Abstract
In this study, we compared the immunogenicities of two lots of meningococcal ACWY-tetanus toxoid conjugate vaccine (MenACWY-TT) that differed in serogroup A polysaccharide (PS) O-acetylation levels and evaluated their immunogenicities and safety in comparison to a licensed ACWY polysaccharide vaccine (Men-PS). In this phase III, partially blinded, controlled study, 1,170 healthy subjects aged 18 to 25 years were randomized (1:1:1) to receive one dose of MenACWY-TT lot A (ACWY-A) (68% O-acetylation), MenACWY-TT lot B (ACWY-B) (92% O-acetylation), or Men-PS (82% O-acetylation). Immunogenicity was evaluated in terms of serum bactericidal activity using rabbit complement (i.e., rabbit serum bactericidal activity [rSBA]). Solicited symptoms, unsolicited adverse events (AEs), and serious AEs (SAEs) were recorded. The immunogenicities, in terms of rSBA geometric mean titers, were comparable for both lots of MenACWY-TT. The vaccine response rates across the serogroups were 79.1 to 97.0% in the two ACWY groups and 73.7 to 94.1% in the Men-PS group. All subjects achieved rSBA titers of ≥1:8 for all serogroups. All subjects in the two ACWY groups and 99.5 to 100% in the Men-PS group achieved rSBA titers of ≥1:128. Pain was the most common solicited local symptom and was reported more frequently in the ACWY group (53.9 to 54.7%) than in the Men-PS group (36.8%). The most common solicited general symptoms were fatigue and headache, which were reported by 28.6 to 30.3% and 26.9 to 31.0% of subjects, respectively. Two subjects reported SAEs; one SAE was considered to be related to vaccination (blighted ovum; ACWY-B group). The level of serogroup A PS O-acetylation did not affect vaccine immunogenicity. MenACWY-TT (lot A) was not inferior to Men-PS in terms of vaccine response and was well tolerated.
INTRODUCTION
Neisseria meningitidis is a major cause of serious invasive bacterial infections, such as meningitis and meningococcemia. These diseases are associated with high morbidity and mortality rates and remain major public health problems globally (1–3). Annually, there are an estimated 1.2 million cases of meningococcal infections and approximately 135,000 deaths worldwide. Today, the overall mortality rate of invasive meningococcal disease (IMD) is 10 to 15% (4). The incidence of IMD is highest in infants, and a second peak is observed among adolescents and young adults (4, 5). Case fatality rates are also highest in infants and young children, although case fatality rates up to 25% have been recorded in adolescents and young adults 15 to 24 years of age (6).
Among 13 serogroups of N. meningitidis identified based on the biochemical composition of the capsular polysaccharide (PS), only six, i.e., A, B, C, W-135, Y, and (more recently) X, account for almost all IMD cases (2, 4, 7, 8). Global serogroup distributions are widely variable. Serogroups A and C are responsible for the majority of cases; however, the prevalence of serogroups Y and W-135 has increased in recent years (2, 9, 10). In particular, serogroup W-135 has recently emerged as a cause of epidemic disease in South Africa and South America (11, 12). In Latin America, outbreaks due to serogroup C in several countries have been reported since the 1970s, whereas serogroups W-135 and Y emerged only recently in some countries (4, 13). In Asia, serogroup A caused large outbreaks in several countries in the past century; more recently, local outbreaks due to serogroups C, W-135, and Y have been also reported (4, 14). Thailand experienced a few cases of IMD due to serogroups A, C, and W-135 between 1994 and 1999, whereas two large outbreaks due to serogroup A occurred in the Philippines between 1989 and 2005 (14). Prevention of meningococcal disease through vaccination with multivalent vaccines that provide broad serogroup coverage is needed, considering the high incidence and mortality rates in children and young adults, the diverse worldwide distribution of meningococcal serogroups, and the increasing rate of global travel.
Plain PS vaccines against serogroups A, C, W-135, and Y have been available since the 1970s. The quadrivalent ACWY PS vaccine (Men-PS) (Mencevax; GlaxoSmithKline, Rixensart, Belgium) is indicated for active immunization of adults and children ≥2 years of age and is broadly used worldwide (15). However, meningococcal PSs are poorly immunogenic in infants and toddlers, do not elicit persistent antibody responses, do not reduce mucosal carriage, and do not confer herd protection (16, 17). The immunogenicity of PS vaccines can be improved by conjugation of the capsular PS to a carrier protein, as demonstrated by monovalent meningococcal serogroup C conjugate vaccines (18–20). Four multivalent meningococcal conjugate vaccines are currently available, including a Haemophilus influenzae type b (Hib) and meningococcal serogroup C and Y tetanus toxoid (TT) conjugate vaccine (Hib-MenCY-TT) (MenHibrix; GlaxoSmithKline, Rixensart, Belgium) licensed in the United States for vaccination of infants in a four-dose series from 6 weeks through 18 months of age (US Food and Drug Administration MenHibrix approval letter [see http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm308573.htm]) and three quadrivalent vaccines against serogroups A, C, W-135, and Y, i.e., a diphtheria toxoid (DT) conjugate vaccine (MenACWY-DT) (Menactra; Sanofi Pasteur, Inc., Swiftwater, PA) licensed in the United States, Canada, Gulf Cooperation States in the Middle East, Australia, and the Philippines for use in individuals 9 months through 55 years of age (21–23) (US Food and Drug Administration, Menactra approval letter [see http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm252511.htm], and Sanofi Pasteur press release for the registration of Menactra by the Health Council for Arab Countries [see http://www.sanofipasteur.com/sanofi-pasteur4/ImageServlet?imageCode=27961&siteCode=SP_CORP4]), a mutant diphtheria toxoid (cross-reactive material 197 [CRM197]) conjugate vaccine (MenACWY-CRM) (Menveo; Novartis Vaccines, Bellaria-Rosia, Italy) licensed in the United States, Canada, Argentina, Pakistan, Saudi Arabia, Australia, the Philippines, and the European Union for active immunization of children (≥2 years of age), adolescents, and adults (24–27) (European Medicines Agency postauthorization summary of opinion on Menveo [see http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/001095/WC500124220.pdf]), and a TT conjugate vaccine (MenACWY-TT) (Nimenrix; GlaxoSmithKline, Rixensart, Belgium) that was approved in 2012 by the European Medicines Agency for the active immunization of individuals from 12 months of age (European Medicines Agency summary for the public for Nimenrix [see http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002226/WC500127665.pdf]).
O-Acetylation of meningococcal serogroup A PS may be important for immunogenicity (28). This study was conducted to compare the immunogenicity of two lots of MenACWY-TT that differed in the percentage of O-acetylation of meningococcal serogroup A PS and to evaluate the immunogenicity and safety of MenACWY-TT versus Men-PS in 18- to 25-year-old adults from Panama, the Philippines, and Thailand.
MATERIALS AND METHODS
Study design.
