Abstract
The cyclic dinucleotides 3'-5'diadenylate (c-diAMP) and 3'-5' diguanylate (c-diGMP) are important bacterial second messengers that have recently been shown to stimulate the secretion of type I Interferons (IFN-Is) through the c-diGMP-binding protein MPYS/STING. Here, we show that physiologically relevant levels of cyclic dinucleotides also stimulate a robust secretion of IL-1β through the NLRP3 inflammasome. Intriguingly, this response is independent of MPYS/STING. Consistent with most NLRP3 inflammasome activators, the response to c-diGMP is dependent on the mobilization of potassium and calcium ions. However, in contrast to other NLRP3 inflammasome activators, this response is not associated with significant changes in mitochondrial potential or the generation of mitochondrial reactive oxygen species. Thus, cyclic dinucleotides activate the NLRP3 inflammasome through a unique pathway that could have evolved to detect pervasive bacterial pathogen-associated molecular patterns associated with intracellular infections.
Keywords: inflammasome, cyclic-di-GMP, cyclic-di-AMP, PAMP, cytokines
INTRODUCTION
Eukaryotes have evolved pattern recognition receptors to recognize essential molecular patterns associated with pathogens (a.k.a., pathogen-associated molecular patterns; [1, 2]). This includes components of the inflammasome, a family of multi-protein complexes that direct the caspase-1-dependent activation and secretion of IL-1β and IL-18 (reviewed in [3–6]). Inflammasomes distinguish themselves from other innate responses by requiring two signals to become activated. The first signal entails the PRR- and NF-κB-dependent expression of pro-IL-1β and pro-IL-18, whereas the second signal involves a distinct PAMP-dependent activation of the inflammasome. The adaptor protein ASC (apoptosis speck-like protein with CARD), a critical component of many inflammasomes, facilitates the interaction between the presumed PAMP ‘sensing’ component of each inflammasome and caspase-1. Sensing components are unique to each inflammasome and include NLRP1b, NLRP3, NLRC4 and AIM2.
The NLRP3 inflammasome has been studied most extensively because of its association with human disease and its response to divergent ‘molecular patterns’. These include molecules associated with tissue damage (for example, monosodium urate; (MSU) and ATP) and environmental stressors (for example, crystalline silica, asbestos, alum, β-amyloid, nigericin and cholesterol; reviewed in [3–5]). Efforts to understand how these pleiotropic ligands promote NLRP3 inflammasome activation have implicated sequential mobilization of potassium (K+) and calcium (Ca++) ions [7–10]. This mobilization also appears to be associated with mitochondrial stress, notably including decreased mitochondrial membrane potential (Δψm), increased mitochondrial reactive oxygen species (mROS) production and the cytosolic accumulation of oxidized mitochondrial (mt)DNA. Recently, studies have championed oxidized mtDNA and MAVS (mitochondrial antiviral signaling protein) as final common mediators of NLRP3 inflammasome activation [7, 9, 11, 12, 13, 14]. In contrast, however, the ability of other microbial pathogen-associated molecular patterns, like poly(dA:dT), flagellin and Bacillus anthracis lethal toxin to promote inflammasome activation appears to involve a direct interaction between these ‘ligands’ and AIM2, NLRC4 and NLRP1b, respectively [4, 15, 16, 17, 18].
Studies exploring the ability of Legionella pneumophila to stimulate IL-1β secretion in infected macrophages determined that flagellin activates the Naip5-NLRC4 inflammasome [19]. Another L. pneumophila PAMP, cyclic 3′-5′ diguanylate (c-diGMP), an important bacterial second messenger [20], was identified as the critical PAMP stimulating activation of the IFN-α/β axis [21, 22]. Intriguingly, closely related cyclic 3′-5′diadenylate (c-diAMP) was also found to direct IFN-α/β expression, during a Listeria monocytogenes infection [23]. Subsequently, MPYS/STING, previously implicated in DNA-dependent IFN-α/β induction [24], was found to bind and direct the type I IFN (IFN-I) response to cyclic dinucleotides [24–28]. Yet, MPYS/STING exhibits a relatively broad pattern of expression, suggesting a role for a more tissue-restricted component in this response, such as DDX41 [29] or cyclic GMP-AMP Synthase [28].
Evidence that poly(dA:dT) stimulates the production of IFN-I and IL-1β through distinct pattern recognition receptors [6, 30] raised the possibility that the ability of c-diGMP to stimulate the expression of these two cytokines might also be mediated through multiple receptors [31]. Consistent with this, c-diAMP and c-diGMP were found to robustly stimulate inflammasome activity in human and murine macrophages. This response was dependent on NLRP3, as well as K+ and Ca++ mobilization. More importantly, this response was not associated with mROS production or a loss in Δψm, two events recently implicated as a common component of NLRP3 inflammasome activation. Thus, cyclic dinucleotides activate the NLRP3 inflammasome in macrophages through a distinct pathway, one that is also independent of MPYS/STING.
