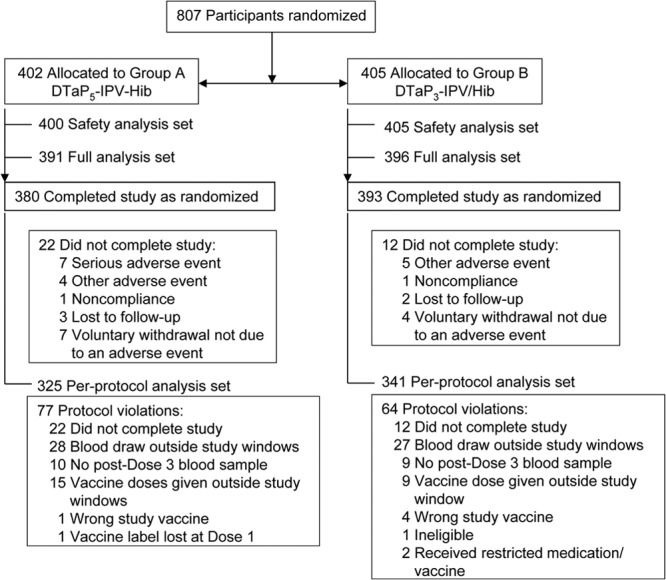
Participant flow throughout the study.
Participants were similarly distributed by gender: 221 (55%) males in group A and 211 (52%) males in group B. The median ages in months were 2.9, 4.9, and 12.2 for doses 1, 2, and 3, respectively, and the median ages were the same for both groups. The median body weight of 6.2 kg at study entry was the same in both groups.
Immunogenicity.
One month post-dose 3 in both groups, the seroprotection rates for PRP, diphtheria and tetanus toxoids, and all poliovirus types were high (>90%) (Table 1). Since the lower bounds of the 95% CI of the differences of the seroprotection rates were greater than the predefined noninferiority margin, group A was noninferior to group B for diphtheria, tetanus, and poliovirus immune responses. For diphtheria, the seroprotection rate for group A was greater than that for group B since the 95% CI of the differences was >0. For PRP, the seroprotection rate for group A was 93.2% (89.9% to 95.7%) and for group B was 96.8% (94.3% to 98.4%), a difference of −3.5% (95% CI, −7.10% to −0.21%); since the lower bound of the 95% CI of the difference was less than −0.05, DTaP5-IPV-Hib did not meet the noninferiority criteria. Using the ≥0.15 μg/ml threshold, the post-dose 3 PRP seroprotection rate was 99.1% (95% CI, 97.3% to 99.8%) for group A and 99.7% (95%CI: 98.4% to 100%) for group B.
Table 1.
Seroprotection rates to study vaccine antigens post-dose 3 (per-protocol analysis set)a
| Antigen (threshold) | Seroprotection rate |
Differenceb (95% CI) | |||
|---|---|---|---|---|---|
| Group A DTaP5-IPV-Hib (N = 325) |
Group B DTaP3-IPV/Hib (N = 341) |
||||
| n/M | % (95% CI) | n/M | % (95% CI) | ||
| PRP (≥1.0 μg/ml) | 303/325 | 93.2 (89.9–95.7) | 330/341 | 96.8 (94.3–98.4) | −3.5 (−7.1 to −0.2) |
| PRP (≥0.15 μg/ml) | 322/325 | 99.1 (97.3–99.8) | 340/341 | 99.7 (98.4–100) | N/A |
| Diphtheria toxoid (≥0.1 IU/ml) | 309/325 | 95.1 (92.1–97.2) | 308/341 | 90.3 (86.7–93.2) | 4.8 (0.8 to 8.8)c |
| Tetanus toxoid (≥0.1 IU/ml) | 325/325 | 100 (98.9–100) | 339/340 | 99.7 (98.4–100) | 0.3 (−0.9 to 1.7)c |
| Poliovirus antigens (≥1:8 dilution) | |||||
| Polio type 1 | 322/324 | 99.4 (97.8–99.9) | 336/336 | 100 (98.9–100) | −0.6 (−2.2 to 0.6)c |
| Polio type 2 | 322/324 | 99.7 (98.3–100) | 336/336 | 100 (98.9–100) | −0.3 (−1.7 to 0.9)c |
| Polio type 3 | 319/323 | 98.8 (96.9–99.7) | 335/335 | 100 (98.9–100) | −1.2 (−3.1 to 0.1)c |
N, number of participants in the per-protocol analysis set; n, number of participants achieving threshold; M, number of participants with at least 1 available value; PRP, polyribosylribitol phosphate; CI, confidence interval; N/A, not applicable.