This was a phase III, partially blinded, randomized, controlled study conducted at three centers in Panama, the Philippines, and Thailand between August and December 2010. Subjects were randomized (1:1:1) to receive a single dose of MenACWY-TT lot A (with 68% O-acetylation of meningococcal serogroup A PS) (ACWY-A group), MenACWY-TT lot B (with 92% O-acetylation of meningococcal serogroup A PS) (ACWY-B group), or Men-PS (with 82% O-acetylation of meningococcal serogroup A PS) (Men-PS group). Treatment allocation was performed using a central randomization call-in system on the Internet. The study was observer-blinded with respect to MenACWY-TT lots but open with respect to the use of MenACWY-TT or Men-PS, due to the different routes of injection (intramuscular for MenACWY-TT and subcutaneous for Men-PS).
Written informed consent was obtained from subjects before any study-specific procedures were performed. The protocol and associated documents were reviewed and approved by local independent ethics committee or institutional review boards. This study was conducted in accordance with good clinical practices and all applicable regulatory requirements, including the Declaration of Helsinki. This study is registered at www.clinicaltrials.gov (NCT01154088).
Study objectives.
The primary objectives were (i) to compare the immunogenicity of MenACWY-TT vaccine lots in terms of serum bactericidal activity using baby rabbit complement (i.e., rabbit serum bactericidal activity [rSBA]) (with an in-house assay) and (ii) to demonstrate the noninferiority of the vaccine response (VR) induced by MenACWY-TT (lot A) versus Men-PS. The secondary objectives included evaluation of the noninferiority of MenACWY-TT lot A versus Men-PS in terms of rSBA (with the Health Protection Agency [HPA] assay) and evaluation of the immunogenicity and safety of MenACWY-TT and Men-PS.
Study subjects.
Healthy adults who were 18 to 25 years of age at the time of vaccination, provided written informed consent, and complied with the requirements of the protocol (completion of the diary cards and return for the follow-up visits) were eligible for the study. Subjects were excluded if they had administration within 30 days prior to the study or planned administration during the study period of any investigational or nonregistered product, administration within 3 months preceding the study of immunoglobulins or any blood products, or administration during the study period of a vaccine not foreseen by the study protocol (with the exception of any licensed inactivated influenza vaccine, including H1N1 vaccine). Subjects who were immunosuppressed for any reason or had been previously vaccinated with a meningococcal conjugate vaccine at any time, with a meningococcal PS vaccine within 5 years prior to the study, or with a TT-containing vaccine within the last month were also excluded. Subjects with a history of meningococcal disease, neurologic disorders (including Guillain-Barré syndrome), bleeding disorders, allergic diseases likely to be exacerbated by any component of the study vaccine, chronic alcohol consumption, and/or drug abuse were ineligible. Moreover, subjects were ineligible if they had major congenital defects, a serious chronic illness, or an acute disease at the time of enrollment. Women of childbearing potential were required to practice adequate contraception for 30 days prior to vaccination, to have a negative pregnancy test result prior to enrollment, and to practice adequate contraception throughout the study period.
Study vaccines.
The MenACWY-TT vaccine was developed and manufactured by GlaxoSmithKline (Rixensart, Belgium). One 0.5-ml dose of the MenACWY-TT vaccine contained 5 μg capsular PS from each meningococcal serogroup (A, C, W-135, and Y) conjugated to TT (approximately 44 μg of TT in each dose); the levels of meningococcal serogroup A PS O-acetylation were 68% for lot A and 92% for lot B. A 0.5-ml dose of the Men-PS vaccine (Mencevax; GlaxoSmithKline, Rixensart, Belgium) contained 50 μg capsular PS from each meningococcal serogroup (A, C, W-135, and Y); the level of meningococcal serogroup A PS O-acetylation was 82%. Each vaccine was administered as a single injection. MenACWY-TT was administered intramuscularly into the nondominant deltoid muscle, and Men-PS was administered subcutaneously into the nondominant upper arm.
Immunogenicity assessment.
For the assessment of vaccine immunogenicity, blood samples were collected from all subjects prior to and 1 month after vaccination. Functional anti-meningococcal serogroup A, C, W-135, and Y activity was determined with a rSBA assay with an antibody titer cutoff 1:8. This cutoff value is considered indicative of seroprotection for serogroup C and has been extended to the other serogroups (29–31). Assays were performed at GlaxoSmithKline for the primary endpoints and at HPA (United Kingdom) laboratories for the secondary endpoints. The proportions of subjects achieving rSBA titers of ≥1:8 and ≥1:128 and antibody geometric mean titers (GMTs) were determined for serogroups A, C, W-135, and Y using both rSBA assays.
Safety and reactogenicity assessment.
Local (pain, redness, and swelling) and general (fever, headache, fatigue, and gastrointestinal symptoms [nausea, vomiting, diarrhea, and/or abdominal pain]) solicited symptoms were recorded for 4 days after vaccination. The intensity of each symptom was graded on a scale of 0 to 3. Symptoms of grade 3 intensity included injection site redness or swelling with a diameter of >50 mm, fever with axillary/oral temperatures of >39.5°C, and all other adverse events (AEs) that prevented normal daily activities. All solicited local (injection site) reactions were considered causally related to vaccination. The causality of all other AEs was assessed by the investigators. All AEs, serious adverse events (SAEs), and cases of new onset of chronic illness (NOCI) that occurred within 1 month (minimum of 30 days) following vaccine administration were recorded.
Statistical analysis.
With 1,050 evaluable subjects (350 per group), the global power to meet both primary objectives was 92.8%. Assuming that up to 10% of enrolled subjects might be excluded from the according-to-protocol (ATP) cohort for immunogenicity, enrollment of 1,170 subjects (390 per group) was planned.
The analysis of safety was performed with the total vaccinated cohort (TVC), which included all vaccinated subjects. The analysis of immunogenicity was performed with the ATP cohort for immunogenicity, which included all subjects who met all eligibility criteria, who complied with the procedures defined in the protocol, and for whom immunogenicity results were available for antibodies against at least one study vaccine antigen.
For each treatment group and for each antibody at pre- and postvaccination time points, rSBA GMTs were calculated with their 95% confidence intervals (CIs). rSBA GMTs were calculated by taking the antilogarithm of the mean of the logarithmic titer transformations. Antibody titers below the cutoff value for the assay were given an arbitrary value of one-half the cutoff value for the purpose of GMT calculations. The proportion (with 95% CI) of subjects with rSBA titers above prespecified cutoff values and the proportion (with 95% CI) of subjects with rSBA VRs also were calculated.
The first primary objective, i.e., the clinical comparability of MenACWY-TT lot A to lot B with respect to rSBA GMTs (as measured at the GlaxoSmithKline laboratory) for serogroups A, C, W-135, and Y, was evaluated through computation of the 95% CIs of the rSBA GMT ratios (ACWY-B/ACWY-A GMT ratios) using an analysis of covariance (ANCOVA) model on the log10 transformation of the titers, including the vaccine group as a fixed effect and using the prevaccination log10 transformation of the titers and the country as covariates. Noninferiority of lot B versus lot A was demonstrated if the upper limit of the two-sided 95% CI for the rSBA GMT ratio was below a limit of 2.
The second primary objective, i.e., the noninferiority of the rSBA VR (as measured at the GlaxoSmithKline laboratory) induced by MenACWY-TT lot A versus Men-PS, was demonstrated for each serogroup if the lower limit of the two-sided standardized asymptotic 95% CI for the group difference (MenACWY-TT lot A value minus Men-PS value) in the percentage of subjects with VRs was at least −10%. A VR was defined as a postvaccination rSBA titer of at least 1:32 for initially seronegative subjects (rSBA titer of <1:8) and a 4-fold increase in the rSBA titer for initially seropositive subjects (rSBA titer of ≥1:8).
