Abstract
Sex pheromones provide an important means of communication to unite individuals for successful reproduction. Although sex pheromones are highly diverse across animals, these signals fulfil common fundamental roles in enabling identification of a mating partner of the opposite sex, the appropriate species and of optimal fecundity. In this review, we synthesize both classic and recent investigations on sex pheromones in a range of species, spanning nematode worms, insects and mammals. These studies reveal comparable strategies in how these chemical signals are produced, detected and processed in the brain to regulate sexual behaviours. Elucidation of sex pheromone communication mechanisms both defines outstanding models to understand the molecular and neuronal basis of chemosensory behaviours, and reveals how similar evolutionary selection pressures yield convergent solutions in distinct animal nervous systems. EMBO reports advance online publication 13 September 2013; doi:10.1038/embor.2013.140
Keywords: sex pheromone, receptor, neural circuit, behaviour, evolution
See the Glossary for abbreviations used in this article.
Glossary.
- daf
dauer formation
- NPR-1
neuropeptide receptor 1
- OR
odorant receptor
- TGF-β
transforming growth factor beta
- Vmn2r116/V2Rp5
vomeronasal 2 receptor 116
- V1R
vomeronasal receptor type 1
- V2R
vomeronasal receptor type 2
Introduction
Nearly 150 years ago, Charles Darwin and the French entomologist Jean-Henri Fabre—although disagreeing on the theory of evolution—both postulated the existence of chemical signals involved in the control of sexual behaviours [1,2]. It was only in the middle of the twentieth century, however, when the German biochemist Adolf Butenandt purified—from half a million scent glands of female silk moths (Bombyx mori)—the first sex pheromone: bombykol [3]. Despite this discovery, in many other animals the precise chemical identity and function of sex pheromones in regulating interactions between males and females have been difficult to define. The limited progress might be due, in part, to the inability of our noses to detect the sophisticated chemical conversations of other species [4].
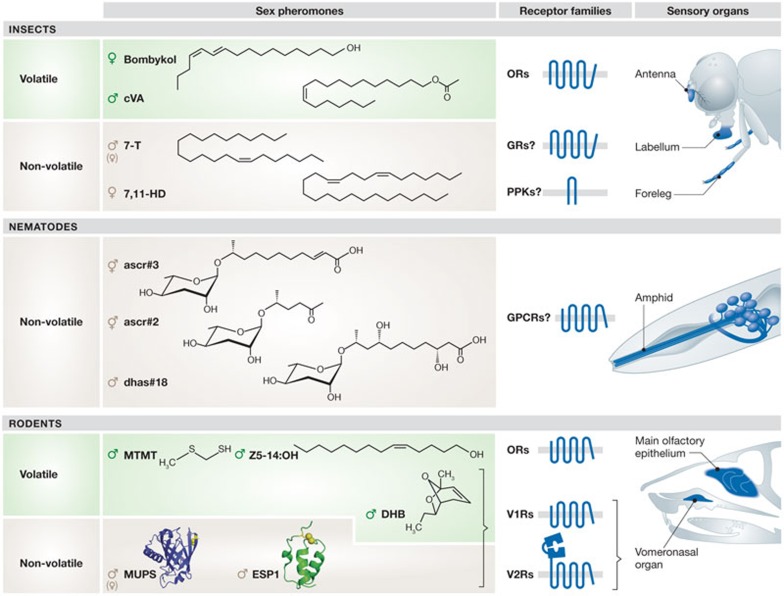
This situation has changed dramatically in the last decade as sex pheromones and receptors have been identified in many species, which have allowed visualization and manipulation of the neural circuits that link these sensory signals with particular behaviours. There is enormous diversity in the chemical nature of sex pheromones, including long-chain hydrocarbons in insects [5], ascaroside (ascr) glycolipids in nematodes [6] and peptides or small proteins in vertebrates (Fig 1; [7,8]). Consistently, the receptors for pheromones have evolved independently in these different animal groups [9,10], and are housed in different types of sensory organ (Fig 1).
Figure 1.
Diversity of sex pheromones, receptors and sensory organs in insects, nematodes and rodents. In insects, many volatile sex pheromones (for example, bombykol from the silk moth Bombyx mori and cVA in drosophilids) are long chain hydrocarbons and are detected by ORs—an unusual class of ionotropic receptors unrelated to GPCRs [108]—which are expressed in the main olfactory organ, the antenna. Non-volatile—or at least less volatile—pheromones (for example, drosophilid 7,11-HD and 7-T) are thought to be detected by GRs (structurally related to ORs) and/or PPK ion channels in the labellum and the forelegs. In nematode worms, sex pheromones are glycolipidic ascarosides and are probably detected by GPCRs expressed in amphid chemosensory neurons, similar to receptors for non-sex pheromone ascarosides. In rodents, many small, volatile sex pheromones, such as DHB, as well as non-volatile protein and peptide pheromones (for example, MUPs and ESP1) might be detected by two different families of GPCRs, V1Rs and V2Rs, respectively, in the vomeronasal organ. A different GPCR family, the ORs, expressed in the main olfactory epithelium detects volatile pheromones, such as MTMT and (Z)-5-tetradecen-1-ol (Z5-14:OH; [109]). 7,11-HD, 7,11-heptacosadiene; 7-T, 7-tricosene; cVA, cis-vaccenyl acetate; DHB, 3,4-dehydro-exo-brevicomin; ESP1, exocrine gland-secreting peptide 1; GPCR, G-protein-coupled receptor; GR, gustatory receptor; MTMT, (methylthio)methanethiol; MUP, major urinary protein; OR, odorant receptor; PPK, Pickpocket; V1R, vomeronasal receptor type 1; V2R, vomeronasal receptor type 2; Z5-14:OH, (Z)-5-tetradecen-1-ol.
Here we consider the crucial roles of sex pheromones and illustrate how diverse animals use and respond to these chemical signals to fulfil them. We focus on the best-described examples of true sex pheromones, defined as chemicals produced by an individual that elicit innate, stereotyped sexual behaviours in conspecifics [11]. We do not cover sexually relevant ‘signature mixtures’, which are complex combinations of chemicals produced by individuals that can vary within a species and must be learned by the receiver [11], or the many other well-established functions of pheromones—for example, as alarm, trail and aggregation signals [4].