RESULTS AND DISCUSSION
Cyclic-dinucleotides activate IL-1β secretion
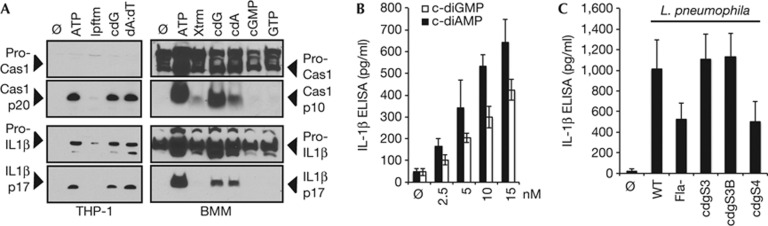
Consistent with previous studies, immunoblotting and ELISA confirmed that stimulation of LPS-pretreated murine bone marrow-derived macrophages (BMMs) or PMA-pretreated human THP-1 cells with ATP, a canonical NLRP3 ligand, led to a marked increase in secretion of activated caspase-1 (p10 and p20, respectively) and IL-1β (that is, p17 kDa; see Fig 1A and supplementary Fig S1A online; [4, 8, 32]). Likewise, transfection of poly(dA:dT), an AIM2 ligand, led to equivalent increases in secreted caspase-1 and IL-1β (Fig 1A). Remarkably, transfection of c-diGMP or c-diAMP, at doses previously shown to promote MPYS/STING-dependent IFNβ expression [21, 22], was associated with an analogous robust and dose-dependent increase in caspase-1 and IL-1β production (Fig 1B). Importantly, these doses were similar to those of transfected poly(dA:dT) required to stimulate the AIM2 inflammasome [33, 34]. As previously reported, c-diGMP appeared to show more potent activity than c-diAMP [21, 23]. Of note, control treatments with other guanine nucleotides (for example, cGMP and GTP), as well as transfection reagents alone (that is, Lipofectamine and X-tremegene), failed to induce IL-1β secretion (Fig 1A and supplementary Fig S1A online). These results demonstrate that cyclic dinucleotides potently stimulate the inflammasome.
Figure 1.
Cyclic dinucleotides activate the inflammasome in THP-1 cells and murine BMMs. (A) PMA-differentiated THP-1 cells (left panel) were treated with ATP (5 mM; 6 h) or transfected (Lipofectamine 2000; lpftm) with c-diGMP (cdG; 5 nmol; 6 h) or poly(dA:dT) (dA:dT; 5 μg/ml; 6 h). LPS-primed BMMs (right panel) were treated with ATP (5 mM; 6 h) or transfected (X-tremegene HP; Xtrm) with c-diGMP (10 nmol; 6 h), c-diAMP (10 nmol; 6 h), cGMP (10 nmol; 6 h) or GTP (10 nmol; 6 h). Supernatants were immunoblotted for immature IL-1β (pro-IL1β), active IL-1β (p17), immature caspase-1 (pro-Cas1) or active caspase-1 (Cas1, p20 and p10, respectively). (B) IL-1β levels were measured by ELISA (BD Biosciences) in 6 h supernatants of LPS-primed BMMs after the indicated treatment with c-diGMP or c-diAMP, as in A. (C) IL-1β levels were measured by ELISA in BMMs infected with IPTG-induced L. pneumophila strains (MOI 10; 6 h), ectopically expressing cdgS3, cdgS3B or cdgS4 [35]. Graphs present means±standard error from three independent experiments. BMMs, bone marrow-derived macrophages; c-diAMP, 3′-5′ diadenylate; c-diGMP, 3′-5′ diguanylate; ELISA, enzyme-linked immunosorbent assay; MOI, multiplicity of infection.
To explore a physiological role for c-diGMP in inflammasome activation, L. pneumophila cdgS strains previously exploited to identify a role for c-diGMP in IFN-I induction, were evaluated [21, 35]. Specifically, these studies examined L. pneumophila strains ectopically expressing: cdgS4 (lpg0156), a phosphodiesterase associated with greater than threefold reduction in c-diGMP levels (that is, from 8.3 to 2.4 fmol/OD of bacteria); cdgS3 (lpg0155), a diguanylate cyclase associated with greater than threefold increase c-diGMP levels (that is, from 8.3 to 24 fmol/OD); and cdgS3B, a point mutant that is resistant to c-diGMP feedback inhibition [35]. Of note, these measured dinucleotide levels correspond closely with endogenous levels of c-diAMP previously reported for intracellular Listeria monocytogenes infection [23]. Additionally, as L. pneumophila flagellin is also known to activate the Naip5-NLRC4 inflammasome [36], it was important to carry out these studies in flagellin-deficient strains. As expected, loss of flagellin led to an approximately two-fold reduction in IL-1β secretion (see Fig 1C). Consistent with changes in c-diGMP levels, IL-1β secretion was significantly increased in BMMs infected with the flagellin null L. pneumophila ectopically expressing cdgS3 or cdgS3B. However, the reduction in IL-1β secretion in L. pneumophila ectopically expressing cdgS4 was not significant (Fig 1C; [35]).