Difference = group A seroprotection rate − group B seroprotection rate.
Noninferiority was achieved if the lower limit of the 95% CI of the difference in seroprotection rates was greater than −5%; noninferiority was not evaluated for pertussis antigens.
The post-dose 2 seroprotection rates for PRP, diphtheria and tetanus toxoids, and polioviruses were similar for each group (Fig. 2).
Fig 2.
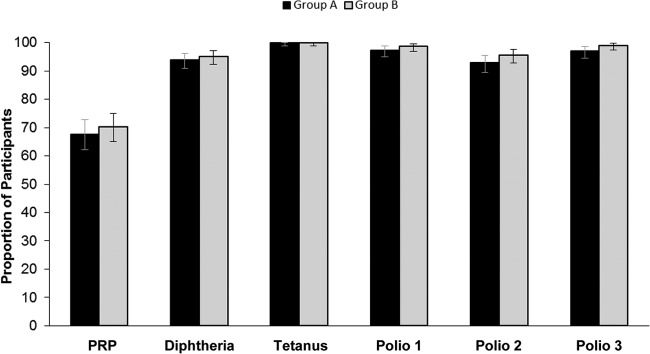
Seroprotection rates post-dose 2 (per-protocol analysis set). Seroprotection rates were based on post-dose 2 antibody concentrations to PRP ≥ 0.15 μg/ml, diphtheria and tetanus toxoids ≥ 0.01 IU/ml, and poliovirus types 1 to 3 ≥ 1:8 dilution in group A (DTaP5-IPV-Hib) and group B (DTaP3-IPV/Hib). Error bars represent 95% confidence intervals.
Rates of seroresponse to pertussis antigens post-dose 2 and post-dose 3 were high and similar between groups for PT and FHA. The seroresponse rates for PRN were higher post-dose 2 in group B (Table 2) but were high (≥96.9%) and similar post-dose 3 in both groups. FIM seroresponse rates were higher in group A after both doses, as expected, since FIM is not a component of DTaP3-IPV/Hib. The proportions of participants achieving ≥2-fold and ≥4-fold seroresponse increases followed a similar pattern, and post-dose 2 and post-dose 3 rates were similar for PT and FHA. PRN rates tended to be higher for group B and FIM rates higher in group A (Table 2).
Table 2.
Proportion of participants with ≥2-fold and ≥4-fold increases in immune responses from prevaccination levels post-dose 2 and post-dose 3 and rates of seroresponses to pertussis antigens (per-protocol analysis sets)a
| Antigen and immune response | Response rate |
|||
|---|---|---|---|---|
| Group A DTaP5-IPV-Hib (N = 325) |
Group B DTaP3-IPV/Hib (N = 341) |
|||
| n/M | % (95% CI) | n/M | % (95% CI) | |
| PT (EU/ml) | ||||
| Post-dose 2 | ||||
| ≥2-fold increase | 300/316 | 94.9 (91.9–97.1) | 320/332 | 96.4 (93.8–98.1) |
| ≥4-fold increase | 277/316 | 87.7 (83.5–91.1) | 300/332 | 90.4 (86.7–93.3) |
| SR | 306/316 | 96.8 (94.3–98.5) | 326/332 | 98.2 (96.1–99.3) |
| Post-dose 3 | ||||
| ≥2-fold increase | 316/323 | 97.8 (95.6–99.1) | 336/341 | 98.5 (96.6–99.5) |
| ≥4-fold increase | 306/323 | 94.7 (91.7–96.9) | 329/341 | 96.5 (93.9–98.2) |
| SR | 318/323 | 98.5 (96.4–99.5) | 340/341 | 99.7 (98.4–100) |
| FHA (EU/ml) | ||||
| Post-dose 2 | ||||
| ≥2-fold increase | 299/315 | 94.9 (91.9–97.1) | 307/331 | 92.7 (89.4–95.3) |
| ≥4-fold increase | 270/315 | 85.7 (81.4–89.4) | 281/331 | 84.9 (80.6–88.6) |