Sex pheromone signalling and gender discrimination
The most important function of sex pheromones is to allow organisms to identify mating partners of the opposite gender. Predictably, chemical signals advertising gender are commonly produced in a sex-specific manner, whether male-specific, female-specific or a combination of the two. Stemming from the identification of bombykol [3], many of the best-characterized insect sex pheromones are female-specific, long-range male attractants [12,13]. This might reflect a historical, rather than taxonomic class, bias: studies of several Hemiptera (true bugs) have revealed principally male-specific sex pheromones [14,15]. Furthermore, in drosophilids, the only identified volatile pheromone is the male-specific cis-vaccenyl acetate (cVA) (Fig 1), which inhibits male–male courtship [16,17]. Non-volatile—or at least less volatile [18]—cuticular hydrocarbon pheromones in insects, detected by contact chemosensation, seem to be combinations of both male-specific and female-specific cues [13]. In Drosophila melanogaster, for example, 7,11-heptacosadiene (7,11-HD), an aphrodisiac for males, is a female-specific cuticular hydrocarbon, whilst male hydrocarbons are enriched for 7-tricosene (7-T), an anti-aphrodisiac for other males (Fig 1; [19,20]).
Nematodes have several different mating systems, including dioecy—males and females—as represented by Panagrellus redivivus, and androdioecy—self-fertilizing hermaphrodites and males—as represented by Caenorhabditis elegans. Although ascr-related sex pheromones are broadly used in nematodes (Fig 1; [6,21]), sex-specific production and responses reflect these different mating systems. For example, P. redivivus females, but not males, produce ascr#1, which is repellent for females but a strong attractant for males. P. redivivus males produce the dihydroxy ascaroside derivative dhas#18, one of the few characterized female-attractants in worms [22]. C. elegans hermaphrodites produce a different pheromone blend to attract males and, usually, to repel other hermaphrodites. Within this blend, #ascr3—also known as C9 [23]—is behaviourally the most potent [24,25]. In contrast to the dioecious species, however, no male-specific sexual attractants are known. This might be because hermaphrodites represent the overwhelming majority in C. elegans (approximately 99.5%, at least in laboratory populations), strongly favouring a male's chances of finding a mate.
In mammals, few definitive cases have been identified in which single pheromone compounds evoke robust sexual behaviours, which might reflect an important contribution of signature mixtures in sexual communication [11,26]. The most compelling example of a sex pheromone is the male-specific exocrine gland-secreting peptide 1 (ESP1; Fig 1). ESP1 is secreted into tear fluids from the extraorbital lacrimal glands [27] and promotes female sexual behaviours, such as lordosis [28]. A second strong sex pheromone candidate is the major urinary protein (MUP) darcin (also known as Mup20), a small protein present in adult male urine ([29]; Fig 1). Darcin can promote attraction of females [29] but also males [30]. In addition, darcin induces spatial learning of other chemical cues, allowing both females and competitor males to relocate sites of previous social interactions [29,30]. Other male pheromones might be volatile chemicals, such as (methylthio)methanethiol (MTMT), 3,4-dehydro-exo-brevicomin (DHB), 2-sec-butyl-4,5-dihydrothiazole (SBT) and (Z)-5-tetradecen-1-ol (Z5-14:OH); these are found in male urine and are attractive to females, either alone or in combination (Fig 1; [31,32,33]). An ESP1-related peptide of unknown function, ESP36, is expressed only in female tear glands, at least in one mouse strain [34]. However, the best candidate for a female sex pheromone in mammals is (Z)-7-dodecen-1-yl acetate in the Asian elephant, which is released in urine during oestrus. In males this compound elicits flehmen behaviour—curling back of the upper lip and inhalation with the nostrils closed—which is thought to facilitate further chemosensation by the vomeronasal organ [35]. Remarkably, this same molecule is also a female-specific pheromone in many moths, presumably reflecting convergent evolution of volatile hydrocarbon derivatives [35].
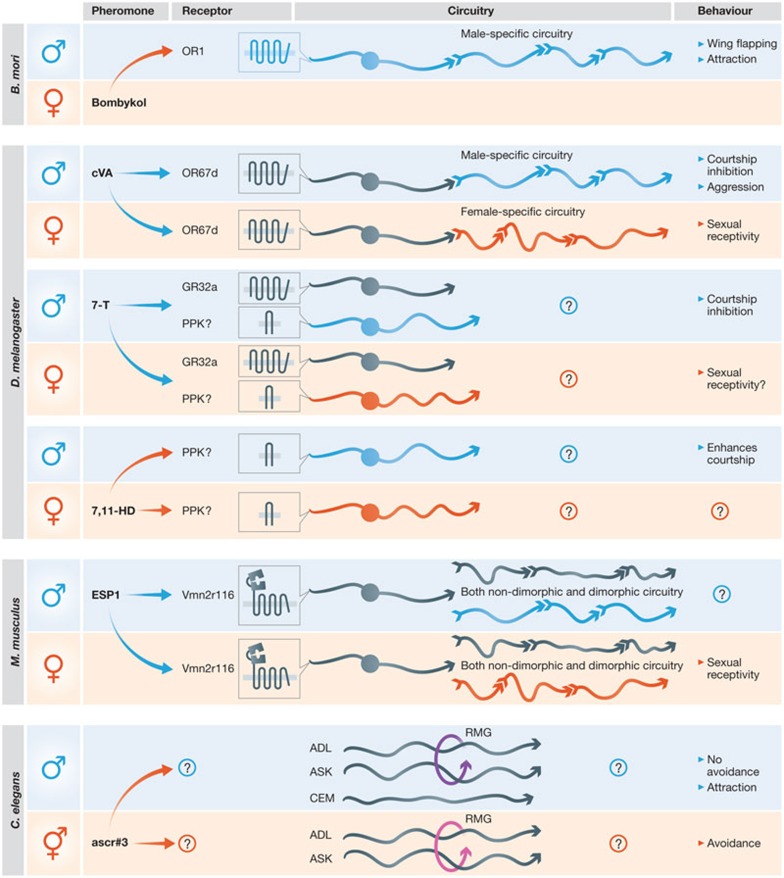
Whilst the pheromones that provide gender information are produced sex-specifically, characterization of the cognate receptors and downstream neural circuits has revealed two different strategies as to how these signals are received. In some cases, the pheromone receptor—and the sensory neurons in which it is expressed—are found exclusively in the opposite sex, rendering individuals of the same sex ‘blind’ to these stimuli. For example, several moth receptors for female pheromones are expressed only in male olfactory organs (antennae), including the Bombyx mori bombykol receptor OR1 (Fig 2; [36]). Such sensory dimorphism provides a simple way to couple precisely a pheromone to sex-specific behavioural responses. In B. mori, males respond to a female pheromone through a series of behaviours, including wing-flapping, orientation and attempted copulation. Remarkably, artificial activation of BmOR1-expressing neurons—by transgenic manipulation of B. mori to express ectopically a pheromone receptor from a different moth species and presentation of its cognate ligand—is sufficient for full induction of these sexual behaviours [37]. Mapping of the neural circuitry responsible for converting detection of a single chemical cue into this range of behaviours will be an exciting, albeit challenging, goal.
