Abstract
If and how neurons remodel their connections after CNS injury critically influences recovery of function. Here, we investigate the role of the growth-initiating transcription factor STAT3 during remodelling of the injured corticospinal tract (CST). Endogenous STAT3 expression in lesioned cortical projection neurons is transient but can be sustained by viral gene transfer. Sustained activation of STAT3 enhances remodelling of lesioned CST fibres and induces de novo formation of collaterals from unlesioned CST fibres. In a unilateral pyramidotomy paradigm, this recruitment of unlesioned fibres leads to the formation of midline crossing circuits that establish ipsilateral forelimb activation and functional recovery.
Keywords: axonal remodelling, corticospinal tract, neuronal plasticity, spinal cord injury, STAT3
Introduction
Lesions to the spinal cord lead to the transection of axonal tract systems and are often followed by devastating motor and sensory deficits. If the lesion of the spinal cord is complete, severe deficits persist. If the lesion is however incomplete, some functional recovery can occur in rodents as well as humans [1–3]. Over the recent years, a number of studies have investigated the anatomical basis of this recovery process using the corticospinal tract (CST) as a model system [2, 4, 5]. The results show that while long-distance regeneration of transected CST fibres generally fails, lesioned CST connections spontaneously attempt to remodel after injury. We have previously identified intraspinal detour circuits that are formed by sprouting of CST collaterals in the cervical cord and the establishment of CST contacts onto long propriospinal neurons as key components of the endogenous remodelling process [2, 6]. Despite the formation of these detour circuits, spontaneous functional recovery in most cases remains incomplete. To further improve functional recovery, we thus need to devise strategies that can extend endogenous remodelling.
How the induction of axonal remodelling is regulated and how it can best be therapeutically supported is so far incompletely understood. As axonal remodelling requires the reorganization of connections distant from the lesion site, strategies that affect the intrinsic growth capability of the entire neuron are conceptually most suited [7]. We have previously identified the sustained activation of the transcription factor STAT3 (signal transducer and activator of transcription 3) as a crucial requirement for the timely induction of the intrinsic growth programme in dorsal root ganglion neurons [8]. A key role for STAT3 during neuronal growth initiation is supported by the following findings: (i) The nuclear accumulation and phosphorylation of STAT3 correlate with the regenerative response of the neuron after injury [8, 9]. (ii) Deletion of STAT3 impairs the timely initiation of PNS regeneration [8] and STAT3 inhibition blocks the growth-promoting effect of a conditioning lesion in the central nervous system [10]. (iii) STAT3 overexpression or the deletion of its inhibitor, SOCS3, can improve sprouting of central dorsal root ganglion projections [8] and promote optic nerve regeneration in vivo [11].
Here, we investigate whether and how growth initiation by STAT3 affects the remodelling of lesioned and unlesioned central nervous system axons and the resulting functional recovery after spinal cord injury.
Results and discussion
Endogenous STAT3 does not contribute to axonal remodelling
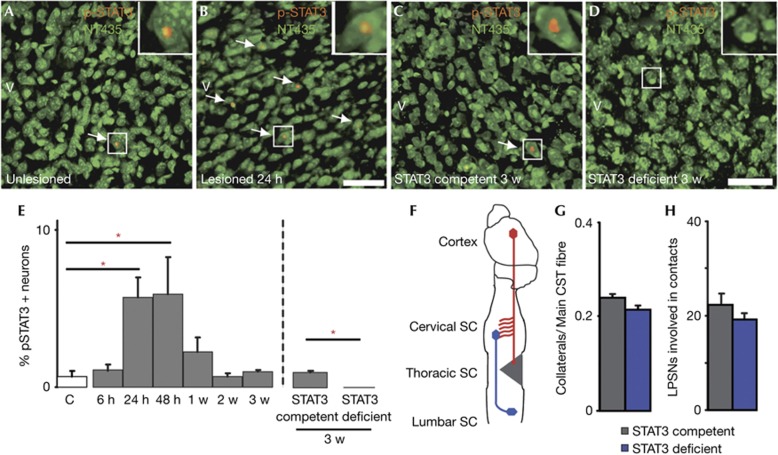
In the peripheral nervous system, the sustained expression and phosphorylation of STAT3 are crucial for the timely initiation of axonal outgrowth after lesion [8]. To examine whether STAT3 is also expressed and activated in lesioned cortical projection neurons, we investigated the expression of STAT3 and its activated phosphorylated form (p-STAT3) immunohistochemically in the hindlimb motor cortices of mice perfused at different timepoints from 6 h to 3 weeks following a dorsal mid thoracic hemisection. In unlesioned animals, only very few layer V pyramidal neurons in the motor cortex (that were identified on the basis of their typical morphology after NeuroTrace labelling) expressed either p-STAT3 (Fig 1A,E) or STAT3 (supplementary Fig S1 online). The number of p-STAT3-positive cortical projection neurons was then significantly increased at 24 h after lesion. However, even at this time, only a small subset of cortical projection neurons expressed STAT3 (supplementary Fig S1 online) or p-STAT3 (Fig 1B,E). Moreover, in these neurons, STAT3 expression and phosphorylation were only transiently induced and had returned to baseline levels at 1 week after injury (Fig 1E and supplementary Fig S1 online).