c-diGMP stimulates cation mobilization
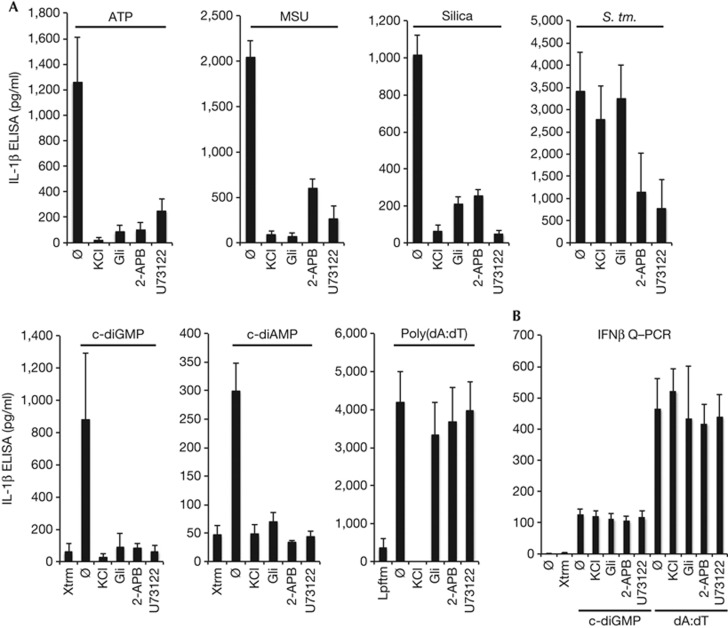
Recent studies have revealed that the ability of divergent ligands to activate the NLRP3 inflammasome is dependent on cation mobilization (that is, K+ and Ca++; [7, 8, 10, 37]). To determine whether this might also be the case for cyclic dinucleotides, BMMs were treated with inhibitors of K+ mobilization (that is, KCl and glibenclamide) or Ca++ mobilization (that is, 2-ABP and U73122) before stimulation with c-diGMP and other well-characterized NLRP3 inflammasome activators. Consistent with prior reports, the ability of ATP, MSU and silica, as well as alum and nigericin (see supplementary Fig S1B online), to stimulate NLRP3 inflammasome activation was sensitive to each of these inhibitors (Fig 2A; [7]). Likewise, stimulation with transfected c-diGMP and c-diAMP was also sensitive to all four inhibitors, underscoring an important mechanistic overlap with known NLRP3 inflammasome activators (Fig 2A; [7, 8]). In contrast, stimulation with Salmonella typhimurium, which activates both the NLRP3 and NLRC4 inflammasomes, was unexpectedly most sensitive to inhibition of Ca++ mobilization [38], whereas poly(dA:dT)-stimulated AIM2 inflammasome activation was uniquely sensitive to extracellular KCl (Fig 2A; [6, 34, 38]). This latter observation raised the possibility that more than one K+ channel might be involved in this response [8], and suggested that cations might have a more complicated and pervasive role in inflammasome activation than previously anticipated. Finally, despite their ability to block inflammasome activation, these inhibitors failed to impede c-diGMP- and poly(dA:dT)-dependent IFN-β expression, drawing attention to important functional distinction between these responses (Fig 2B; [21, 22, 26, 30]).
Figure 2.
Cation mobilization during c-diGMP NLRP3 inflammasome activation.(A) IL-1β levels were measured by ELISA in LPS-primed BMMs treated with KCl (130 mM), Glibenclamide (Gli; 50 μM), 2-APB (100 μM) or U73122 (10 μM) 30 min before stimulation with ATP (5 mM; 30 min), MSU (100 μg/ml; 4 h) and silica (500 μg/ml, 6 h); or transfection with c-diGMP (10 nmol; 6 h), c-diAMP (10 nmol; 6 h) and poly(dA:dT) (5 μg/ml; 6 h); or infected with S. typhimurium (MOI 25; 6 h). (B) IFN-β expression was evaluated by Q–PCR in LPS-primed BMMs treated as in panel A. Results were normalized to GAPDH and reported as relative fold change. Graphs present means±standard error from three independent experiments. BMMs, bone marrow-derived macrophages; c-diAMP, 3′-5′ diadenylate; c-diGMP, 3′-5′ diguanylate; ELISA, enzyme-linked immunosorbent assay; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; IFN-β, interferon beta; MOI, multiplicity of infection; Q–PCR, quantitative polymerase chain reaction.