The noninferiority of MenACWY-TT lot A versus Men-PS with respect to rSBA GMTs tested by the HPA was evaluated through computation of the 95% CIs of the rSBA GMT ratios (Men-PS/ACWY-A GMT ratios) using an ANCOVA model on the log10 transformation of the titers, including the vaccine group as a fixed effect and using the prevaccination log10 transformation of the titers and the country as covariates. The noninferiority of ACWY-A versus Men-PS was demonstrated if the upper limit of the 95% CI for the GMT ratio was lower than 2.
In the exploratory analysis, the two groups were considered to be statistically significantly different in terms of percentages of subjects with titers above the specified cutoff values if the 95% CI for the difference in rates between the two vaccine groups did not contain the value 0. Two groups were considered to be statistically significantly different in terms of antibody titers if the 95% CI for the GMT ratio between the two groups did not contain the value 1. Exploratory analyses were not adjusted for multiplicity; therefore, the results should be interpreted cautiously.
The percentages of subjects reporting each solicited local or general symptom (any grade, grade 3, and [for general symptoms] related and grade 3 related symptoms), unsolicited AEs (grade 3, related, and grade 3 related), SAEs, and cases of NOCI were tabulated with exact 95% CIs. SAEs and withdrawals due to AEs were described in detail. All statistical analyses were performed using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA) and StatXact 7.0 (Cytel, Cambridge, MA).
RESULTS
Study subjects.
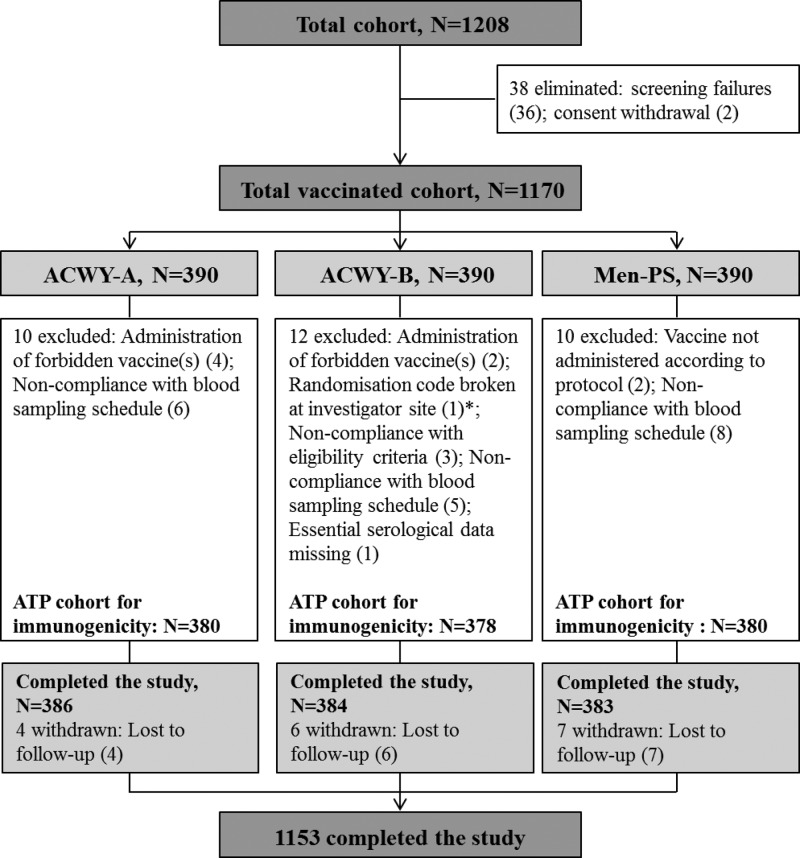
Of the 1,172 subjects enrolled, 1,170 were vaccinated. Subjects were distributed equally among the three study centers (130 subjects per center per treatment group). A total of 1,153 subjects completed the study; all 17 withdrawn subjects were lost to follow-up after receipt of the study vaccine (Fig. 1). The demographic characteristics were comparable between the study groups with respect to mean age and racial distribution. There were more female versus male subjects in the ACWY-B group than in the other groups (Table 1). Almost all subjects recruited from Thailand and the Philippines were South-East Asian, whereas the majority of subjects from Panama were of Hispanic and mixed racial backgrounds. There was no major difference in the ages of subjects recruited from the different countries. More females than males were recruited from Panama (52.6%) and Thailand (59.0%), whereas the reverse was true for the Philippines (49.0%) (see Table S1 in the supplemental material).
Fig 1.
Subject flow chart showing the number of subjects enrolled, the number who completed the study, and the reasons for exclusion from the ATP cohort for immunogenicity. *, the randomization code was broken at the investigator site (to determine which vaccine had been administered) for one subject because the subject experienced a serious adverse event (blighted ovum) that was assessed by the investigators as possibly being related to the study vaccine. ACWY-A, subjects vaccinated with MenACWY-TT lot A; ACWY-B, subjects vaccinated with MenACWY-TT lot B; Men-PS, subjects vaccinated with Men-PS; ATP, according-to-protocol; N, total number of subjects. Numbers in parentheses indicate numbers of subjects.
Table 1.
Summary of demographic characteristics (total vaccinated cohort)
| Characteristics | ACWY-Aa | ACWY-B | Men-PS |
|---|---|---|---|
| No. | 390 | 390 | 390 |
| Age (mean ± SD) (yr) | 20.8 ± 2.14 | 20.9 ± 2.10 | 20.6 ± 1.94 |
| Gender (n [%]) | |||
| Female | 197 (50.5) | 225 (57.7) | 204 (52.3) |
| Male | 193 (49.5) | 165 (42.3) | 186 (47.7) |
| Race (n [%]) | |||
| Asian, southeast Asian heritage | 258 (66.2) | 259 (66.4) | 257 (65.9) |
| Asian, east Asian heritage | 2 (0.5) | 1 (0.3) | 2 (0.5) |
| Asian, central/south Asian heritage | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| Asian, Japanese heritage | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| African heritage/African-American | 16 (4.1) | 13 (3.3) | 17 (4.4) |
| White, Caucasian/European heritage | 13 (3.3) | 14 (3.6) | 13 (3.3) |
| White, Arabic/North African heritage | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Otherb | 100 (25.6) | 102 (26.2) | 100 (25.6) |
ACWY-A, subjects vaccinated with MenACWY-TT lot A; ACWY-B, subjects vaccinated with MenACWY-TT lot B; Men-PS, subjects vaccinated with Men-PS.
Hispanic, indigenous, Kuna (indigenous), Latin, mixed, or Panamanian Lebanese.
Immunogenicity. (i) Assays performed at GlaxoSmithKline.
The primary criterion to demonstrate the noninferiority of the immunogenicity of MenACWY-TT lot A (68% O-acetylation of serogroup A PS) versus lot B (92% O-acetylation of serogroup A PS) at 1 month after vaccination was reached, as the upper limits of the 95% CIs of the rSBA GMT ratios (ACWY-B over ACWY-A) were below the limit of 2 for all serogroups. Exploratory analyses suggested that the postvaccination rSBA GMTs for serogroup Y were statistically significantly higher in the ACWY-A group than in the ACWY-B group (Table 2).