| SR | 311/315 | 98.7 (96.8–99.7) | 320/331 | 96.7 (94.1–98.3) |
| Post-dose 3 | ||||
| ≥2-fold increase | 320/324 | 98.8 (96.9–99.7) | 339/340 | 99.7 (98.4–100) |
| ≥4-fold increase | 310/324 | 95.7 (92.9–97.6) | 327/340 | 96.2 (93.6–97.9) |
| SR | 321/324 | 99.1 (97.3–99.8) | 340/340 | 100 (98.9–100) |
| PRN (EU/ml) | ||||
| Post-dose 2 | ||||
| ≥2-fold increase | 219/311 | 70.4 (65.0–75.4) | 282/330 | 85.5 (81.2–89.1) |
| ≥4-fold increase | 172/311 | 55.3 (49.6–60.9) | 248/330 | 75.2 (70.1–79.7) |
| SR | 246/311 | 79.1 (74.2–83.5) | 300/330 | 90.9 (87.3–93.8) |
| Post-dose 3 | ||||
| ≥2-fold increase | 301/321 | 93.8 (90.5–96.2) | 330/338 | 97.6 (95.4–99.0) |
| ≥4-fold increase | 280/321 | 87.2 (83.1–90.7) | 319/338 | 94.4 (91.4–96.6) |
| SR | 311/321 | 96.9 (94.3–98.5) | 335/338 | 99.1 (97.4–99.8) |
| FIM (EU/ml) | ||||
| Post-dose 2 | ||||
| ≥2-fold increase | 283/311 | 91.0 (87.3–93.9) | 2/324 | 0.6 (0.1–2.2) |
| ≥4-fold increase | 266/311 | 85.5 (81.1–89.2) | 0/324 | 0(0–1.1) |
| SR | 297/311 | 95.5 (92.6–97.5) | 8/324 | 2.5 (1.1–4.8) |
| Post-dose 3 | ||||
| ≥2-fold increase | 309/322 | 96.0 (93.2–97.8) | 8/334 | 2.4 (1.0–4.7) |
| ≥4-fold increase | 304/322 | 94.4 (91.3–96.7) | 1/334 | 0.3 (0.0–1.7) |
| SR | 310/322 | 96.3 (93.6–98.1) | 12/334 | 3.6 (1.9–6.2) |
SR (seroresponse), post-dose 2 or 3 antibody concentration ≥ lower limit of quantitation (LLOQ = 4 EU/ml for PT, PRN, and FIM and 3 EU/ml for FHA) when baseline concentrations were <LLOQ or at least maintenance of antibody concentration in participants whose values were initially ≥LLOQ; N, number of participants in the per-protocol analysis set; n, number of participants who met the criteria; M, number of participants with at least 1 available per-protocol value; PT, pertussis toxoid; FHA, filamentous hemagglutinin; PRN, pertactin; FIM, fimbria types 2 and 3; CI, confidence interval.
Antibody GMCs/GMTs against all antigens in the study vaccines post-dose 2 and post-dose 3 are shown in Table 3. The GMC ratio (95% CI) of PRP antibodies comparing group A to group B was 0.70 (95% CI, 0.57 to 0.85). Since the lower limit of the 95% CI of the ratio was <0.67, DTaP5-IPV-Hib did not meet the noninferiority criteria for PRP responses. Some numerical differences in the GMCs/GMTs were also observed between groups post-dose 2 and post-dose 3. The values for diphtheria toxoid, PT, and FIM tended to be higher in group A, and those for tetanus, poliovirus, FHA, and PRN tended to be higher in group B.
Table 3.