c-diGMP activates the NLRP3 inflammasome
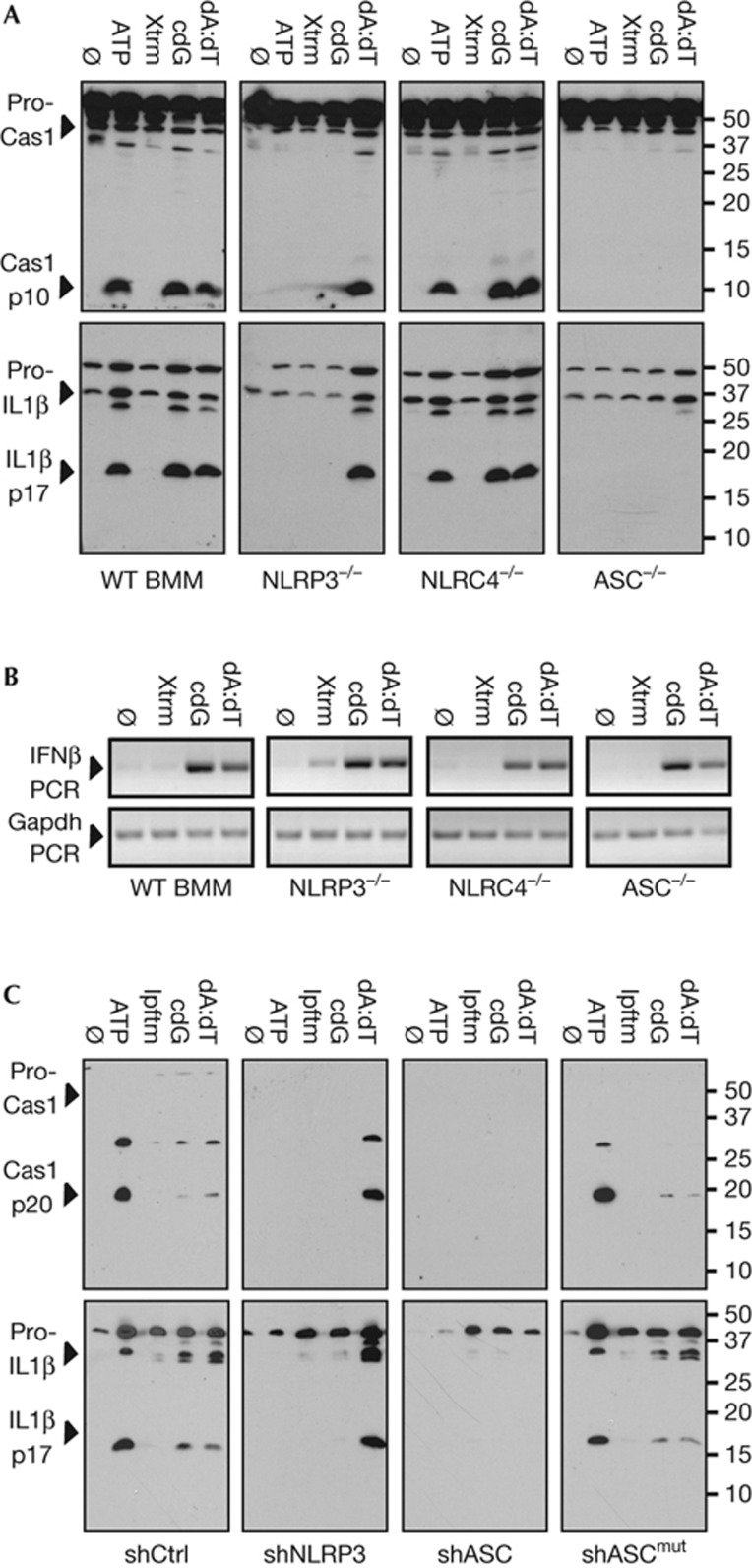
To more rigorously investigate the role of the NLRP3 inflammasome in the innate response to cyclic dinucleotides, macrophages defective in specific inflammasome components were evaluated. As anticipated, ATP failed to activate the inflammasome in BMMs prepared from NLRP3[−/−] and ASC[−/−] mice, whereas poly(dA:dT)-dependent inflammasome activity was defective in ASC[−/−] and AIM2[−/−] macrophages (see Fig 3A; supplementary Fig S2A,B online). Notably, the ability of c-diGMP to stimulate capase-1 and IL-1β activation was abrogated in NLRP3[−/−] and ASC[−/−] BMMs, but not NLRC4[−/−], AIM2[−/−] or NLRP1b[−/−] BMMs, which are defective in their response to flagellin, poly(dA:dT) and B. anthracis lethal toxin (LT; [16–18]), respectively (see Fig 3A, supplementary Fig S2A–C online). As previously reported, when these culture supernatants were assayed for IL-18, there was a parallel, but less robust accumulation of this cytokine (see supplementary Fig S2E online; [39, 40]). Intriguingly, LT-stimulated IL-18 production was proportionately more robust. Although discordance between IL-1β and IL-18 production has been previously reported, the mechanism has not been fully elucidated [41].