Table 2.
Adjusted ACWY-B/ACWY-A GMT ratios for MenA, MenC, MenW-135, and MenY titers 1 month after vaccination, with the GlaxoSmithKline rSBA assay (ATP cohort for immunogenicity)
| Antibody | ACWY-Ba |
ACWY-A |
Adjusted GMT ratio (ACWY-B/ACWY-A) (95% CI)b | ||
|---|---|---|---|---|---|
| N | Adjusted GMT | N | Adjusted GMT | ||
| MenA | 298 | 5,195.3 | 302 | 4,997.0 | 1.04 (0.92–1.17)c |
| MenC | 342 | 6,874.5 | 346 | 5,988.0 | 1.15 (0.96–1.37)c |
| MenW-135 | 327 | 9,202.5 | 338 | 10,115.0 | 0.91 (0.80–1.04)c |
| MenY | 353 | 10,339.0 | 358 | 11,757.9d | 0.88 (0.78–0.99)c |
ACWY-A, subjects vaccinated with MenACWY-TT lot A; ACWY-B, subjects vaccinated with MenACWY-TT lot B; N, number of subjects with both pre- and postvaccination results available; adjusted GMT, geometric mean antibody titer adjusted for country and baseline titer.
CI, confidence interval. The ANCOVA model included adjustment for country and baseline titer, with pooled variance.
The upper limit of the 95% CI is below the noninferiority limit of 2.
Statistically significantly higher value in the ACWY-A group than in the ACWY-B group (exploratory analysis).
The primary criterion to demonstrate the noninferiority of MenACWY-TT lot A versus Men-PS in terms of VRs was reached; for each serogroup separately, the lower limit of the 95% CI for the group difference (ACWY-A minus Men-PS) was greater than the predefined limit of −10%. Exploratory analyses suggested that rSBA VR rates for serogroups W-135 and Y were statistically significantly higher in the ACWY-A group than in the Men-PS group (Table 3).
Table 3.
Differences between the ACWY-A and Men-PS groups in the percentages of subjects with rSBA vaccine responses to antibodies 1 month after vaccination, with the GlaxoSmithKline rSBA assay (ATP cohort for immunogenicity)
| Antibody | No. (% with VR) with available resultsa |
Difference in vaccine response rate (ACWY-A − Men-PS) (% [95% CI]) | |
|---|---|---|---|
| ACWY-A | Men-PS | ||
| MenA | 302 (79.1) | 293 (73.7) | 5.42 (−1.41 to 12.25)b |
| MenC | 346 (93.6) | 353 (94.1) | −0.41 (−4.11 to 3.25)b |
| MenW-135 | 338 (97.0)c | 337 (90.2) | 6.83 (3.28–10.78)b |
| MenY | 358 (93.3)c | 357 (86.3) | 7.02 (2.63–11.58)b |
ACWY-A, subjects vaccinated with MenACWY-TT lot A; Men-PS, subjects vaccinated with Men-PS; CI, confidence interval (standardized asymptotic). Vaccine responses were defined as follows: for initially seronegative subjects (rSBA titer of <1:8), postvaccination titer of ≥1:32; for initially seropositive subjects (rSBA titer of ≥1:8), postvaccination titer ≥4 times the prevaccination antibody titer.
The lower limit of the 95% CI for the group difference between the ACWY-A and Men-PS groups is above the noninferiority limit of −10%.
Statistically significantly higher value in the ACWY-A group than in the Men-PS group (exploratory analysis).
At 1 month after vaccination, VR rates for each of the four serogroups ranged from 79.1% to 97.0% in the MenACWY-TT groups and from 73.7% to 94.1% in the Men-PS group (Table 4). The percentages of subjects with rSBA titers of ≥1:8 for the four serogroups increased to 100% in all three groups. All subjects in the MenACWY-TT groups and 99.5 to 100% of subjects in the Men-PS group achieved rSBA titers of ≥1:128 (Table 4).
Table 4.
Percentages of subjects with rSBA titers of ≥1:8 and ≥1:128, GMTs (prior to and 1 month after vaccination), and vaccine responses, with the GlaxoSmithKline rSBA assay (ATP cohort for immunogenicity)
| Antibody and groupa | Time point | No. of subjects with available results | % (95% CI) of subjects with rSBA titers of: |
GMT (95% CI)b | No. (% with VR [95% CI]) of subjects with pre- and postvaccination results availablec | |
|---|---|---|---|---|---|---|
| ≥1:8 | ≥1:128 | |||||
| MenA | ||||||
| ACWY-A | Prevaccination | 320 | 92.5 (89.0–95.1) | 85.0 (80.6–88.7) | 348.4 (293.0–414.2) | |
| Postvaccination | 359 | 100 (99.0–100) | 100 (99.0–100) | 4,846.0 (4,459.8–5,265.7)d | 302 (79.1 [74.1–83.6]) | |
| ACWY-B | Prevaccination | 328 | 95.7 (92.9–97.6) | 89.0 (85.1–92.2) | 401.4 (347.2–464.1) | |
| Postvaccination | 343 | 100 (98.9–100) | 100 (98.9–100) | 5,064.6 (4,657.5–5,507.2)d | 298 (79.9 [74.9–84.3]) | |
| Men-PS | Prevaccination | 310 | 95.2 (92.1–97.3) | 88.7 (84.6–92.0) | 424.4 (362.9–496.5) | |
| Postvaccination | 356 | 100 (99.0–100) | 100 (99.0–100) | 3,421.0 (3,134.7–3,733.4) | 293 (73.7 [68.3–78.7]) | |
| MenC | ||||||
| ACWY-A | Prevaccination | 351 | 60.1 (54.8–65.3) | 37.9 (32.8–43.2) | 36.3 (29.3–45.0) | |
| Postvaccination | 374 | 100 (99.0–100) | 100 (99.0–100) | 6,025.4 (5,269.0–6,890.2) | 346 (93.6 [90.5–96.0]) | |
| ACWY-B | Prevaccination | 350 | 60.9 (55.5–66.0) | 42.9 (37.6–48.2) | 44.7 (35.6–56.2) | |
| Postvaccination | 370 | 100 (99.0–100) | 100 (99.0–100) | 7,070.7 (6,225.3–8,030.9) | 342 (95.6 [92.9–97.5]) | |
| Men-PS | Prevaccination | 361 | 62.0 (56.8–67.1) | 44.0 (38.9–49.3) | 46.9 (37.2–59.0) | |
| Postvaccination | 372 | 100 (99.0–100) | 99.5 (98.1–99.9) | 5,953.1 (5,188.2–6,830.7) | 353 (94.1 [91.0–96.3]) | |
| MenW-135 | ||||||
| ACWY-A | Prevaccination | 343 | 86.3 (82.2–89.8) | 71.1 (66.0–75.9) | 185.9 (153.1–225.6) | |
| Postvaccination | 375 | 100 (99.0–100) | 100 (99.0–100) | 9,836.7 (8,939.2–10,824.3)d | 338 (97.0 [94.6–98.6])d | |
| ACWY-B | Prevaccination | 333 | 85.9 (81.7–89.4) | 72.1 (66.9–76.8) | 191.2 (155.9–234.4) | |
| Postvaccination | 370 | 100 (99.0–100) | 100 (99.0–100) | 8,855.5 (8,021.8–9,775.9)d | 327 (95.4 [92.5–97.4])d | |
| Men-PS | Prevaccination | 345 | 86.4 (82.3–89.8) | 70.7 (65.6–75.5) | 205.5 (167.6–252.0) | |
| Postvaccination | 372 | 100 (99.0–100) | 99.7 (98.5–100) | 4,675.1 (4,145.9–5,272.0) | 337 (90.2 [86.5–93.2]) | |
| MenY | ||||||
| ACWY-A | Prevaccination | 363 | 92.3 (89.0–94.8) | 84.6 (80.4–88.1) | 387.5 (324.9–462.2) | |
| Postvaccination | 375 | 100 (99.0–100) | 100 (99.0–100) | 11,632.5 (10,675.1–12,675.8)d | 358 (93.3 [90.2–95.7])d | |
| ACWY-B | Prevaccination | 359 | 94.2 (91.2–96.3) | 85.8 (81.7–89.2) | 426.8 (362.2–502.8) | |
| Postvaccination | 371 | 100 (99.0–100) | 100 (99.0–100) | 10,386.7 (9,557.5–11,287.9)d | 353 (91.8 [88.4–94.4])d | |
| Men-PS | Prevaccination | 364 | 92.3 (89.1–94.8) | 86.0 (82.0–89.4) | 412.6 (346.8–490.8) | |
| Postvaccination | 373 | 100 (99.0–100) | 100 (99.0–100) | 6,315.9 (5,787.9–6,892.1) | 357 (86.3 [82.3–89.7]) | |
ACWY-A, subjects vaccinated with MenACWY-TT lot A; ACWY-B, subjects vaccinated with MenACWY-TT lot B; Men-PS, subjects vaccinated with Men-PS; CI, confidence interval; prevaccination, month 0; postvaccination, month 1.