Figure 2.
Sexually dimorphic pheromone processing. Sex pheromones induce gender-specific behaviours through different neural processing strategies. In some cases, such as the female sex pheromone in the silk moth B. mori, the cognate receptor is expressed only in the opposite sex. In others, sex-specific pheromones are detected by both sexes but processed differently—either peripherally or centrally—to produce distinct behavioural outputs. cVA, in Drosophila melanogaster, and ESP1 in the mouse, are detected by both sexes but might evoke distinct behaviours through sexually dimorphic circuitry (blue: male-specific; red: female-specific) in the central brain. Cuticular hydrocarbon pheromones in D. melanogaster (for example, 7,11-HD and 7-T) are detected by circuits that have sexual dimorphisms in sensory neuron projections. In C. elegans, ascaroside ascr#3 evokes avoidance in hermaphrodites and attraction in males, in part through a male-specific sensory neuron (CEM) and in part through differential processing of sensory signals from neurons present in both sexes (ASL, ASK) by the RMG interneuron. In many of these examples, the identity of the pheromone receptors is unknown. 7,11-HD, 7,11-heptacosadiene; 7-T, 7-tricosene; ascr, ascaroside; cVA, cis-vaccenyl acetate; ESP1, exocrine gland-secreting peptide 1; GR, gustatory receptor; OR, odorant receptor; PPK, pickpocket.
In other cases, sex-specific pheromones are detected by both genders, and can elicit distinct behaviours. The D. melanogaster male-specific pheromone cVA is detected by OR67d, which is expressed in both male and female antennae (Fig 2; [16,38]). In males, activation of OR67d by cVA inhibits courtship behaviour, thereby preventing males from fruitlessly courting other males. Similarly to B. mori OR1-expressing neurons, artificial activation of OR67d neurons is also sufficient to suppress courtship [16]. In females, activation of OR67d neurons is necessary for full sexual receptivity [16]. How cVA can reduce sexual behaviour in males, but increase it in females is an intriguing problem. OR67d-expressing sensory neurons have largely non-dimorphic anatomical and physiological properties. However, higher-order neurons have sexually dimorphic connectivity [39,40], which might underlie sex-specific behavioural responses to the same pheromone (reviewed in [41]).
Discrete sensory neuron populations for D. melanogaster cuticular hydrocarbon sex pheromones of females (7,11-HD) and males (7-T) have been identified. These express different combinations of Pickpocket (PPK) receptors (Fig 2; [42,43,44,45,46]). PPKs belong to the degenerin/epithelial sodium channel class of ion channels, but whether these are the pheromone receptors themselves is unclear. A second, apparently distinct, population of sensory neurons for 7-T has been described, which express the gustatory receptors GR32a and GR33a [47,48]. All of these sets of neurons are present in male and female labella as well as in their forelegs, with which flies ‘taste’ mating partners (Fig 1). Importantly, the PPK-expressing neurons have sexually dimorphic projection patterns (Fig 2; [42,43,44,45,46]). Physiological and behavioural analysis of these neurons has so far been restricted to males [20,42,46,47,48], but these anatomical observations raise the possibility that females also detect these sex pheromones, but process and respond to them differently. Indeed, females show greater receptivity to males perfumed with additional 7-T [49]. Beyond studies of the roles of individual sex pheromones in D. melanogaster, recent work has also begun to explore how volatile and contact chemical cues are integrated to reinforce or refine selection of the right gender for mating [50,51,52], although the underlying neural basis for sensory integration remains obscure.
Anatomical evidence for sex-specific pheromone processing has also come from studying mouse ESP1. Although exclusively male-specific, this pheromone is recognized by the receptor Vmn2r116—also known as V2Rp5—expressed in vomeronasal organ neurons present in both males and females (Fig 2; [28]). Consistently, ESP1 exposure activates neurons in the vomeronasal organ and several higher centres of the vomeronasal system in both genders [28]. However, at least some ESP1-evoked activity is sexually dimorphic; for example, only females show responses in the posteromedial cortical amygdaloid nucleus and the ventromedial hypothalamic nucleus. ESP1 promotes sexual receptivity in females, notably lordosis behaviour [28]. Although the effect of this peptide on males has yet to be described, the differential activation patterns in the brain are suggestive that this pheromone, as with cVA in D. melanogaster, could evoke sex-specific behaviours.
In C. elegans, dissection of the neural pathways underlying detection of the hermaphrodite pheromone ascr#3 has revealed a combination of sex-specific sensory detection and central processing properties, which might explain why this pheromone is attractive to males but aversive to hermaphrodites (Fig 2; [23]). ascr#3 stimulates, through an unidentified receptor, the ADL class of sensory neurons in hermaphrodites; these neurons are well-characterized, multi-functional noxious stimuli detectors and their activation promotes avoidance behaviour—although this response can be modulated, as described below [23]. By contrast, males—who also possess ascr#3-sensitive ADL neurons—are attracted by low concentrations of ascr#3 [24]. This marked switch in valence of behavioural response has been related to three sexually dimorphic neural properties. First, ADL physiological responses to pheromone are reduced in magnitude and temporally delayed in males [23]. Second, another class of ascr3#-responsive neurons, ASK, antagonize ADL-mediated avoidance in males, but not hermaphrodites, through the RMG interneuron—a ‘hub’ that integrates many sensory cues [23,53]. Finally, a third, male-specific neuron, CEM, is necessary for attraction to ascr#3, although it is unknown whether it is directly activated by this pheromone [24]. Thus, both peripheral and central dimorphisms, in part anatomical and in part neurophysiological, seem to underlie the sex-specific behavioural responses to ascr#3.
Despite the relatively incomplete knowledge of the identity and function of sex pheromones in any animal species, these examples indicate that gender identification depends on sex-specific pheromonal cues produced by both males and females. These might indicate both whom to and whom not to mate with, through sexually dimorphic peripheral and central neural pathways. Several striking observations suggest that sex-specific behaviours might be founded on relatively limited differences in these sensory circuits. In insects, female tobacco hornworm moths (Manduca sexta) grafted with male antennae follow plumes of female pheromones (detected by the male-specific receptors; [54]). In C. elegans, mutation of a single developmental regulator, daf-7, encoding a TGF-β related protein, is sufficient to render hermaphrodites attracted to pheromones that are normally only attractive to males [55]. Finally, female mice with impaired vomeronasal signal transduction display not only reduced female-specific behaviours but also new behaviours characteristic of males, such as mounting and pelvic thrusting [56]. These studies reveal the existence of ‘latent’ neural pathways capable of driving behaviours normally shown only by the opposite sex. Such properties might reflect the evolution of sex pheromone-sensing circuits from non-dimorphic pathways through relatively limited developmental modifications.