Figure 3.
c-diGMP-stimulated inflammasome activation is dependent on NLRP3. (A) Secreted IL-1β and Cas1 levels were evaluated by immunoblotting in LPS-primed WT, NLRP3−/−, NLRC4−/− or ASC−/− BMMs, as in Fig 1. (B) IFN-β and GAPDH expression were evaluated by PCR, in BMMs treated as in panel A. (C) Secreted IL-1β and caspase-1 levels were evaluated in PMA-differentiated THP-1 cells stably expressing control shRNA (shCtrl), shNLRP3, shASC or the re-expression of a knockdown resistant ASC cDNA (shASCmut), as above. Data are representative of more than three independent experiments. ASC, apoptosis speck-like protein with CARD; BMMs, bone marrow-derived macrophages; Cas1, caspase-1; c-diGMP, 3′-5′ diguanylate; IFN-β, interferon beta; IL-1β, interleukin 1 beta; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; PCR, polymerase chain reaction; shctrl, control short hairpin RNA; WT, wild type.
Again, c-diGMP-stimulated IFN-β expression was not affected by the loss of NLRP3, NLRC4 or ASC (Fig 3B), underscoring essential differences in these two inflammatory responses. A parallel set of studies, examining THP-1 cells knocked down for NLRP3 or ASC expression (supplementary Fig S2F online), revealed the same pattern of inhibition (Fig 3C; [32]). Reassuringly, the responses to ATP, poly(dA:dT) and c-diGMP were fully restored in ASC knockdown cells expressing an short hairpin RNA resistant ASC cDNA (that is, shASCmut; Fig 3C; supplementary Fig S2F online). Consistent with a dependence on cation mobilization, these genetic studies provide compelling evidence that c-diGMP activates the NLRP3 inflammasome.
Recent studies have implicated several additional components in the regulation of the NLRP3 inflammasome, including a pivotal role for mitochondrial stress [7, 11, 12, 13, 42, 43, 44]. To explore whether cyclic dinucleotide-dependent inflammasome activation was also associated with mitochondrial stress, c-diGMP-stimulated BMMs were stained with mitoSox to measure mROS production and tetramethyl rhodamine methyl ester (TMRM) to evaluate Δψm. Consistent with previous studies, ATP stimulation led to a significant increase in mitoSox staining (that is, mROS production; see supplementary Fig S3A,B online) and a notable decrease in TMRM incorporation (that is, Δψm; supplementary Fig S3C–E online; [7, 11, 12, 13]). As expected, the AIM2-dependent response to poly(dA:dT) was neither associated with changes in mitoSox staining (supplementary Fig.S3A,B online) nor TMRM incorporation (supplementary Fig S3C–E online). Intriguingly, stimulation with c-diGMP was also not associated with any significant changes in either mitoSox or TMRM staining (supplementary Fig S3A–E online). Consistent with recent reports, there was also no evidence that cyclic dinucleotide-stimulated inflammasome activation was associated with the double-stranded RNA kinase PKR or HMBG1 release (see supplementary Figs S2D and S4A online; data not shown; [45]). Finally, studies with an inhibitor of BRCC3, a deubiquitinase implicated in NLRP3 inflammasome activation, revealed the anticipated defect in response to ATP (supplementary Fig S4B online; [42]). This inhibitor also blocked the ability of cyclic dinucleotides to activate the inflammasome, suggesting that BRCC3 broadly regulates NLRP3 inflammasome activation. These observations are consistent with the notion that c-diGMP-directed NLRP3 inflammasome activation is dependent on BRCC3-mediated deubiquitinylation, but independent of mitochondrial perturbation.
Inflammasome activation is independent of STING
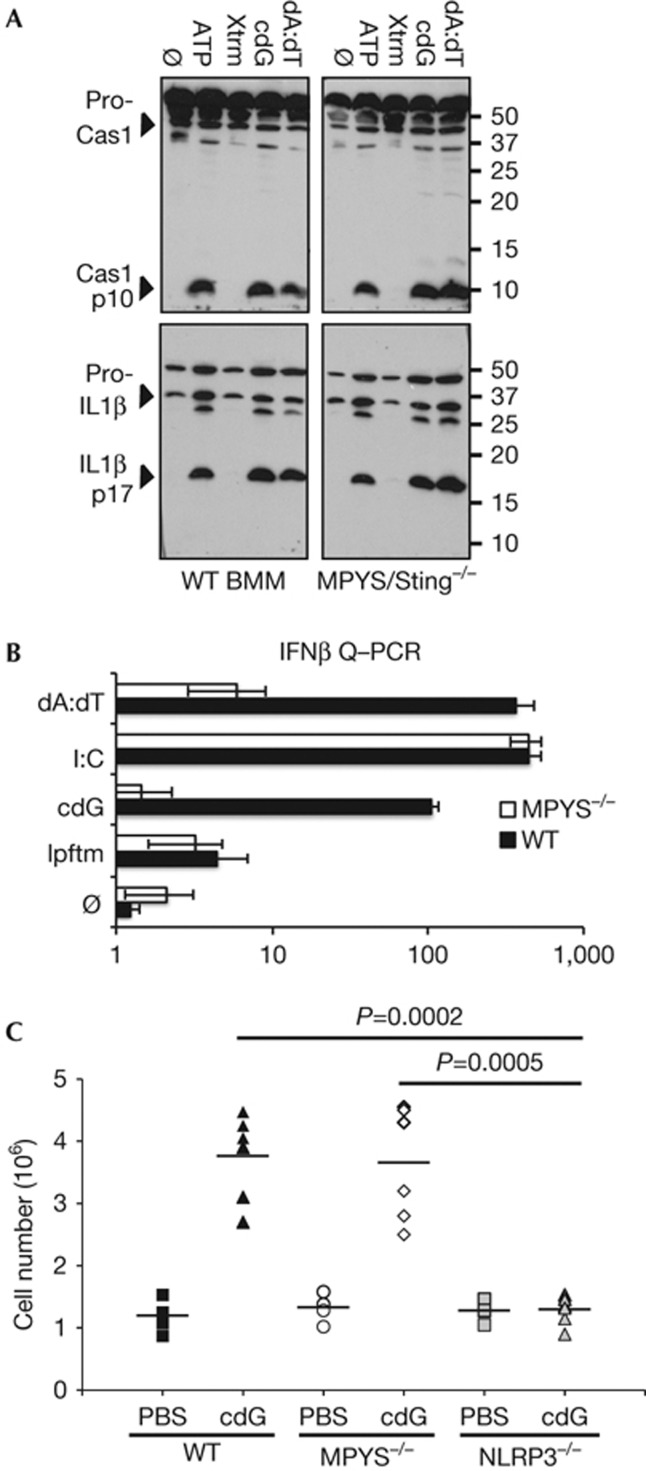