The geometric mean antibody titers have not been adjusted for baseline titers or country.
Vaccine responses were defined as follows: for initially seronegative subjects (rSBA titer of <1:8), postvaccination titer of ≥1:32; for initially seropositive subjects (rSBA titer of ≥1:8), postvaccination titer ≥4 times the prevaccination antibody titer.
Statistically significantly higher value in the ACWY-A or ACWY-B group than in the Men-PS group (exploratory analysis).
In all groups, rSBA GMTs for each serogroup were at least 8-fold higher 1 month after vaccination than before vaccination. Exploratory analyses suggested that the rSBA GMTs for serogroups A, W-135, and Y were statistically significantly higher in the MenACWY-TT groups than in the Men-PS group 1 month postvaccination (Table 4).
The percentages of subjects with prevaccination rSBA titers of ≥1:8 for serogroup C appeared lower in Panama (41.2 to 47.8%) than in Thailand (61.1 to 67.7%) or the Philippines (69.6 to 77.4%). There were no major between-country differences in terms of percentages of subjects with pre- or postvaccination rSBA titers of ≥1:8 or ≥1:128 for the other serogroups (see Table S2 in the supplemental material).
(ii) Assays performed at the Health Protection Agency.
At 1 month after vaccination, the noninferiority of ACWY-A versus Men-PS in terms of rSBA GMTs measured by the HPA assay was demonstrated, since the upper limits of the 95% CIs of the rSBA GMT ratios (Men-PS/ACWY-A GMT ratios) were below the limit of 2 for all serogroups. In both vaccine groups, the rSBA GMTs for each serogroup were over 200-fold higher 1 month postvaccination than prevaccination. Exploratory analyses suggested that the rSBA GMTs for serogroups A, W-135, and Y were statistically significantly higher in the ACWY-A group than in the Men-PS group (see Table S3 in the supplemental material).
The VR rates for each of the four serogroups ranged from 97.8% to 98.9% in the ACWY-A group and from 95.3% to 98.1% in the Men-PS group (see Table S4 in the supplemental material). Exploratory analyses suggested that, for serogroups W-135 and Y, rSBA VR rates were statistically significantly higher in the ACWY-A group than in the Men-PS group. One month postvaccination, the percentages of subjects with rSBA titers of ≥1:8 for the four serogroups reached 98.9 to 100% in the ACWY-A group and 96.8 to 99.7% in the Men-PS group. The proportions of subjects with rSBA titers of ≥1:128 were 98.4 to 100% in the ACWY-A group and 96.2 to 98.4% in the Men-PS group. Exploratory analyses suggested that the percentages of subjects with rSBA titers of ≥1:8 for serogroups C and Y and rSBA titers of ≥1:128 for serogroups A, W-135, and Y were statistically significantly higher in the ACWY-A group than in the Men-PS group. Exploratory analyses suggested that the rSBA GMTs for serogroups A, W-135, and Y were statistically significantly higher in the ACWY-A group than in the Men-PS group 1 month postvaccination (see Table S4 in the supplemental material). Similar to the results obtained with the GlaxoSmithKline rSBA assay, there were no major differences in pre- or postvaccination seropositivity rates between the countries, aside from a trend for lower prevaccination seropositivity rates for serogroup C in Panama (data not shown).
Safety and reactogenicity.
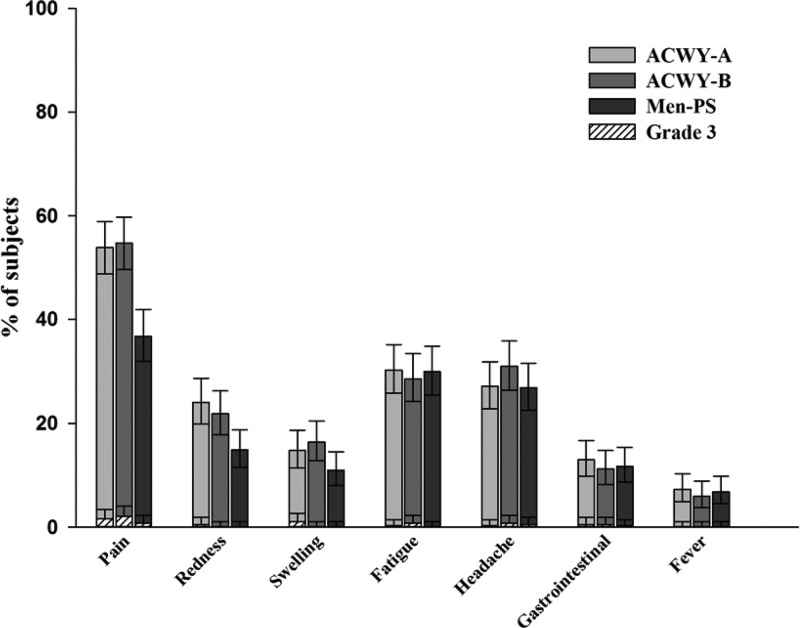
The most commonly reported solicited local symptom was pain at the injection site, which was reported by 53.9% of subjects in the ACWY-A group, 54.7% of subjects in the ACWY-B group, and 36.8% of subjects in the Men-PS group. The percentages of patients reporting pain tended to be higher in both MenACWY-TT groups than in the Men-PS group, as indicated by the nonoverlapping 95% CIs (Fig. 2). Overall, solicited local symptoms seemed to be reported more frequently in the MenACWY-TT groups than in the Men-PS group. The most common solicited general symptoms were fatigue and headache, with incidences of 28.6 to 30.3% and 26.9 to 31.0%, respectively (Fig. 2). The incidences of fever (≥37.5°C) ranged from 6.0% to 7.3% across the groups; fever above 39.5°C was not reported. The proportions of subjects reporting general symptoms were comparable for all three groups (Fig. 2). Grade 3 solicited local and general symptoms were reported by ≤2.1% of subjects.