Discrimination of species, strains and individuals
Successful reproduction also requires the correct matching of conspecifics or, in some cases, individuals of particular strains within species. Many sensory modalities contribute to prevent cross-species and strain mating, including visual and auditory cues, as well as ecological and habitat constraints, which can depend on non-pheromonal chemosensory adaptations [57]. However, sex pheromone signals might be of particular importance to permit species discrimination—both for sexual and non-sexual behaviours—in closely related, morphologically similar species that occupy overlapping niches. Sex pheromones are easily ‘mutable’, whether by changes in the function or expression of enzymes required for their biosynthesis, or—for peptide/protein pheromones—changes in their sequence. This flexibility can provide a rapid way to evolve private communication between members of the same species, strain or even individuals [57].
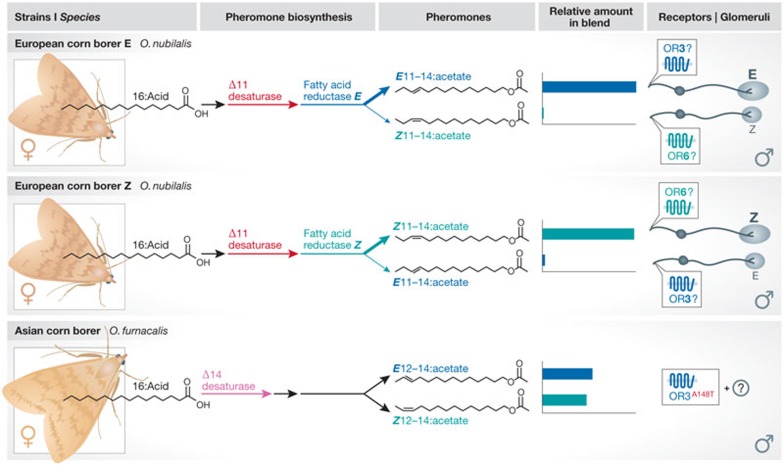
Moths offer the best-described examples of sex pheromone-dependent discrimination in phylogenetically close species (reviewed in [57]). Female pheromones typically comprise a species-specific blend of chemicals rather than just a single pheromone. Unique identity is conferred by varying minor components of the blend, or by varying the ratio between components. The latter strategy is exemplified by the European corn borer moth (Ostrinia nubilalis), in which two sympatric strains (referred to as E and Z ‘races’) produce pheromone blends with opposite ratios of isomers of the major female sex pheromone components, E11–14:acetate and Z11-14:acetate (Fig 3; [58]). The genetic basis for this variation has been mapped to allelic variation in a single gene that encodes a fatty-acyl reductase required for pheromone biosynthesis [59]. Such apparently simple changes have a powerful behavioural influence: in the laboratory, these two strains can be mated to produce fertile offspring, but in the wild they do not freely interbreed. The distinct behavioural sensitivity to these pheromone blends is correlated with changes in the peripheral physiological sensitivity of two populations of olfactory sensory neurons that sense these two isomers [60], which could potentially be achieved by a simple switch in OR expression (Fig 3). The molecular basis of this strain specificity remains to be fully worked out. In heterologous cells, several Ostrinia ORs respond to pheromone components, such as OR6, which recognizes Z11–14:acetate, and OR3, which is broadly tuned to several pheromone components, including E11–14:acetate [60,61]. However, precise matching of ORs to specific neuronal populations to firmly link in vitro and in vivo responses awaits. A distinct Ostrinia species, the Asian corn borer (O. furnacalis), has evolved a pheromone with a small but significant change in the position of the double bond in the hydrocarbon tail (E/Z12–14:acetate), by using a Δ14-desaturase (Fig 3; [62]). Interestingly, the Asian corn borer OR3 orthologue has drastically reduced sensitivity to the European corn borer pheromone component E11–14:acetate, which can be accounted for by a single amino acid polymorphism (A148T; [63]). This receptor mutation might therefore have contributed to the process of speciation by reducing the efficiency of crossbreeding between the ancestors of O. nubilalis and O. furnacalis.
Figure 3.
Species- and strain-specific pheromone recognition. The European corn borer Ostrinia nubilalis exists as two distinct strains (or races) ‘E’ and ‘Z’. The female sex pheromone blends of these strains contain different ratios of E and Z isomers of the 11–14:acetate pheromone component, due to allelic differences in a fatty acid reductase. The underlying molecular basis of pheromone recognition in vivo is not clear, but in heterologous cells OR3 responds to several pheromone components (including E11–14:acetate), whilst OR6 is selectively tuned to Z11–14:acetate. In both strains, the major and minor pheromone components activate the larger and smaller glomeruli, respectively, within the pheromone processing centre in the brain, indicating that a switch in peripheral receptor expression might have occurred between strains. A distinct species, the Asian corn borer O. furnacalis uses a different desaturase enzyme during pheromone biosynthesis to produce a structurally distinct E/Z12–14:acetate pheromone mixture. In this species, the OR3 orthologue has greater specificity for 12–14:acetate pheromone compounds, which can be explained by a single amino acid mutation, A148T. OR, odorant receptor.
Species-specific cuticular hydrocarbon blends are well-described in drosophilids. For example, D. melanogaster females produce a set of dienes (including 7,11-HD) that are distinct from those found in D. erecta females, and essentially absent from other species, such as D. simulans [19]. As in moths, such pheromone variation might have a relatively simple genetic basis, with rapidly evolving fatty acid desaturases being prominent candidates [64,65]. Genetic ablation of pheromone-producing cells (oenocytes) in D. melanogaster leads to these females being courted inappropriately by D. erecta and D. simulans males, indicating that these dienes help species discrimination [50]. Artificially perfuming these D. melanogaster females with 7,11-HD is sufficient to prevent their courtship by D. simulans males, thereby restoring this species barrier [50]. It is noteworthy that this single pheromone seems to act both to promote male courtship within D. melanogaster (through PPK-expressing foreleg neurons, as described above) and to inhibit interspecific courtship through an unknown sensory pathway. Formally, this latter function of 7,11-HD in signalling between species is not as a pheromone. A recent study implicated other cuticular hydrocarbon sex pheromones, including 7-T, in preventing male (but not female) D. melanogaster from mating with other species, such as D. virilis [66]. Here, these signals seem to act through GR32a-expressing neurons in the male forelegs. As described above, this same pheromone and sensory neuron population have also been implicated in preventing inappropriate male–male courtship in D. melanogaster [47,48,51]. However, the central neural circuits underlying these behaviours seem to be different [66]. The intertwined relationship of pheromones specifying sex and species discrimination in drosophilids is intriguing and prompts consideration of whether this is a general phenomenon in animals.