MPYS/STING has been identified as a critical component in cyclic dinucleotide-stimulated IFN-I expression [25–27]. To explore whether this receptor has a similar role in cyclic dinucleotide-stimulated inflammasome activation, BMMs were prepared from MPYS/STING knockout mice [25]. Intriguingly, analogous to poly(dA:dT)-dependent IL-1β and caspase-1 activation, MPYS/STING was not required for the response to c-diGMP (Fig 4A; [6, 24, 30]). Yet, as previously reported, c-diGMP and poly(dA:dT)-stimulated IFN-β expression were highly dependent on MPYS/STING, whereas the response to poly(I:C) was MPYS/STING independent (Fig 4B; [24, 30]). These observations, as well as the lack of correlation between the cellular expression of MPYS/STING and c-diGMP response, raised the possibility that an unknown PRR both senses and directs the ability of c-diGMP to activate the NLRP3 inflammasome [46].
Figure 4.
c-diGMP-stimulated inflammasome activation is independent of MPYS/STING. (A) Secreted IL-1β and Cas1 were evaluated in LPS-primed WT* or MPYS/STING[−/−] BMMs, as in Fig 1. *(Note, the WT data (right panel) is a replicate of WT data presented in Fig 3A.) (B) IFN-β expression was evaluated by Q–PCR in WT or MPYS/STING[−/−] BMMs 6 h after transfection with c-diGMP (cdG; 10 nmol), poly(I:C) (I:C; 1 μg/ml) or poly(dA:dT) (dA:dT; 1 μg/ml) as in Fig 2. Results are reported as relative log fold change with respect to untreated controls and graphed as means±standard error from three independent experiments. (C) Peritoneal leukocytes were enumerated by FACS (see supplementary Fig S5A,B online) 15 hrs after IP injection of PBS or c-diGMP (cdG; 160 nmols). The scatter plot represents data from two independent experiments (n=6 mice for c-di-GMP and n=4 mice for PBS treatment). Statistical significance was evaluated by student’s t-test (Graphpad). BMMs, bone marrow-derived macrophages; Cas1, caspase-1; c-diGMP, 3′-5′ diguanylate; FACS, fluorescence-activated cell sorting; IP, intraperitoneal; IFN-β, interferon beta; IL-1β, interleukin 1 beta; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; WT, wild type.
Subsequent studies explored the ability of c-diGMP to stimulate inflammasome activation in vivo, absent priming (that is, signal 1), as has previously been reported for MSU, silica, alum and hemozoin, [47–50]. Peritoneal instillation of c-diGMP was associated with a robust accumulation of leukocytes (PMNs and macrophages) in wild-type mice (Fig 4C; supplementary Fig S5A online [31]). As anticipated, pretreatment of mice with an IL-1β blocking antibody prevented this response (supplementary Fig S5B online). Consistent with our in vitro analysis, the IL-1β-mediated recruitment of leukocytes was dependent on NLRP3. Even though the overall peritoneal response was only minimally affected by the loss of MPYS/STING, a more detailed analysis revealed that this was associated with an altered distribution in accumulating leukocytes (Fig 4C; supplementary Fig S5A online). The recruitment of PMNs was significantly reduced, likely reflecting a loss in IL-1β production. These findings suggest that MPYS/STING functions upstream of NLRP3 in vivo. This contrasts our observations in cultured macrophages in which both signal 1 (that is, PRR-dependent NF-κB activation) and signal 2 (that is, c-diGMP, ATP, nigericin, MSU, poly(dA:dT), and so on) were required to activate the inflammasome independently of MPYS/STING. Consistent with its response in vivo, MPYS/STING is required for c-diGMP-induced canonical NF-κB activation in myeloid cells (signal 1) (Jin Lei, manuscript in preparation). These observations implicate STING in a c-diGMP-dependent activation of NF-κB and might explain why NLRP3 ‘ligands’ are able to direct inflammasome activation in vivo absent signal 1.