Fig 2.
Percentages of subjects who reported local or general solicited symptoms within the 4-day postvaccination period (total vaccinated cohort). ACWY-A, subjects vaccinated with MenACWY-TT lot A; ACWY-B, subjects vaccinated with MenACWY-TT lot B; Men-PS, subjects vaccinated with Men-PS. Error bars represent 95% confidence intervals.
In all groups, the incidences of grade 3 unsolicited AEs ranged from 1.8 to 3.6%; the most commonly reported AEs were upper respiratory tract infection (0.3 to 1.0%), headache (0.3 to 0.5%), and dysmenorrhea (0.0 to 0.8%). Grade 3 unsolicited symptoms causally related to vaccination were reported by four subjects, one in the ACWY-A group (rash), one in the ACWY-B group (blighted ovum, also reported as an SAE; see below), and two in the Men-PS group (one reported migraine and one reported atopic dermatitis).
Two subjects reported SAEs, one in the ACWY-B group (blighted ovum) and one in the Men-PS group (acute appendicitis). The blighted ovum was considered by the investigators to be potentially related to the study vaccination. This event was resolved when the subject underwent dilation and curettage. The appendicitis was considered not related to the study vaccination. The episode was completely resolved after the subject was hospitalized and underwent an appendectomy. No subjects reported NOCI, and no fatal SAEs were reported during the study.
DISCUSSION
It has been shown that removal of the O-acetyl groups reduced the immunogenicity of the meningococcal serogroup A capsular PS in mice (28). The meningococcal serogroup A capsular PS is 70 to 90% O-acetylated at carbon 3, and O-acetylation is susceptible to alkaline hydrolysis (28, 32, 33). Potential differences in O-acetylation levels between different lots may occur if O-acetyl groups are partially removed in the process of PS conjugation. This study was conducted to ensure that a lower level (68%) of O-acetylation of the meningococcal serogroup A PS in MenACWY-TT vaccine lot A did not unduly affect the immunogenicity of the vaccine, compared to MenACWY-TT lot B, with a higher level of serogroup A PS O-acetylation (92%), and to a licensed PS vaccine with 82% serogroup A PS O-acetylation.
The primary criterion for noninferiority of MenACWY-TT lot A versus lot B was met. Comparable immunogenicity of the two lots of MenACWY-TT with different levels of meningococcal serogroup A capsular PS O-acetylation (68% and 92% O-acetylation) was demonstrated in terms of rSBA GMTs for all serogroups. Our results indicated that the lower level of O-acetylation of the meningococcal serogroup A capsular PS in MenACWY-TT did not result in reduced VRs. The primary criteria for noninferiority of Men-ACWY-TT (lot A) versus a licensed Men-PS vaccine in terms of VRs for meningococcal serogroups A, C, W-135, and Y were met.
At 1 month postvaccination, high VR rates for all four serogroups were observed in all groups. Moreover, when the GlaxoSmithKline rSBA assays were used, all subjects had rSBA titers of ≥1:8 and at least 99.5% had rSBA titers of ≥1:128, with large fold increases in rSBA GMTs. These observations are consistent with the results of previous studies evaluating the immune responses to MenACWY-TT in adolescents and young adults (34, 35).
The results of the immunogenicity analyses performed at the HPA laboratories were in line with those obtained with rSBA assays performed at GlaxoSmithKline. The MenACWY-TT lot with the lowest level of O-acetylation of the meningococcal serogroup A PS was shown to be noninferior to Men-PS in terms of rSBA GMTs. Furthermore, high VR rates and percentages of subjects with rSBA titers of ≥1:8 and ≥1:128 were observed in both groups. Exploratory analyses showed that the rSBA GMTs evaluated with both GlaxoSmithKline and HPA assays for meningococcal serogroups A, W-135, and Y were higher in subjects vaccinated with MenACWY-TT than in those who received the Men-PS vaccine. These results are consistent with those obtained previously for adults 18 to 55 years of age (36). Exploratory analysis done on the results obtained with the HPA assay showed that the percentages of subjects with rSBA titers of ≥1:8 for serogroups C and Y and with rSBA titers of ≥1:128 for serogroups A, W-135, and Y were statistically significantly higher among subjects vaccinated with the MenACWY-TT lot with the lowest level of O-acetylation of the meningococcal serogroup A PS than among those who received the Men-PS vaccine.
In this study, the majority of subjects were seropositive for rSBA against all four serogroups before vaccination, as shown with the GlaxoSmithKline assay, although the HPA assay indicated that approximately one-third as many subjects were seropositive for any serogroup before vaccination. Different technical parameters used in the GlaxoSmithKline assay render the assay more sensitive to naturally acquired antibodies (37). Thus, interlaboratory differences may explain the lower reported rSBA titers measured with the HPA assay than with the GlaxoSmithKline in-house assay.
High prevaccination rSBA titers have also been reported in other studies with meningococcal conjugate vaccines conducted with adults (36, 38, 39) and are consistent with the knowledge that immunity to meningococcal strains increases with age (40). It is likely that circulating meningococcal strains causing asymptomatic nasopharyngeal carriage and acquisition of functional antibodies due to natural immunity may contribute to these high observed seropositivity rates. Therefore, VR was chosen as a primary endpoint of this study, as it measures the ability of subjects to respond to the vaccine regardless of their prevaccination serostatus. Interestingly, the percentage of subjects with prevaccination serogroup C rSBA titers of ≥1:8 appeared lower for Panama than for Thailand or the Philippines. It is possible that this might result from different levels of asymptomatic carriage of serogroup C in these countries. However, as the carriage data from these countries are scarce and the prevalence of serogroup C in other parts of South America (e.g., Brazil) is high, these possible differences in prevaccination rSBA titers should be interpreted with caution (13, 41).
All vaccines administered in this study were well tolerated. The safety profile of MenACWY-TT was comparable to that observed in previous studies conducted with adolescents and young adults (42, 43). However, a lower incidence of pain at the injection site was observed in two other studies, one conducted with adolescents from India, Taiwan, and the Philippines (26.2%) (44) and the other with adults from Lebanon and the Philippines (19.4%) (36). It is possible that the lower incidence of pain observed in the two latter studies reflects cultural differences in the reporting of symptoms. Pain at the injection site tended to be reported more frequently for participants who received the MenACWY-TT vaccine than the Men-PS vaccine. This observation is consistent with previous studies showing that injection site reactions are more frequent in individuals vaccinated with MenACWY-TT versus Men-PS (35, 36, 45, 46) and those vaccinated with other quadrivalent meningococcal conjugate vaccines versus plain PS vaccines (47, 48). It is likely that the increased reactogenicity following vaccination with the MenACWY-TT vaccine is due to the TT component (49). Grade 3 unsolicited symptoms as well as unsolicited symptoms considered related to vaccination were infrequent, and only two SAEs were reported.