Many other insects (such as ants [67] and beetles [68]), as well as various nematodes [21,69] produce species-specific chemical blends. In most cases it is unclear whether these include true pheromones or whether these blends are more appropriately classified as signature mixtures [11]. Regardless, little is known about their role (if any) in determining correct sexual pairing of conspecifics or strains. Insect sex pheromone blends do seem to provide signals to distinguish inbred and outbred individuals: inbred male African butterflies (Bicyclus anynana; [70]) and mealworm beetles (Tenebrio molitor; [71]) produce chemical signals that are much less attractive to female conspecifics than those produced by outbred animals. Although the mechanism by which inbreeding alters pheromonal blends is unclear, this phenomenon might be advantageous to species by minimizing inbreeding depression within populations [72].
In mammals, clear evidence for sex pheromones in defining species recognition is also lacking. However, the two known male pheromones, ESP1 and the MUP darcin, belong to larger families that display both inter- and intra-species genomic diversity [34,73], raising the possibility that these proteinaceous pheromones could provide this type of information. Indeed, MUPs have been implicated as chemical signals that allow the recognition of genetic heterozygosity—a sign of phenotypic vigour—and enable avoidance of inbreeding, as well as in the distinction of individuals of the same or different species [74,75,76,77]. Moreover, some of these proteins can (directly or indirectly) stimulate neurons in the vomeronasal organ [74,78]. Disentangling precisely which MUPs are important, and through which sensory pathways they act, remain important unsolved problems. A third genetically variable family, the major histocompatibility complex (MHC) class I receptors, has also been implicated in defining individual identity [79] (however, by contrast, see [75]). As part of their role in the immune system, these receptors present short peptides (derived from endogenous proteins) on the cell surface. Unexpectedly, such peptides, synthesized in vitro, can activate neurons both in the vomeronasal organ and main olfactory epithelium [80,81]. These observations prompted the hypothesis that the MHC genotype of an animal could define a repertoire of associated peptides potentially available to act as pheromonal cues [79]. The natural source of such peptides is, however, unclear as recent proteomic analysis of mouse urine—a rich source of behaviourally relevant chemical cues—identified only one MHC-dependent peptide [82]. Nevertheless, this analysis identified in urine many other genetically variable MHC-independent peptides, which also activated vomeronasal neurons [82]. Although biological functions for such ligands are unknown, the blends of genetically variable peptides—analogous to the chemically diverse hydrocarbon profiles of insects—could potentially provide precise information on species, strain and even individuals during mate choice.
Discrimination of age, fecundity and mating status
Although sex pheromones, by definition, evoke innate, stereotyped sexual behaviours, there can be substantial plasticity in pheromone production and the responses they evoke to provide greater nuance in how these chemicals control interactions between conspecific males and females. The best-documented role for plasticity in sex pheromone signalling is in indicating the age of individuals, a crucial property to ensure mating occurs only after sexual maturation, but before the onset of senescence. For example, in nematodes, sex pheromone production by Caenorhabditis remanei females or by C. elegans hermaphrodites peaks in young adults [69,83]. Notably, in C. remanei, the attraction of male worms to this pheromone also reaches maximum levels at this time [69]. Similarly, in the noctuid moth Agrotis ipsilon, male behavioural responses to female pheromones are observed only 3–5 days after eclosion. This maturation correlates with increased physiological responses of olfactory interneurons to pheromones but not to plant volatiles [84,85]. Finally, in mice, ESP1 expression is not observed before four weeks of age [27]—the time when these animals become sexually mature—and initiation (but not the maintenance) of expression is dependent on the male sex hormone testosterone [34].
Pheromone levels can also change in sexually mature adults. In D. melanogaster, cuticular hydrocarbon profiles of both males and females vary with age in adults [86,87]. Interestingly, the aphrodisiac 7,11-HD declines over time, which might underlie, in part, a preference of males for younger females [86]. Youthfulness is not always favoured, however: in the European corn borer moth O. nubilalis, females have a mating preference for older males [88], and females of the African butterfly B. anynana prefer middle-aged (14-day old) over young (three-day old) males [89]. In both cases, these inclinations correlate with changes in proportions of the main components of the male sex pheromone blend. Furthermore, perfuming young B. anynana with synthetic ‘young’ and ‘old’ male pheromone blends is sufficient to recapitulate the difference in attractiveness, suggesting a direct role for age-dependent pheromone signals in controlling mating success [89].
Ageing also influences production of pheromones, or putative pheromones, in mammals [90,91,92]. In inbred male mice the urine levels of some androgen-dependent urinary volatiles are increased in middle-aged compared with young adult animals. These changes seem to be behaviourally relevant as female mice are more attracted to the urine of middle-aged animals. The precise pertinent cues are unclear, but MUPs and volatiles bound by MUPs are good candidates, as female preference is abolished when urine is depleted of these proteins [91]. Consistent with this hypothesis, during senescence, male urine becomes less attractive to females and shows declining MUP levels [92], reflecting a potentially honest signal of decreasing fecundity.
In addition to the progressive increases or decreases in sex pheromone signalling that occur with age, shorter-term variation in sex pheromone production and/or responses has been described in several species, including diurnal fluctuations [93,94,95], seasonal changes [96,97] and, in mammals, during the female reproductive cycle [35,98]. Although specific adaptive advantages of these variations are often easy to accommodate into models reflecting the lifestyle and sexual behaviour of individual species, they are often difficult to prove experimentally as many other phenotypic traits change coordinately over these timescales.
A second major influence on sex pheromone signalling is mating itself. In many species, the chemical profile of the female changes depending on their mating status (reviewed in [99]). This phenomenon has been mostly characterized in insects. For example, in some tortricid moths, mated females suppress production of pheromones that would attract new suitors [100]. In many species, males ‘apply’ new pheromones to females during mating. In D. melanogaster, two male-specific pheromones, cVA and CH503, are transferred to the female during copulation through the seminal fluid, thereby marking her non-virginal status. cVA suppresses further courtship of the mated female by other males through OR67d [16], in the same way as this pheromone prevents male–male courtship (see above). CH503 also inhibits male courtship, through an unknown sensory pathway. Because it perdures longer on females than cVA—perhaps due to its lower volatility—CH503 might suppress remating over a longer time period [101].
Mating experiences can also modify pheromone responses. As with many animals, male A. ipsilon moths have a post-ejaculatory refractory period, in order to refill their sex glands [102]. This quiescent period seems to be linked to reduced sensitivity to female sex pheromones. Whilst peripheral pheromone sensation is unchanged, olfactory interneurons show markedly diminished pheromone responsiveness, suggesting a role for neuromodulatory factors in this physiological regulation [102]. A different type of pheromone-sensing plasticity has been observed in D. melanogaster males, which show enhanced behavioural sensitivity to cVA after sexual rejection by mated—cVA-scented—females. This ‘courtship learning’ helps males focus their subsequent mating attempts on more receptive, virgin females [103,104]. Although dopaminergic neuron input to the mushroom body—a site of learning and memory in insects—is required for modulating pheromone responses [104], where and how this intersects with the cVA-sensing circuitry has not yet been determined.