In summary, these studies demonstrate that in addition to their ability to induce IFN-I expression through an interaction with MPYS/STING [21, 22, 26, 27], cyclic dinucleotides also activate the NLRP3 inflammasome at levels corresponding to those observed during an intracellular bacterial infection [23, 35]. Analogous to other NLRP3 stimulating agents, the response to cyclic dinucleotides was dependent on K+ and Ca++ mobilization, as well as the BRCC3 deubiquitinase. Surprisingly, the c-diGMP-stimulated inflammasome activation was neither associated with a loss in Δψm nor with increases in mROS production, as has recently been reported for other NLRP3 ‘triggers’ [10]. These observations exclude a significant role for mitochondrial damage during c-diGMP-dependent inflammasome activation and suggest this response is also likely to be independent of mitochondrial antiviral signalling protein [14]. Equally remarkable, in vitro cyclic dinucleotide-stimulated inflammasome activation was found to be independent of MPYS/STING, revealing that a distinct, unknown host receptor is directing the ability of cyclic dinucleotides to provide signal 2 for inflammasome activation. This receptor may also participate in other c-diGMP-stimulated responses [22, 25]. Although, preliminary studies have largely excluded a role for GBP5 in the response to cyclic dinucleotides (data not shown; [46]), it will be interesting to explore whether NLRP3 itself or DDX41 contribute to this response [29]. These observations suggest that mammals evolved at least two distinct innate responses to bacterial-delivered c-diGMP, one that promotes the IL-1β-dependent recruitment of inflammatory cells [50, 51], and another that entails IFN-I and potentially NF-κB-dependent activation of these immune cells. This intriguing dichotomy will be an important area of future investigation.
METHODS
Cell culture. THP-1 cells were cultured as previously reported [21]. ASC (shASC), NLRP3 (shNLRP3) knockdown and ASC re-expressing (shASCmut) THP-1 lines were previously characterized [32]. Primary bone marrow-derived macrophages (BMMs) were prepared from 129, C57Bl6/J, NLRP3[−/−], NLRC4[−/−], ASC[−/−], MPYS/STING[−/−], AIM2[−/−] and NLRP1b[−/−] mice [17, 25, 40, 52], as previously reported [21]. In some studies, cells were treated with inhibitors of K+ efflux (that is, KCl; or Glibenclamide, Sigma-Aldrich, St Louis, MO) or Ca++ mobilization (that is, phospholipase C inhibitor U73122, Sigma-Aldrich; or store-operated Ca2+ entry channel inhibitor 2-aminoethoxydiphenyl borate, 2-APB, Sigma-Aldrich). RT and Q–PCR were preformed as previously reported ([21]; see Supplementary Table S1 online for primers). All murine studies were IACUC approved (see supplementary Materials and methods online section for additional details).
Inflammasome stimulation. THP-1 cells were primed with phorbol myristate acetate (PMA; 100 nM, over night; Sigma-Aldrich) and BMMs were primed with LPS (1 μg/ml, 3 h; E. coli; type 025:86; Sigma-Aldrich) before stimulation with ATP (Sigma-Aldrich), MSU (Enzo Life Sciences, Farmingdale, NY), crystalline silica (Min-U-Sil 5, U.S. Silica, Berkeley Springs, WV), S. typhimurium (see supplementary Materials and methods online), L. pneumophila (see supplementary Materials and methods online; [26]), or transfection with synthetic c-diGMP or c-diAMP (> 99% pure; [53]), cGMP (Enzo Life Sciences), GTP (Sigma-Aldrich) or poly(dA:dT) (Sigma-Aldrich), as indicated. Transfections were carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or X-tremeGENE HP (Roche; Indianapolis, IN), respectively.
Immune assays. The level of IL-1β and caspase-1 in culture supernatants was measured by immunoblotting with anti-IL-1β (AF-401-NA; R&D System; Minneapolis, MN), anti-human-caspase1 (06-503; Millipore; Billerica, MA), anti-murine-caspase1 (SC-514; Santa Cruz; Santa Cruz, CA; [32]), or by IL-1β ELISA (BD Biosciences, San Jose, California).