Antibody geometric mean concentrations and titers post-dose 2 and post-dose 3 for study vaccine antigens (per-protocol analysis sets)
| Antigen and immune response | Group A DTaP5-IPV-Hib (N = 325) |
Group B DTaP3-IPV/Hib (N = 341) |
||
|---|---|---|---|---|
| M | GMC/GMT (95% CI) | M | GMC/GMT (95% CI) | |
| PRP | ||||
| Post-dose 2 | 316 | 0.40 μg/ml (0.33–0.50) | 333 | 0.44 μg/ml (0.36–0.54) |
| Post-dose 3 | 325 | 12.20 μg/ml (10.46–14.24) | 341 | 17.54 μg/ml (15.38–20.01) |
| Diphtheria toxoid | ||||
| Post-dose 2 | 316 | 0.07 μg/ml (0.06 −0.08) | 334 | 0.05 μg/ml (0.04; 0.05) |
| Post-dose 3 | 325 | 1.28 μg/ml (1.09 −1.50) | 341 | 0.70 μg/ml (0.60–0.82) |
| Tetanus toxoid | ||||
| Post-dose 2 | 316 | 0.43 μg/ml (0.39–0.47) | 334 | 0.66 μg/ml (0.61–0.72) |
| Post-dose 3 | 325 | 3.63 μg/ml (3.35–3.93) | 340 | 3.91 μg/ml (3.63–4.22) |
| Poliovirus type 1 | ||||
| Post-dose 2 | 312 | 100.9 (1/dilution) (83.5–122.0) | 327 | 173.1 (1/dilution) (142.5; 210.3) |
| Post-dose 3 | 324 | 1,260.2 (1/dilution) (1,081.6–1,468.3) | 336 | 3,419.5 (1/dilution) (2,987.5–3,914.0) |
| Poliovirus type 2 | ||||
| Post-dose 2 | 312 | 34.5 (1/dilution) (29.1–40.9) | 326 | 37.8 (1/dilution) (32.0–44.6) |
| Post-dose 3 | 323 | 853.3 (1/dilution) (709.4–1026.3) | 336 | 1,870.3 (1/dilution) (1,584.0–2,208.3) |
| Poliovirus type 3 | ||||
| Post-dose 2 | 309 | 89.5 (1/dilution) (74.1–108.2) | 324 | 158.7 (1/dilution) (129.6; 194.3) |
| Post-dose 3 | 323 | 1,204.1 (1/dilution) (991.4–1462.5) | 335 | 3,536.4 (1/dilution) (2,984.8–4,189.9) |
| PT | ||||
| Post-dose 2 | 316 | 77.3 (EU/ml) (71.2–83.9) | 332 | 72.8 (EU/ml) (67.6–78.3) |
| Post-dose 3 | 323 | 150.3 (EU/ml) (138.5–163.1) | 341 | 118.6 (EU/ml) (110.4–127.3) |
| FHA | ||||
| Post-dose 2 | 316 | 61.5 (57.1–66.3) | 316 | 73.72 (EU/ml) (68.29; 79.57) |
| Post-dose 3 | 325 | 149.5 (EU/ml) (138.5–161.5) | 340 | 215.6 (EU/ml) (200.4–231.9) |
| PRN | ||||
| Post-dose 2 | 315 | 25.2 (EU/ml) (22.0–28.9) | 333 | 56.9 (EU/ml) (50.7–63.9) |
| Post-dose 3 | 325 | 98.1 (EU/ml) (89.0–108.1) | 341 | 206.7 (EU/ml) (188.4–226.8) |
| FIM | ||||
| Post-dose 2 | 313 | 131.0 (EU/ml) (115.1–149.1) | 325 | 2.6 (EU/ml) (2.4; 2.8) |
| Post-dose 3 | 324 | 439.6 (EU/ml) (384.4–502.8) | 335 | 2.3 (EU/ml) (2.2–2.4) |
N, number of participants in the per-protocol analysis set; M, number of participants with at least 1 available value; GMC/GMT, geometric mean concentration/geometric mean titer; CI, confidence interval; PRP, polyribosylribitol phosphate; PT, pertussis toxoid; FHA, filamentous hemagglutinin; PRN, pertactin; FIM, fimbria types 2 and 3; CI confidence interval.
Solicited reactions.