Finally, other social conditions might indirectly modulate sex pheromone-evoked behaviours. In C. elegans, behavioural responses to the hermaphrodite sex pheromone ascr#3 are modified by changes in the neuromodulatory state, as revealed by different alleles of the neuropeptide receptor npr-1 [23]. Low activity alleles of npr-1—which are thought to recapitulate a state of metabolic or crowding stress—reduce hermaphrodite avoidance but increase male attraction. NPR-1 functions predominantly in the RMG ‘hub’ interneuron, which coordinates sexually dimorphic processing of sex pheromones [23]. The adaptive advantage of these changes in pheromone-evoked behaviours is unknown.
Closing remarks
Within the complex universe of chemosensory stimuli that control the interactions of animals with their environment, sex pheromones provide outstanding examples where specific chemical signals are tightly linked to particular species, receptors, sensory circuits and behavioural responses. Fascinatingly, this close relationship has led to sex pheromones being coopted for several other non-sexual functions. For example, larvae of the cotton leafworm moth, Spodoptera littoralis, are attracted by an adult sex pheromone, which might help guide them to suitable food sources [105]. Nematophagous fungi sense the ascaroside pheromones produced by their prey as a trigger to set ‘traps’ [106]. Most famously, sexually deceptive orchids produce remarkably good chemical mimics of female sex pheromone blends of particular insect species in order to attract the corresponding males as unwitting pollinators [107].
Considering the principal roles of sex pheromones, as reviewed here—in uniting conspecific, fecund males and females—there is clearly still much to discover (Sidebar A). Progress will depend on a continued integration of chemistry, molecular genetics, neurophysiology, behavioural analysis and ecology, in both traditional laboratory models and those with better-defined natural histories. Nevertheless, pheromone-sensing pathways have become firmly established as premier models to understand how external information is processed in the nervous system to evoke adaptive behaviours. Current knowledge clearly demonstrates how common functions of sex pheromones are met with common solutions as to how these chemical cues are produced and detected.
Sidebar A | In need of answers.
How many different sex pheromones are produced by a given animal and what are their chemical identities? How and where are these signals synthesized, and how is pheromone production modified during development and ageing?
What are the specific receptors for each sex pheromone, and how are their downstream sensory circuits organized in the brain? How does activation of a specific receptor lead to a particular behavioural response?
What are the similarities and differences between circuit anatomy and function in males and females, and what are the crucial distinctions that define sexually dimorphic behavioural responses to pheromones? How are these dimorphic properties established during development?
How do sex pheromone signals integrate with other sensory modalities, such as signature mixtures of chemicals or visual cues, to control behaviour?
Do common strategies in sex pheromone production and detection between organisms reflect evolutionary conservation or convergence? How does the use of distinct strategies between animals reflect their different lifestyles in nature? How do changes in pheromone signalling contribute to speciation?

Carolina Gomez-Diaz

Richard Benton
Acknowledgments
We apologize to our colleagues whose work could not be cited due to space constraints. We thank Jean-Christophe Billeter, Teun Dekker, Darren Logan, Patrick McGrath, Pavan Ramdya, Michael Saina and Kevin Wanner for comments on the manuscript. C.G.-D. was supported by the Post-doctoral Foundation for the Development of Applied Scientific Research and Technology Clarin Programme from the Government of the Principado de Asturias. Research in the laboratory of R.B. is supported by the University of Lausanne, a European Research Council Starting Independent Researcher Grant and the Swiss National Science Foundation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Darwin C (1874) The Descent of Man and Selection in Relation to Sex. London, UK: Murray [Google Scholar]
- Fabre J-H (1912) Social Life in the Insect World. London, UK: T.F. Unwin [Google Scholar]
- Butenandt A, Beckmann R, Stamm D (1961) Uber den Sexuallockstoff des Seidenspinners. II. Konstitution und Konfiguration des Bombykols. Hoppe Seylers Z Physiol Ch 324: 84–87 [DOI] [PubMed] [Google Scholar]
- Wyatt TD (2003) Pheromones and Animal Behaviour: Communication by Smell and Taste. Oxford, UK: Oxford University Press [Google Scholar]
- Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50: 371–393 [DOI] [PubMed] [Google Scholar]
- Ludewig AH, Schroeder FC (2013) Ascaroside signaling in C. elegans. WormBook 18: 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins SF, Bowie JH (2012) Pheromones, attractants and other chemical cues of aquatic organisms and amphibians. Nat Prod Rep 29: 642–658 [DOI] [PubMed] [Google Scholar]
- Touhara K (2008) Sexual communication via peptide and protein pheromones. Curr Opin Pharmacol 8: 759–764 [DOI] [PubMed] [Google Scholar]
- Silbering AF, Benton R (2010) Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design’? EMBO Rep 11: 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB (2009) Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol 71: 307–332 [DOI] [PubMed] [Google Scholar]
- Wyatt TD (2010) Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 196: 685–700 [DOI] [PubMed] [Google Scholar]
- Leal WS (2005) Pheromone reception. Top Curr Chem 240: 1–36 [Google Scholar]
- Blomquist GJ, Vogt RG (2003) Insect Pheromone Biochemistry and Molecular Biology. London, UK: Elsevier Academic Press [Google Scholar]
- Moraes MC, Pareja M, Laumann RA, Borges M (2008) The chemical volatiles (semiochemicals) produced by neotropical stink bugs (Hemiptera: Pentatomidae). Neotrop Entomol 37: 489–505 [DOI] [PubMed] [Google Scholar]
- Soldi RA, Rodrigues MA, Aldrich JR, Zarbin PH (2012) The male-produced sex pheromone of the true bug, Phthia picta, is an unusual hydrocarbon. J Chem Ecol 38: 814–824 [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ (2007) A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446: 542–546 [DOI] [PubMed] [Google Scholar]
- Benton R (2007) Sensitivity and specificity in Drosophila pheromone perception. Trends Neurosci 30: 512–519 [DOI] [PubMed] [Google Scholar]
- Farine JP, Ferveur JF, Everaerts C (2012) Volatile Drosophila cuticular pheromones are affected by social but not sexual experience. PLoS ONE 7: e40396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur JF (2005) Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet 35: 279–295 [DOI] [PubMed] [Google Scholar]