Intraperitoneal injections. Control C57BL/6J, MPYS/STING[−/−] or NLRP3[−/−] mice were injected (IP; 200 μl) with either phosphate-buffered saline or c-diGMP (160 nmols). Cells were then collected (350 ×g; 10 min; 4 °C) from the peritoneal lavage (2 × 5 ml cold phosphate-buffered saline) of killed mice and evaluated by fluorescence-activated cell sorting (F4/80, clone BM8, Biolegend, San Diego, CA; and Ly6G, clone 1A8, BD Biosciences).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
Anthrax lethal toxin was a kind gift from Jeremy Mogridge (Department of Laboratory Medicine & Pathobiology, University of Toronto, ON, Canada). These studies were supported by the NIH grants AI096088 (C.S.), AI058211 (C.S.), AI062739 (J.C.C.), T32AI074491 (L.J.), AR061491 (B.H.K.), AI057157 (J.P.-Y.T.) and R37-AI029564 (J.P.-Y.T.).
Author contributions
A.A.A.-S, I.T., L.J., J.C.C., S.E.G. and C.S. designed the experiments; A.A.A.-S, I.T. and L.J. performed the research; A.G., S.L.B., I.C.A., B.H.K., K.A.F., A.L., S.E.G. and J.P.-Y.T. contributed valuable reagents and advice; A.A.A.-S., L.J., J.C.C., S.E.G. and C.S. analysed the data; and A.A.A.-S. and C.S. wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Kumar H, Kawai T, Akira S (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30: 16–34 [DOI] [PubMed] [Google Scholar]
- Magalhaes JG, Sorbara MT, Girardin SE, Philpott DJ (2011) What is new with Nods? Curr Opin Immunol 23: 29–34 [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R (2012) Inflammasomes in health and disease. Nature 481: 278–286 [DOI] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM (2012) Inflammasomes and their roles in health and disease. Ann Rev Cell Dev Biol 28: 137–161 [DOI] [PubMed] [Google Scholar]
- Schroder K, Muruve DA, Tschopp J (2009) Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol 19: R262–R265 [DOI] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T (2012) Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci of USA 109: 11282–11287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM (2009) Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 187: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ (2012) The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492: 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G (2013) K(+) Efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K et al. (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K et al. (2012) Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225 [DOI] [PubMed] [Google Scholar]
- Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN (2013) The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153: 348–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoed EM, Vance RE (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ED, Dietrich WF (2006) Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38: 240–244 [DOI] [PubMed] [Google Scholar]
- Kovarova M et al. (2012) NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J Immunol 189: 2006–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam A, Der SD, Mogridge J (2005) Differentiation of human monocytic cell lines confers susceptibility to Bacillus anthracis lethal toxin. Cell Microbiol 7: 281–292 [DOI] [PubMed] [Google Scholar]
- Lightfield KL et al. (2008) Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9: 1171–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva PV, Giglio KM, Sondermann H (2012) Sensing the messenger: the diverse ways that bacteria signal through c-di-GMP. Protein Sci 21: 929–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Sater AA et al. (2012) The overlapping host responses to bacterial cyclic dinucleotides. Microbes Infect 14: 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter SM et al. (2009) A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med 206: 1899–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Iavarone AT, Portnoy DA (2010) c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328: 1703–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Barber GN (2011) The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cell Mol Life Sci 68: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL (2011) MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol 187: 2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE (2011) STING is a direct innate immune sensor of cyclic di-GMP. Nature 478: 515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S et al. (2012) Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity 36: 1073–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatiyar K et al. (2012) The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 13: 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ (2011) The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol 12: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaolis DK et al. (2007) Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol 178: 2171–2181 [DOI] [PubMed] [Google Scholar]
- Willingham SB et al. (2007) Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J (2008) The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452: 103–107 [DOI] [PubMed] [Google Scholar]
- Levi A, Folcher M, Jenal U, Shuman HA (2011) Cyclic diguanylate signaling proteins control intracellular growth of Legionella pneumophila. mBio 2: e00310–00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfield KL et al. (2011) Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun 79: 1606–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ (2012) The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca(2+) and cAMP. Nature 492: 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM (2010) Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207: 1745–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S et al. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232 [DOI] [PubMed] [Google Scholar]
- Rathinam VA et al. (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11: 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RL, Lenz LL (2012) Distinct licensing of IL-18 and IL-1beta secretion in response to NLRP3 inflammasome activation. PLoS One 7: e45186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49: 331–338 [DOI] [PubMed] [Google Scholar]
- Lu B et al. (2012) Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488: 670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD (2012) GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336: 481–485 [DOI] [PubMed] [Google Scholar]
- He Y, Franchi L, Nunez G (2013) The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur J Immunol 43: 1147–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, Macmicking JD (2012) GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336: 481–485 [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241 [DOI] [PubMed] [Google Scholar]
- Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, Rock KL (2006) MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest 116: 2262–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS et al. (2006) MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24: 79–91 [DOI] [PubMed] [Google Scholar]
- Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP (2009) The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30: 556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajkowski A, Cieslak J, Gapeev A, Schindler C, Beaucage SL (2010) Convenient synthesis of a propargylated cyclic (3′-5′) diguanylic Acid and its ‘click’ conjugation to a biotinylated azide. Bioconjug Chem 21: 2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.