Figure 1.
Transient upregulation of p-STAT3 expression in cortical neurons does not contribute to endogenous CST remodelling after injury. (A–D) Confocal images of the expression of the activated form of STAT3, p-STAT3, in layer V cortical neurons (green, NeuroTrace 435; red, p-STAT3) of unlesioned (A), lesioned STAT3-competent (B, 24 h after lesion; C, 3 w after lesion) and STAT3-deficient mice (D). Arrows indicate p-STAT3-positive neurons (magnified in insets). (E) Quantification of p-STAT3 expression in layer V cortical neurons of unlesioned mice (white bar, n=5) and mice perfused at different timepoints following thoracic hemisection (grey bars, STAT3-competent mice; blue bar, STAT3-deficient mice; n=5–6 for each timepoint). (F) Schematic representation of the analysis of CST remodelling after a mid thoracic hemisection. (G,H) Quantification of axonal sprouting in the cervical spinal cord (G) and of the percentage of long propriospinal neurons contacted by CST fibres (H) in STAT3-competent (grey bars, n=9) and STAT3-deficient (blue bars, n=12) mice perfused 3 w following thoracic hemisection. All bars and error bars in this figure represent mean±s.e.m. Statistical analysis was performed using a one-way ANOVA with Tukey test for E (left panel) and t-tests for E (right panel), G, H. *P<0.05. Scale bars equal 50 μm in B (also for A) and in D (also for C). ANOVA, analysis of variance; CST, corticospinal tract; SC, spinal cord; STAT3, signal transducer and activator of transcription 3.
To assess whether those neurons that transiently expressed STAT3 are primarily responsible for endogenous attempts of axonal growth and remodelling, we selectively deleted STAT3 expression in cortical projection neurons. For this purpose, we crossed Emx-Cre mice, which express Cre recombinase in the forebrain [12], with STAT3fl/fl mice [13]. As expected, no STAT3 or p-STAT3 expressions are detected in cortical projection neurons of Cre-positive STAT3fl/fl mice (Fig 1C–E; supplementary Fig S1 online). Deletion of STAT3 did not appear to affect neuronal survival as similar densities of neurons were present in layer V of the motor cortex in Cre-positive and Cre-negative littermates (supplementary Fig S2 online). We then performed mid thoracic dorsal hemisections in STAT3-competent (Cre-negative) and conditional STAT3-deficient (Cre-positive) mice and examined the effects of STAT3 deletion on CST growth, remodelling and functional recovery. Our analysis revealed no differences between STAT3-competent and conditional STAT3-deficient mice in all parameters analysed (Fig 1F–H; supplementary Fig S3 online). In particular, we did not detect differences in the formation of intraspinal detour circuits as similar amounts of cervical collaterals formed at 1 week (supplementary Fig S3 online) and 3 weeks (Fig 1G) after injury, which did not differ in length, complexity and number of boutons (data not shown) and contacted similar proportions of long propriospinal neurons (Fig 1H). Consistent with these observations, no differences in the recovery of hindlimb locomotion were detected between STAT3-competent and conditional STAT3-deficient mice (supplementary Fig S4 online). These results show that the transient expression of STAT3 in cortical projection neurons is unlikely to contribute to the induction of the endogenous remodelling processes.