Potential limitations of the study include the partial blinding due to the different routes of administration for MenACWY-TT vaccine (intramuscular) and the meningococcal PS control vaccine (subcutaneous). Theoretically, this might have impacted the evaluation of safety objectives, although it is most likely that any bias would have occurred in favor of the control group. The immunogenicity evaluations are very unlikely to have been impacted by the level of study blinding, since laboratory personnel remained blinded to treatment group throughout the study. Multiplicity adjustments were not made for the numerous exploratory statistical analyses; therefore, the results of the exploratory comparisons should be interpreted cautiously.
In conclusion, this study showed that the level of meningococcal serogroup A PS O-acetylation did not affect the immunogenicity of the vaccine and the vaccine lot of MenACWY-TT with the lower level of O-acetylation was noninferior to the lot with the higher level of O-acetylation. A single dose of MenACWY-TT was noninferior to a single dose of Men-PS in terms of the VR.
Supplementary Material
ACKNOWLEDGMENTS
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and publishing of this article.
The institutes of Pornthep Chanthavanich (Mahidol University) and Nestor Sosa (Medical and Research Center) received grants from the GlaxoSmithKline group of companies. Pornthep Chanthavanich received support for meetings, travel, or accommodation expenses from the GlaxoSmithKline group of companies. Nestor Sosa is a board member of the Latin American HIV advisory board and received payment for lectures from Novartis and Pfizer. The institution of Nestor Sosa received grants from Bristol Myers Squibb and Tibotec (antiretroviral clinical trial) and from Pfizer (pneumonia clinical trial). Veronique Bianco, Yaela Baine, Marie Van der Wielen, and Jacqueline Miller are employees of GlaxoSmithKline Vaccines. Marie Van der Wielen declares stock ownership in the GlaxoSmithKline group of companies. Yaela Baine and Jacqueline Miller declare restricted shares in the GlaxoSmithKline group of companies. Socorro Lupisan and Kriengsak Limkittikul declare that they have no competing interests.
We are indebted to the study subjects, clinicians, nurses, and laboratory technicians at the study sites and to the clinical investigators for their contributions to this study. We also thank the following employees of GlaxoSmithKline Vaccines for their valuable contributions: Salvacion Gatchalian, Maria Mercedes Castrejon, and Yongyuth Wangroongsarb for assisting in coordination of the study; Emmanuel Aris and Anne Sumbul for contributing to the statistical analysis; and Koen Maleux for conducting laboratory assays. Finally, we thank Urszula Miecielica (XPE Pharma & Science) and Wouter Houthoofd and Virginie Durbecq (XPE Pharma & Science, on behalf of GlaxoSmithKline Vaccines) for providing medical writing services and editorial support in the preparation of the manuscript.
S. Lupisan, K. Limkittikul, N. Sosa, and P. Chanthavanich were involved in supervision of the study, administrative, logistic, and technical support, recruitment and medical evaluation of subjects, evaluation of reported AEs/SAEs for severity and causality, collection and interpretation of the data, and drafting and approval of the manuscript. Y. Baine, J. M. Miller, M. Van der Wielen (clinical development scientists), and V. Bianco (biostatistician) are employed by GlaxoSmithKline Vaccines and were involved in all stages of the study (study design, data analysis and interpretation, and drafting and approval of the manuscript).
Footnotes
Published ahead of print 24 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00162-13.
REFERENCES
- 1.Findlow J, Balmer P, Yero D, Niebla O, Pajon R, Borrow R. 2007. Neisseria vaccines 2007. Expert Rev. Vaccines 6:485–489 [DOI] [PubMed] [Google Scholar]
- 2.Harrison LH, Trotter CL, Ramsay ME. 2009. Global epidemiology of meningococcal disease. Vaccine 27(Suppl 2):B51–B63 [DOI] [PubMed] [Google Scholar]
- 3.Pollard AJ. 2004. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr. Infect. Dis. J. 23:S274–S279 [PubMed] [Google Scholar]
- 4.Rouphael NG, Stephens DS. 2012. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol. Biol. 799:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatami A, Pollard AJ. 2010. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev. Vaccines 9:285–298 [DOI] [PubMed] [Google Scholar]
- 6.Harrison LH, Pass MA, Mendelsohn AB, Egri M, Rosenstein NE, Bustamante A, Razeq J, Roche JC. 2001. Invasive meningococcal disease in adolescents and young adults. JAMA 286:694–699 [DOI] [PubMed] [Google Scholar]
- 7.Harrison LH. 2010. Epidemiological profile of meningococcal disease in the United States. Clin. Infect. Dis. 50(Suppl 2):S37–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens DS. 2007. Conquering the meningococcus. FEMS Microbiol. Rev. 31:3–14 [DOI] [PubMed] [Google Scholar]
- 9.Caugant DA, Maiden MC. 2009. Meningococcal carriage and disease: population biology and evolution. Vaccine 27(Suppl 2):B64–B70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan LK, Carlone GM, Borrow R. 2010. Advances in the development of vaccines against Neisseria meningitidis. N. Engl. J. Med. 362:1511–1520 [DOI] [PubMed] [Google Scholar]
- 11.Efron AM, Sorhouet C, Salcedo C, Abad R, Regueira M, Vázquez JA. 2009. W135 invasive meningococcal strains spreading in South America: significant increase in incidence rate in Argentina. J. Clin. Microbiol. 47:1979–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Gottberg A, du Plessis M, Cohen C, Prentice E, Schrag S, de Gouveia L, Coulson G, de Jong G, Klugman K, Group for Enteric Respiratory and Meningeal Disease Surveillance in South Africa 2008. Emergence of endemic serogroup W135 meningococcal disease associated with a high mortality rate in South Africa. Clin. Infect. Dis. 46:377–386 [DOI] [PubMed] [Google Scholar]
- 13.Sáfadi MAP, González-Ayala S, Jäkel A, Wieffer H, Moreno C, Vyse A. 2013. The epidemiology of meningococcal disease in Latin America 1945–2010: an unpredictable and changing landscape. Epidemiol. Infect. 141:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyse A, Wolter JM, Chen J, Ng T, Soriano-Gabarro M. 2011. Meningococcal disease in Asia: an under-recognized public health burden. Epidemiol. Infect. 139:967–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao PL, Chang LY, Hsieh SM, Chang SC, Pan SC, Lu CY, Hsieh YC, Lee CY, Dobbelaere K, Boutriau D, Tang H, Bock HL, Huang LM. 2009. Safety and immunogenicity of a tetravalent polysaccharide vaccine against meningococcal disease. J. Formos. Med. Assoc. 108:539–547 [DOI] [PubMed] [Google Scholar]
- 16.Harrison LH. 2006. Prospects for vaccine prevention of meningococcal infection. Clin. Microbiol. Rev. 19:142–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terranella A, Cohn A, Clark T. 2011. Meningococcal conjugate vaccines: optimizing global impact. Infect. Drug Resist. 4:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borrow R, Miller E. 2006. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev. Vaccines 5:851–857 [DOI] [PubMed] [Google Scholar]