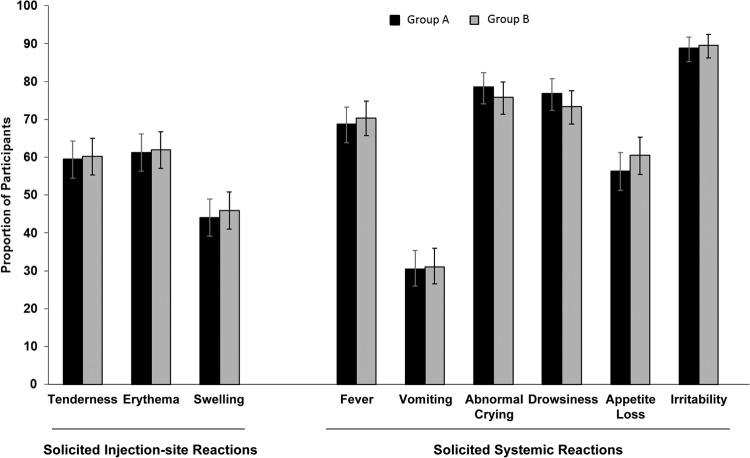
Group A and group B exhibited similar rates of solicited injection site and systemic reactions at ≤7 days after vaccination (Fig. 3). Erythema was the most frequently reported solicited injection site reaction; irritability was the most frequently reported solicited systemic reaction. Most solicited injection site reactions were grade 1 in intensity; reactions occurred and resolved within 1 to 3 days.
Fig 3.
Solicited injection site and systemic reactions. Data represent the proportions of participants with solicited injection site reactions and solicited systemic reactions reported for days 0 through 7 in group A (DTaP5-IPV-Hib) and group B (DTaP3-IPV/Hib). Error bars represent the 95% confidence interval.
Other adverse events.
Overall, the proportions of participants reporting unsolicited AEs at ≤28 days postvaccination were similar: 72.5% (95% CI, 67.8% to 76.8%) in group A and 75.8% (95% CI, 71.1% to 79.7%) in group B. The most frequently reported unsolicited AEs in both groups were pyrexia, rhinitis, and otitis media. While the rate of reported SAEs were slightly higher in group A (8.5% [95% CI, 6.0% to 11.7] compared to 5.4% [95% CI, 3.4% to 8.1%] in group B), no SAEs were considered to be vaccination related. The higher rate of SAEs was attributable to the 7 (1.7%) participants who withdrew from the study after an SAE in group A (Fig. 1); the reasons for discontinuing included developmental delay (n = 2), mild arrested hydrocephalus (n = 1), congenital atrial septal defect (n = 1), thrombocytopenia (n = 1), diagnosis of epilepsy (n = 1), and an eye disorder (n = 1). All were followed until symptom resolution or the conclusion of the study.
The antipyretic/analgesic use rate 3 days postvaccination ranged from 33.1% to 36.6% in group A and from 24.2% to 44.3% in group B.
DISCUSSION
This study demonstrated that the fully liquid DTaP5-IPV-Hib vaccine can be administered using the 3-, 5-, and 12-month vaccination schedule. DTaP5-IPV-Hib elicited a robust immune response to all vaccine antigens and had a safety profile similar to that of DTaP3-IPV/Hib. DTaP5-IPV-Hib was noninferior to DTaP3-IPV/Hib as assessed by post-dose 3 rates of seroprotection against diphtheria and tetanus toxoids and poliovirus antigens; the response to diphtheria was found to be higher among DTaP5-IPV-Hib recipients. Post-dose 3 PRP seroprotection rates (≥0.15 μg/ml and ≥1.0 μg/ml) were high (99.1% and 93.2% after DTaP5-IPV-Hib and 99.7% and 96.8% after DTaP3-IPV/Hib, respectively); however, predefined noninferiority criteria for DTaP5-IPV-Hib were not met for the seroprotection rate at ≥1.0 μg/ml or GMCs compared with DTaP3-IPV/Hib. Post-dose 2, PRP seroprotection rates (≥0.15 μg/ml) and GMCs also tended to be numerically lower for those receiving DTaP5-IPV-Hib. Whether this was due to differences in prevaccination levels is not known since PRP was not assessed at baseline.