STAT3 enhances sprouting of lesioned CST fibres
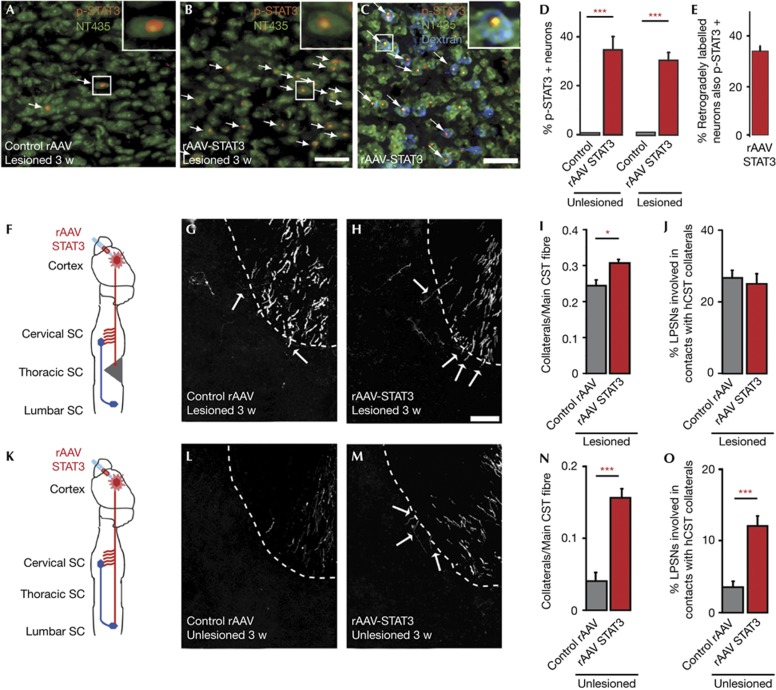
As the transient induction of STAT3 expression in a subset of cortical neurons after central lesions is thus insufficient to induce a robust neuronal growth response, we next investigated whether the exogenous induction of sustained STAT3 expression would be sufficient to promote axonal growth. To induce sustained STAT3 expression in cortical neurons, we injected recombinant adeno-associated viruses (rAAV) expressing STAT3 into the hindlimb motor cortex. Immunohistochemical analysis of STAT3 expression and phosphorylation confirmed the efficiency of viral gene transfer to corticospinal projection neurons (Fig 2A–E). To assess the effect of sustained STAT3 expression on the response of lesioned CST fibres, we injected rAAV-STAT3 or Control rAAV into the hindlimb motor cortex and lesioned the main dorsal and the minor dorsolateral component of the CST by a mid thoracic dorsal hemisection. Our results show that while sustained STAT3 expression did not affect lesion volume (0.307±0.02 mm3 versus 0.316±0.03 mm3 in mice injected with Control rAAV and rAAV-STAT3 respectively, n=7 mice per group) it significantly increased CST sprouting at the lesion site and moderately improved axonal growth beyond the lesion site (supplementary Fig S5 online). To assess to what extent sustained STAT3 expression can also support the remodelling of CST fibres distant from the lesion site, we quantified the formation of intraspinal detour circuits [2] after mid thoracic hemisections in mice injected with rAAV-STAT3 and Control rAAV. Indeed mice injected with rAAV-STAT3 showed an increased formation of cervical CST collaterals at 3 weeks after injury (Fig 2F–I). The intraspinal contact pattern of these collaterals, however, was not affected by this rather moderate increase in collateral number and a similar proportion of long propriospinal neurons were contacted in mice injected with rAAV-STAT3 and Control rAAV (Fig 2J). Taken together, this suggests that the endogenous growth response of lesioned CST projection neurons is sufficient to promote the formation of detour circuits at an ‘optimal’ rate that is not improved further by the presence of more CST collaterals. We noted, however, that unlesioned mice injected with rAAV-STAT3 showed a marked increase in the formation of cervical collaterals (Fig 2K–N) that resulted in a significantly higher number of contacts onto the long propriospinal neurons (Fig 2O). This indicated that sustained STAT3 expression might be able to also induce the remodelling of fibres that have not been primed to grow by their previous transection.
Figure 2.