- Lacaille F et al. (2007) An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE 2: e661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe A, von Reuss SH, Kogan D, Gasser RB, Platzer EG, Schroeder FC, Sternberg PW (2012) Ascaroside signaling is widely conserved among nematodes. Curr Biol 22: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe A et al. (2012) Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proc Natl Acad Sci USA 109: 20949–20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Kim K, Neal SJ, Macosko E, Kim D, Butcher RA, Zeiger DM, Bargmann CI, Sengupta P (2012) Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron 75: 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J et al. (2008) A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454: 1115–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J et al. (2012) A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol 10: e1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A (2013) Chemosignals, hormones and mammalian reproduction. Horm Behav 63: 723–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K (2005) Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature 437: 898–901 [DOI] [PubMed] [Google Scholar]
- Haga S et al. (2010) The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 466: 118–122 [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL (2010) Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. BMC Biol 8: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL (2012) Pheromonal induction of spatial learning in mice. Science 338: 1462–1465 [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT (2003) Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci 4: 551–562 [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC (2005) Encoding social signals in the mouse main olfactory bulb. Nature 434: 470–477 [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Nakagawa H, Mori N, Watanabe H, Touhara K (2013) An unsaturated aliphatic alcohol as a natural ligand for a mouse odorant receptor. Nat Chem Biol 9: 160–162 [DOI] [PubMed] [Google Scholar]
- Kimoto H, Sato K, Nodari F, Haga S, Holy TE, Touhara K (2007) Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr Biol 17: 1879–1884 [DOI] [PubMed] [Google Scholar]
- Rasmussen LE, Lee TD, Roelofs WL, Zhang A, Daves GD Jr. (1996) Insect pheromone in elephants. Nature 379: 684. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sakurai T, Nishioka T, Touhara K (2005) Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307: 1638–1642 [DOI] [PubMed] [Google Scholar]
- Sakurai T et al. (2011) A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genet 7: e1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS, Smith DP (2006) A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci 26: 8727–8733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR et al. (2008) The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452: 473–477 [DOI] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R (2010) A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468: 686–690 [DOI] [PubMed] [Google Scholar]
- Manoli DS, Fan P, Fraser EJ, Shah NM (2013) Neural control of sexually dimorphic behaviors. Curr Opin Neurobiol 23: 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Zhao X, Dickson BJ (2012) The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep 1: 599–607 [DOI] [PubMed] [Google Scholar]
- Liu T, Starostina E, Vijayan V, Pikielny CW (2012) Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J Neurosci 32: 11879–11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, Pikielny CW (2012) A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J Neurosci 32: 4665–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Lamora A, Sun Y, Welsh MJ, Ben-Shahar Y (2012) ppk23-dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet 8: e1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K (2012) Contact chemoreceptors mediate male–male repulsion and male–female attraction during Drosophila courtship. Cell 149: 1140–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H (2008) Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci 11: 874–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C (2009) A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol 19: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet M, Dartevelle L, Ferveur JF (2006) A Drosophila male pheromone affects female sexual receptivity. Proc Biol Sci 273: 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD (2009) Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461: 987–991 [DOI] [PubMed] [Google Scholar]
- Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ (2011) Hierarchical chemosensory regulation of male–male social interactions in Drosophila. Nat Neurosci 14: 757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D (2011) Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69: 498–508 [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI (2009) A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458: 1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman AM, Hildebrand JG, Brennan MM, Tumlinson JH (1986) Trans-sexually grafted antennae alter pheromone-directed behaviour in a moth. Nature 323: 801–803 [DOI] [PubMed] [Google Scholar]
- White JQ, Jorgensen EM (2012) Sensation in a single neuron pair represses male behavior in hermaphrodites. Neuron 75: 593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C (2007) A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448: 1009–1014 [DOI] [PubMed] [Google Scholar]
- Smadja C, Butlin RK (2009) On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity (Edinb) 102: 77–97 [DOI] [PubMed] [Google Scholar]
- Lassance JM (2010) Journey in the Ostrinia world: from pest to model in chemical ecology. J Chem Ecol 36: 1155–1169 [DOI] [PubMed] [Google Scholar]
- Lassance JM, Groot AT, Lienard MA, Antony B, Borgwardt C, Andersson F, Hedenstrom E, Heckel DG, Lofstedt C (2010) Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature 466: 486–489 [DOI] [PubMed] [Google Scholar]
- Karpati Z, Dekker T, Hansson BS (2008) Reversed functional topology in the antennal lobe of the male European corn borer. J Exp Biol 211: 2841–2848 [DOI] [PubMed] [Google Scholar]
- Wanner KW, Nichols AS, Allen JE, Bunger PL, Garczynski SF, Linn CE, Robertson HM, Luetje CW (2010) Sex pheromone receptor specificity in the European corn borer moth, Ostrinia nubilalis. PLoS ONE 5: e8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs WL, Rooney AP (2003) Molecular genetics and evolution of pheromone biosynthesis in Lepidoptera. Proc Natl Acad Sci USA 100: 9179–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary GP, Allen JE, Bunger PL, Luginbill JB, Linn CE Jr, Macallister IE, Kavanaugh MP, Wanner KW (2012) Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. Proc Natl Acad Sci USA 109: 14081–14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keays MC, Barker D, Wicker-Thomas C, Ritchie MG (2011) Signatures of selection and sex-specific expression variation of a novel duplicate during the evolution of the Drosophila desaturase gene family. Mol Ecol 20: 3617–3630 [DOI] [PubMed] [Google Scholar]
- Shirangi TR, Dufour HD, Williams TM, Carroll SB (2009) Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol 7: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P et al. (2013) Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell 154: 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Drijfhout F (2009) A review of ant cuticular hydrocarbons. J Chem Ecol 35: 1151–1161 [DOI] [PubMed] [Google Scholar]
- Blomquist GJ, Figueroa-Teran R, Aw M, Song M, Gorzalski A, Abbott NL, Chang E, Tittiger C (2010) Pheromone production in bark beetles. Insect Biochem Mol Biol 40: 699–712 [DOI] [PubMed] [Google Scholar]