In contrast, a study conducted in France and Poland (4) found that DTaP5-IPV-Hib recipients achieved higher PRP seroprotection rates and GMCs than DTaP3-IPV/Hib recipients using a 2-, 3-, and 4-month schedule as well as after the fourth booster dose at 12 to 18 months of age (heptavalent pneumococcal conjugate vaccine [PCV7] was coadministered to both groups). After the 3-dose primary series, PRP seroprotection rates at ≥0.15 μg/ml were 91.0% versus 80.8%, at ≥1.0 μg/ml were 63.3% versus 38.9%, and GMCs were 1.38 μg/ml versus 0.59 μg/ml. Post-dose 4, PRP seroprotection rates at ≥1.0 μg/ml were 99.1% versus 95.2% and GMCs were 32.4 μg/ml versus 19.26 μg/ml (4). Similarly, in a German study (9), 100% of toddlers aged 11 to 18 months (primed with a hexavalent vaccine primary series) who received DTaP5-IPV-Hib or DTaP3-HBV-IPV/Hib (both coadministered with PCV7) as a fourth booster dose achieved PRP seroprotection rates ≥ 1.0 μg/ml in both groups; GMCs were higher in those receiving DTaP5-IPV-Hib (37.16 μg/ml versus 30.27 μg/ml for DTaP3-HBV-IPV/Hib recipients). In both of these studies, the same laboratory and analysis methods were used as in the current study.
Variability in PRP immune responses is well recognized and may have contributed to the observed differences across studies (15, 16). Given the clinically similar point estimates, and the high seroprotection rates obtained post-dose 3, it would be difficult to assign much clinical significance to the PRP numerical differences between groups.
Post-dose 2, seroprotection rates elicited against diphtheria, tetanus, and poliovirus type 1 to 3 antigens were similar. For pertussis, the post-dose 2 and 3 PT and FHA seroresponse rates and 2- and 4-fold rise rates were generally similar. For PRN, the post-dose 3 rates were high in both groups, although some variability in the immune response after each dose was observed. This was likely due to differences in vaccine antigen content. Rates for FIM were higher in group A as expected since FIM is not a component of the DTaP3-IPV/Hib. The lack of a response in group B demonstrates the low background levels of antibody against FIM in the community. Overall, the development of an established correlate of protection for pertussis would assist in the clinical interpretation of pertussis responses.
For the post-dose 2 and 3 GMCs/GMTs, robust immune responses to the vaccine antigens were observed. Numerical differences were observed, but they are unlikely to be clinically relevant given the high seroprotection and seroresponse/fold-rise rates achieved.
One advantage of this study was the ability to directly compare 2 licensed pentavalent pediatric combination vaccines. While differences exist in the composition of the vaccines (e.g., antigen concentrations and aluminum adjuvant), the potential for independent effects and/or interactions of these differences is not clearly apparent. Overall, however, this study did demonstrate robust immune responses after administration of each vaccine.
Compared to a 3-dose primary administration series at 2, 3, and 4 months using the same vaccines (coadministered with PCV7) and laboratory methods (4), 2 doses of vaccine at 3 and 5 months of age (i.e., a 2-dose primary series) tended to elicit lower immune responses (seroprotection rates and GMCs/GMTs). However, a third booster dose administered to participants at 12 months of age resulted in seroprotection rates similar to those seen after a fourth booster dose was administered at 12 to 18 months of age, even though GMCs/GMTs tended to be higher after 4 vaccine doses (4). Still, with both schedules, high seroprotection rates for most antigens were achieved at established levels for the primary and booster series.
Similar safety profiles were observed in both study groups, and there were no unexpected safety issues identified. Although there were a higher number of participants reporting SAEs among those receiving DTaP5-IPV-Hib, no SAEs were considered vaccination related. Adverse reactions were generally mild (grade 1 intensity) and of short duration (≤3 days).
One potential study limitation was the challenge for participants to meet the study windows defined for vaccinations and blood sampling. A total of 70% of 141 protocol violations resulted from nonadherence to such issues (including lack of a post-dose 3 blood draw), but these were distributed similarly in the two study groups. Results from the full analysis set (not shown) confirmed the per-protocol findings.
Overall, the safety and immunogenicity data from this study support DTaP5-IPV-Hib administration to infants and toddlers using the 3-, 5-, and 12-month schedule. DTaP5-IPV-Hib was shown to elicit a robust immune response and had a safety profile similar to the DTaP3-IPV/Hib safety profile. While some numerical differences were observed in the immune responses post-dose 3 between vaccines, clinical significance is unlikely given the high seroprotection and seroresponse rates achieved in both groups.