- 19.Khatami A, Peters A, Robinson H, Williams N, Thompson A, Findlow H, Pollard AJ, Snape MD. 2011. Maintenance of immune response throughout childhood following serogroup C meningococcal conjugate vaccination in early childhood. Clin. Vaccine Immunol. 18:2038–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, Ala'aldeen DA, Crook DW, Cann K, Harrison S, Cunningham R, Baxter D, Kaczmarski E, Maclennan J, Cameron JC, Stuart JM. 2008. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention 2011. Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb. Mortal. Wkly. Rep. 60:1391–1392 [PubMed] [Google Scholar]
- 22.Sanofi Pasteur 2011. Menactra (meningococcal [groups A, C, Y and W-135] polysaccharide diphtheria toxoid conjugate vaccine) prescribing information. Sanofi Pasteur, Swiftwater, PA: http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm131170.pdf [Google Scholar]
- 23.Australian Government Department of Health and Ageing 2011. Australian public assessment report for groups A, C, Y and W-135 meningococcal polysaccharide diphtheria toxoid conjugate vaccine. Australian Government Department of Health and Ageing, Woden, Australia: http://www.tga.gov.au/pdf/auspar/auspar-menactra-110929.pdf [Google Scholar]
- 24.Cooper B, DeTora L, Stoddard J. 2011. MenveoR: a novel quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135 and Y. Expert Rev. Vaccines 10:21–33 [DOI] [PubMed] [Google Scholar]
- 25.Deeks ED. 2010. Meningococcal quadrivalent (serogroups A, C, W135, and Y) conjugate vaccine (Menveo): in adolescents and adults. BioDrugs 24:287–297 [DOI] [PubMed] [Google Scholar]
- 26.Lee B. 2012. Meningococcal vaccines. Novartis Vaccines and Diagnostics, Basel, Switzerland: http://orion.pleksus.com.tr/puader/v2/files/file/pdf/sunum_2012/PUADER_Congress_Meningococcal_Vaccines_LEE_FINAL.pdf. [Google Scholar]
- 27.Australian Government Department of Health and Ageing 2010. Australian public assessment report for meningococcal conjugated vaccine. Australian Government Department of Health and Ageing, Woden, Australia: http://www.tga.gov.au/pdf/auspar/auspar-menveo.pdf [Google Scholar]
- 28.Berry DS, Lynn F, Lee CH, Frasch CE, Bash MC. 2002. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 70:3707–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews N, Borrow R, Miller E. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borrow R, Balmer P, Miller E. 2005. Meningococcal surrogates of protection: serum bactericidal antibody activity. Vaccine 23:2222–2227 [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention 2006. Inadvertent misadministration of meningococcal conjugate vaccine: United States, June–August 2005. MMWR Morb. Mortal. Wkly. Rep. 55:1016–1017 [PubMed] [Google Scholar]
- 32.Jennings HJ, Bhattacharjee AK, Bundle DR, Kenny CP, Martin A, Smith IC. 1977. Structures of the capsular polysaccharides of Neisseria meningitidis as determined by 13C-nuclear magnetic resonance spectroscopy. J. Infect. Dis. 136(Suppl 1):S78–S83 [DOI] [PubMed] [Google Scholar]
- 33.Lemercinier X, Jones C. 1996. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides from Neisseria meningitidis used in vaccine production. Carbohydr. Res. 296:83–96 [DOI] [PubMed] [Google Scholar]
- 34.Dbaibo G, Van der Wielen M, Reda M, Medlej F, Tabet C, Boutriau D, Sumbul A, Anis S, Miller JM. 2012. The tetravalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine is immunogenic with a clinically acceptable safety profile in subjects previously vaccinated with a tetravalent polysaccharide vaccine. Int. J. Infect. Dis. 16:e608–e615 [DOI] [PubMed] [Google Scholar]
- 35.Ostergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. 2009. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15–25 years. Vaccine 27:161–168 [DOI] [PubMed] [Google Scholar]
- 36.Dbaibo G, Macalalad N, Aplasca-De Los Reyes MR, Dimaano E, Bianco V, Baine Y, Miller J. 2012. The immunogenicity and safety of an investigational meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate vaccine (ACWY-TT) compared with a licensed meningococcal tetravalent polysaccharide vaccine: a randomized, controlled non-inferiority study. Hum. Vaccin. Immunother. 8:873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechevin I, Le Bras V, Lestrate PR, Wauters D. 2012. Identification of key parameters that impact the sensitivity of the MenC-rSBA assay to natural antibodies, abstr P 145 Programme XVIIIth Int. Pathogenic Neisseria Conf., Würzburg, Germany, 9 to 14 September 2012 [Google Scholar]
- 38.Campbell JD, Edelman R, King JC, Jr, Papa T, Ryall R, Rennels MB. 2002. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J. Infect. Dis. 186:1848–1851 [DOI] [PubMed] [Google Scholar]
- 39.Kshirsagar N, Mur N, Thatte U, Gogtay N, Viviani S, Preziosi MP, Elie C, Findlow H, Carlone G, Borrow R, Parulekar V, Plikaytis B, Kulkarni P, Imbault N, LaForce FM. 2007. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine 25(Suppl 1):A101–A107 [DOI] [PubMed] [Google Scholar]
- 40.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibarz-Pavón AB, Lemos AP, Gorla MC, Regueira M, Gabastou JM. 2012. Laboratory-based surveillance of Neisseria meningitidis isolates from disease cases in Latin American and Caribbean countries, SIREVA II 2006–2010. PLoS One 7:e44102. 10.1371/journal.pone.0044102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. 2011. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr. Infect. Dis. J. 30:e41–e48 [DOI] [PubMed] [Google Scholar]
- 43.Ostergaard L, Silfverdal SA, Berglund J, Flodmark CE, West C, Bianco V, Baine Y, Miller JM. 2012. A tetravalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine is immunogenic and well-tolerated when co-administered with TwinrixR in subjects aged 11–17 years: an open, randomised, controlled trial. Vaccine 30:774–783 [DOI] [PubMed] [Google Scholar]
- 44.Bermal N, Huang LM, Dubey AP, Jain H, Bavdekar A, Lin TY, Bianco V, Baine Y, Miller JM. 2011. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum. Vaccin. 7:239–247 [DOI] [PubMed] [Google Scholar]
- 45.Knuf M, Kieninger-Baum D, Habermehl P, Muttonen P, Maurer H, Vink P, Poolman J, Boutriau D. 2010. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine 28:744–753 [DOI] [PubMed] [Google Scholar]
- 46.Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, Bianco V, Van der Wielen M, Gatchalian S, Miller JM. 2011. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr. Infect. Dis. J. 30:e56–e62 [DOI] [PubMed] [Google Scholar]
- 47.Black S, Klein NP, Shah J, Bedell L, Karsten A, Dull PM. 2010. Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in children 2–10 years of age. Vaccine 28:657–663 [DOI] [PubMed] [Google Scholar]
- 48.Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, Sullivan K, Gilmet G, Reinhardt A. 2005. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch. Pediatr. Adolesc. Med. 159:907–913 [DOI] [PubMed] [Google Scholar]
- 49.Wassilak SG, Roper MH, Murphy TV, Orenstein WA. 2004. Tetanus toxoid, p 766 In Plotkin SA, Orenstein WA. (ed), Vaccines, 4th ed. WB Saunders Co., Philadelphia, PA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.