- Chasnov JR, So WK, Chan CM, Chow KL (2007) The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Natl Acad Sci USA 104: 6730–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen E, Brakefield PM, Heuskin S, Zwaan BJ, Nieberding CM (2013) The scent of inbreeding: a male sex pheromone betrays inbred males. Proc Biol Sci 280: 20130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkki M, Krams I, Kangassalo K, Rantala MJ (2012) Inbreeding affects sexual signalling in males but not females of Tenebrio molitor. Biol Lett 8: 423–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Willis JH (2009) The genetics of inbreeding depression. Nat Rev Genet 10: 783–796 [DOI] [PubMed] [Google Scholar]
- Logan DW, Marton TF, Stowers L (2008) Species specificity in major urinary proteins by parallel evolution. PLoS ONE 3: e3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L (2010) The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell 141: 692–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherborne AL, Thom MD, Paterson S, Jury F, Ollier WE, Stockley P, Beynon RJ, Hurst JL (2007) The genetic basis of inbreeding avoidance in house mice. Curr Biol 17: 2061–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham SA, Thom MD, Jury F, Ollier WE, Beynon RJ, Hurst JL (2007) The genetic basis of individual-recognition signals in the mouse. Curr Biol 17: 1771–1777 [DOI] [PubMed] [Google Scholar]
- Thom MD, Stockley P, Jury F, Ollier WE, Beynon RJ, Hurst JL (2008) The direct assessment of genetic heterozygosity through scent in the mouse. Curr Biol 18: 619–623 [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L (2007) Identification of protein pheromones that promote aggressive behaviour. Nature 450: 899–902 [DOI] [PubMed] [Google Scholar]
- Chamero P, Leinders-Zufall T, Zufall F (2012) From genes to social communication: molecular sensing by the vomeronasal organ. Trends Neurosci 35: 597–606 [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T et al. (2004) MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306: 1033–1037 [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F (2006) Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci 26: 1961–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm T et al. (2013) Mouse urinary peptides provide a molecular basis for genotype discrimination by nasal sensory neurons. Nat Commun 4: 1616. [DOI] [PubMed] [Google Scholar]
- Kaplan F et al. (2011) Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS ONE 6: e17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner B, Gadenne C, Anton S (2002) Central processing of plant volatiles in Agrotis ipsilon males is age-independent in contrast to sex pheromone processing. Chem Senses 27: 45–48 [DOI] [PubMed] [Google Scholar]
- Jarriault D, Barrozo RB, de Carvalho Pinto CJ, Greiner B, Dufour MC, Masante-Roca I, Gramsbergen JB, Anton S, Gadenne C (2009) Age-dependent plasticity of sex pheromone response in the moth, Agrotis ipsilon: combined effects of octopamine and juvenile hormone. Horm Behav 56: 185–191 [DOI] [PubMed] [Google Scholar]
- Kuo TH, Yew JY, Fedina TY, Dreisewerd K, Dierick HA, Pletcher SD (2012) Aging modulates cuticular hydrocarbons and sexual attractiveness in Drosophila melanogaster. J Exp Biol 215: 814–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts C, Farine JP, Cobb M, Ferveur JF (2010) Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5: e9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassance JM, Lofstedt C (2009) Concerted evolution of male and female display traits in the European corn borer, Ostrinia nubilalis. BMC Biol 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieberding CM, Fischer K, Saastamoinen M, Allen CE, Wallin EA, Hedenstrom E, Brakefield PM (2012) Cracking the olfactory code of a butterfly: the scent of ageing. Ecol Lett 15: 415–424 [DOI] [PubMed] [Google Scholar]
- Osada K, Yamazaki K, Curran M, Bard J, Smith BP, Beauchamp GK (2003) The scent of age. Proc Biol Sci 270: 929–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada K, Tashiro T, Mori K, Izumi H (2008) The identification of attractive volatiles in aged male mouse urine. Chem Senses 33: 815–823 [DOI] [PubMed] [Google Scholar]
- Garratt M, Stockley P, Armstrong SD, Beynon RJ, Hurst JL (2011) The scent of senescence: sexual signalling and female preference in house mice. J Evol Biol 24: 2398–2409 [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Kent C, Billeter JC, Azanchi R, So AK, Schonfeld JA, Smith BP, Lucas C, Levine JD (2008) Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol 18: 1373–1383 [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Billeter JC, Wong A, Choi C, Nitabach MN, Levine JD (2013) Pigment-dispersing factor modulates pheromone production in clock cells that influence mating in Drosophila. Neuron 79: 54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Zada A, Nestel D, Fefer D, Nemni-Lavy E, Deloya-Kahane I, David M (2012) Analyzing diurnal and age-related pheromone emission of the olive fruit fly, Bactrocera oleae by sequential SPME-GCMS analysis. J Chem Ecol 38: 1036–1041 [DOI] [PubMed] [Google Scholar]
- Parker MR, Mason RT (2009) Low temperature dormancy affects the quantity and quality of the female sexual attractiveness pheromone in red-sided garter snakes. J Chem Ecol 35: 1234–1241 [DOI] [PubMed] [Google Scholar]
- Lemmen J, Evenden M (2009) Peripheral and behavioral plasticity of pheromone response and its hormonal control in a long-lived moth. J Exp Biol 212: 2000–2006 [DOI] [PubMed] [Google Scholar]
- Stopka P, Janotova K, Heyrovsky D (2007) The advertisement role of major urinary proteins in mice. Physiol Behav 91: 667–670 [DOI] [PubMed] [Google Scholar]
- Thomas ML (2011) Detection of female mating status using chemical signals and cues. Biol Rev Camb Philos Soc 86: 1–13 [DOI] [PubMed] [Google Scholar]
- Foster SP, Roelofs WL (1994) Regulation of pheromone production in virgin and mated females of two tortricid moths. Arch Insect Biochem Physiol 25: 271–285 [Google Scholar]
- Yew JY, Dreisewerd K, Luftmann H, Muthing J, Pohlentz G, Kravitz EA (2009) A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol 19: 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrozo RB, Jarriault D, Deisig N, Gemeno C, Monsempes C, Lucas P, Gadenne C, Anton S (2011) Mating-induced differential coding of plant odour and sex pheromone in a male moth. Eur J Neurosci 33: 1841–1850 [DOI] [PubMed] [Google Scholar]
- Griffith LC, Ejima A (2009) Courtship learning in Drosophila melanogaster: diverse plasticity of a reproductive behavior. Learn Mem 16: 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleman K, Vrontou E, Kruttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ (2012) Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489: 145–149 [DOI] [PubMed] [Google Scholar]
- Poivet E, Rharrabe K, Monsempes C, Glaser N, Rochat D, Renou M, Marion-Poll F, Jacquin-Joly E (2012) The use of the sex pheromone as an evolutionary solution to food source selection in caterpillars. Nat Commun 3: 1047. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Mahanti P, Schroeder FC, Sternberg PW (2013) Nematode-trapping fungi eavesdrop on nematode pheromones. Curr Biol 23: 83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskett AC (2011) Orchid pollination by sexual deception: pollinator perspectives. Biol Rev Camb Philos Soc 86: 33–75 [DOI] [PubMed] [Google Scholar]
- Benton R (2008) Chemical sensing in Drosophila. Curr Opin Neurobiol 18: 357–363 [DOI] [PubMed] [Google Scholar]
- Duan X et al. (2012) Crucial role of copper in detection of metal-coordinating odorants. Proc Natl Acad Sci USA 109: 3492–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]