ACKNOWLEDGMENTS
This work was sponsored by Sanofi Pasteur.
We thank the following A5I15 Investigators and their staffs at the participating trial centers: Anitta Ahonen, Inge Axelsson, Anna-Maija Hanni, Anna Hyvärinen, Tarja Järvinen, Tiina Karppa, Aino Karvonen, Teija Kauti, Tiina Korhonen, Niklas Lindblad, Federico Nasta, Jaana Neuvonen, Anne Salomäki, Ilkka Seppä, Heli Silvennoinen, and Reeta Vehmas. We also thank Julie Boyer, Anne Fiquet, Armelle Marais, and Anne Schuyleman of Sanofi Pasteur MSD and Julien Bertrand, Lizbeth Carmona, Avi Collins, Xavier DaCosta, Leona Fernandes, Santiago Ferro, Subha Indrarajah, Peter Lashley, Jacqueline Pennycook, Ellen Snell, and Florence Venant of Sanofi Pasteur. We thank Robert Lersch of Sanofi Pasteur, who coordinated the writing of the manuscript; we also thank Julia R. Gage of Gage Medical Writing, LLC, who, on behalf of Sanofi Pasteur, assisted with writing the manuscript.
We wish to declare the following conflicts of interest: Timo Vesikari was the principal investigator of this trial and is a member of advisory boards for Sanofi Pasteur MSD, Merck, and Novartis and a consultant for Pfizer. Timo Vesikari has received honoraria or lecture fees from the same and GlaxoSmithKline Biologicals. Sven Arne Silverdal has received financial support to conduct studies from Sanofi Pasteur, GlaxoSmithKline Biologicals, Wyeth, and Pfizer. Sven Arne Silverdal has also received some travel and accommodation expense reimbursement and board membership fees from GlaxoSmithKline Biologicals. Florence Boisnard and Stéphane Thomas are employees of Sanofi Pasteur MSD. Grace Mwawasi and Donna Reynolds were employees of Sanofi Pasteur Limited at the time the study was conducted. Grace Mwawasi is now an employee of PharmaNet/i3. Donna Reynolds is now an adjunct professor at the University of Toronto.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Kitchin N, Southern J, Morris R, Hemme F, Cartwright K, Watson M, Miller E. 2006. A randomised controlled study of the reactogenicity of an acellular pertussis-containing pentavalent infant vaccine compared to a quadrivalent whole-cell pertussis-containing vaccine and oral poliomyelitis vaccine, when given concurrently with meningococcal group C conjugate vaccine to healthy UK infants at 2, 3 and 4 months of age. Vaccine 24:3964–3970 [DOI] [PubMed] [Google Scholar]
- 2.Kitchin NR, Southern J, Morris R, Hemme F, Thomas S, Watson MW, Cartwright K, Miller E. 2007. Evaluation of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b vaccine given concurrently with meningococcal group C conjugate vaccine at 2, 3 and 4 months of age. Arch. Dis. Child. 92:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slack MH, Cade S, Schapira D, Thwaites RJ, Crowley-Luke A, Southern J, Borrow R, Miller E. 2005. DT5aP-Hib-IPV and MCC vaccines: preterm infants' response to accelerated immunisation. Arch. Dis. Child. 90:338–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimprel E, Wysocki J, Boisnard F, Thomas S, Mwawasi G, Reynolds D. 2011. Immunogenicity and safety of fully liquid DTaP(5)-IPV-Hib compared with DTaP(3)-IPV/Hib when both coadministered with a heptavalent pneumococcal conjugate vaccine (PCV7) at 2, 3, 4, and 12 to 18 months of age: a phase III, single-blind, randomised, controlled, multicentre study. Vaccine 29:7370–7378 [DOI] [PubMed] [Google Scholar]
- 5.Castaneda JL, Gonzalez N, Chavez R, Lavigne P, Barreto L. 15–20 August 2004. Enhanced inactivated poliovaccine (IPV) in a fully liquid pentavalent DTaP-IPV-PRP-T combination: immunogenicity and safety in Mexican infants. Abstr. 24th Int. Congr. Pediatr., Cancun, Mexico, poster 346 [Google Scholar]
- 6.Castaneda JL, Gonzalez N, Lavigne P, Ortiz E. 2004. Immunogenicity and safety of a fully liquid DTaP-IPV-Hib vaccine with full primary IPV or sequential IPV-OPV in Mexican infants. Abstr. 11th Int. Congr. Infect. Dis., Cancun, Mexico, 4 to 7 March 2004, poster 18.010 [Google Scholar]
- 7.Lin TY, Wang YH, Chang LY, Huang YC, Kao HT, Lin PY, Lu HK, Chavand P, Ortiz E. 2007. A fully liquid diphtheria-tetanus-five component acellular pertussis-inactivated poliomyelitis–Haemophilus influenzae type b conjugate vaccine: immunogenicity and safety of primary vaccination in Taiwanese infants. Int. J. Infect. Dis. 11:129–136 [DOI] [PubMed] [Google Scholar]
- 8.Capeding MR, Unalivia L, Victoria MV, Pietrobon P, Young L, Fievet-Groyne F. 2002. Immunogenicity and safety of a fully liquid DTaP-IPV-HiB vaccine in infants at 6, 10, and 14 weeks of age (EPI schedule). Abstr. 3rd World Congr. Pediat. Infect. Dis. World Soc. Pediatr. Infect. Dis., Santiago de Chile, Chile, 19 to 23 November 2002, poster 59 [Google Scholar]
- 9.Berner R, Boisnard F, Thomas S, Mwawasi G, Reynolds D. 2012. Safety and immunogenicity of fully liquid DTaP5-IPV-Hib pediatric combination vaccine (Pediacel) compared to DTaP3-HBV-IPV/Hib (Infanrix Hexa) when coadministered with heptavalent pneumococcal conjugate vaccine (PCV7) as a booster at 11–18 months of age: a phase III, modified double-blind, randomized, controlled, multicenter study. Vaccine 30:5270–5277 [DOI] [PubMed] [Google Scholar]
- 10.Gold R, Barreto L, Ferro S, Thippawong J, Guasparini R, Meekison W, Russell M, Mills E, Harrison D, Lavigne P. 2007. Safety and immunogenicity of a fully liquid vaccine containing five-component pertussis-diphtheria-tetanus-inactivated poliomyelitis-Haemophilus influenzae type b conjugate vaccines administered at two, four, six and 18 months of age. Can. J. Infect. Dis. Med. Microbiol. 18:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin TY, Wang YH, Huang YC, Chiu CH, Lin PY, Chen CJ, Chavand P, Ortiz E. 2007. One-year post-primary antibody persistence and booster immune response to a fully liquid five-component acellular pertussis, diphtheria, tetanus, inactivated poliomyelitis, Haemophilus influenzae type b conjugate vaccine. Int. J. Infect. Dis. 11:488–495 [DOI] [PubMed] [Google Scholar]
- 12.US National Institutes of Health 2012. April 13, posting date Comparison of safety and immunogenicity of Pediacel™ and Infanrix™ IPV+Hib (Penta) given in a 3 dose schedule in infants. http://clinicaltrial.gov/ct2/show/NCT00287092?term=A5I15&rank=1
- 13.Newcombe RG. 1998. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat. Med. 17:873–890 [DOI] [PubMed] [Google Scholar]
- 14.Collett D. 1991. Modelling binary data. Chapman & Hall, London, United Kingdom [Google Scholar]
- 15.Vidor E, Hoffenbach A, Fletcher MA. 2001. Haemophilus influenzae type b vaccine: reconstitution of lyophilised PRP-T vaccine with a pertussis-containing paediatric combination vaccine, or a change in the primary series immunisation schedule, may modify the serum anti-PRP antibody responses. Curr. Med. Res. Opin. 17:197–209 [DOI] [PubMed] [Google Scholar]
- 16.Ward JI, Greenberg DP, Anderson PW, Burkart KS, Christenson PD, Gordon LK, Kayhty H, Kuo JS, Vella P. 1988. Variable quantitation of Haemophilus influenzae type b anticapsular antibody by radioantigen binding assay. J. Clin. Microbiol. 26:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]