Sustained STAT3 expression induces sprouting of lesioned and unlesioned fibres in the cervical spinal cord following injury. (A,B) Confocal images of p-STAT3 expression in layer V cortical neurons (green, NeuroTrace 435; red, p-STAT3) of mice injected with Control rAAV (A) or rAAV-STAT3 (B) and perfused 3 w following a mid thoracic hemisection. Arrows indicate p-STAT3-positive neurons (magnified in insets). (C) Confocal images of p-STAT3 expression in hindlimb CST projection neurons that were retrogradely labelled from the lesion site (blue, neurons retrogradely labelled with dextran tetramethylrhodamine; green, NeuroTrace 435; red, p-STAT3) in mice injected with rAAV-STAT3 and perfused 3 w following a mid thoracic hemisection. (D) Quantification of p-STAT3 expression in layer V neurons in the transduced area of the hindlimb motor cortex of unlesioned mice (left panel) either untreated (C same as in Fig 1E) or injected with rAAV-STAT3 (red bar) and lesioned mice (right panel, perfused 3 w after injury) injected with Control rAAV (grey bar) or rAAV-STAT3 (red bar, n=3–5 mice per group). (E) Quantification of p-STAT3 expression in hindlimb CST projection neurons that were retrogradely labelled from the lesion site in mice injected with rAAV-STAT3 (n=4). (F) Schematic representation of the analysis of cervical CST sprouting and remodelling following a mid thoracic spinal cord injury. (G,H) Confocal images of cervical hindlimb CST collaterals in lesioned mice injected with Control rAAV (G) or rAAV-STAT3 (H) and perfused 3 w following injury. Arrows indicate collaterals as they exit into the grey matter. (I,J) Quantification of the number of collaterals exiting the hindlimb CST in the cervical spinal cord (I) and of the percentage of long propriospinal neurons contacted by CST fibres (J) in mice injected with Control rAAV (grey bars, n=9) or rAAV-STAT3 (red bars, n=9) 3 w following spinal cord injury. (K) Schematic representation of the analysis of cervical CST sprouting and remodelling in unlesioned mice. (L,M) Confocal images of cervical hindlimb CST collaterals in unlesioned mice injected with Control rAAV (L) or rAAV-STAT3 (M). (N,O) Quantification of the number of collaterals exiting the hindlimb CST in the cervical spinal cord (N) and of the percentage of long propriospinal neurons contacted by CST fibres (O) in unlesioned mice injected with Control rAAV (grey bars, n=5) or rAAV-STAT3 (red bars, n=8). All bars and error bars in this figure represent mean±s.e.m. Statistical analysis was performed using t-tests. *P<0.05; ***P<0.001. Scale bar equals 50 μm in B (also for A), 25 μm in C and 50 μm in H (also for G,L,M). CST, corticospinal tract; rAAV, recombinant adeno-associated viruses; SC, spinal cord; STAT3, signal transducer and activator of transcription 3.
STAT3 recruits unlesioned CST connections
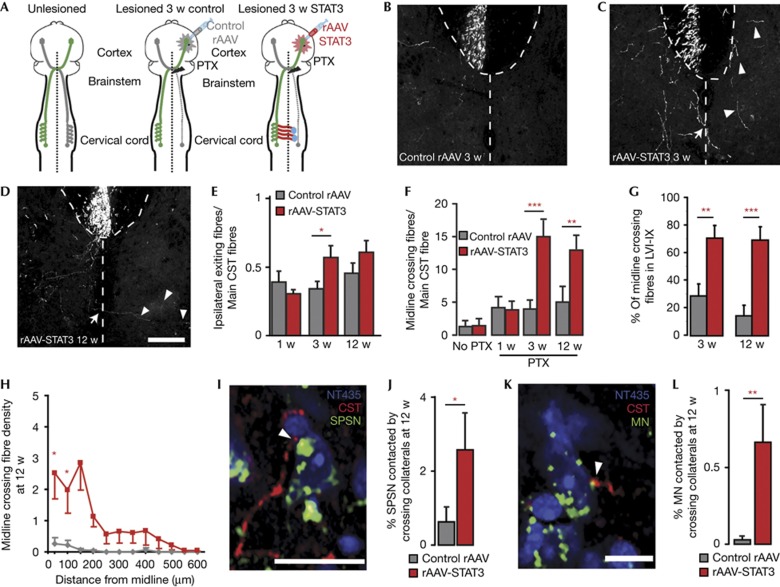
To further investigate the capability of STAT3 to recruit unlesioned fibres to the remodelling process, we induced a unilateral lesion of the left CST at the level of the medulla oblongata (‘unilateral pyramidotomy,’ Fig 3A). We then assessed whether and how unlesioned fibres from the contralateral, right forelimb portion of the CST remodel in response to the unilateral denervation. In animals injected with Control rAAV, no significant difference in the number of CST fibres that exited the right CST is detected at 1, 3 or 12 weeks after pyramidotomy (Fig 3B,E). Further, CST fibres that exit the CST rarely crossed the spinal midline (Fig 3B,F). In contrast, in animals injected with rAAV-STAT3, more CST collaterals exited the CST at 3 weeks after injury (Fig 3C,E). These newly formed collaterals extended towards the denervated side of the spinal cord resulting in a significant increase in the number of midline crossing fibres that was first detected at 3 weeks (Fig 3C,F) and persisted for at least 12 weeks after lesion (Fig 3D,F). We next examined the projection pattern of these newly formed midline crossing CST collaterals and found that in animals injected with rAAV-STAT3, CST collaterals extended significantly further into the denervated spinal cord and often projected to the intermediate and ventral laminae of the spinal cord (Fig 3G,H). As the cell bodies of short propriospinal neurons and spinal motoneurons that control forelimb movement are located in these laminae, we next labelled these cell populations using retrograde tracing and assessed whether they are targeted by midline crossing CST collaterals at 12 weeks after pyramidotomy. Our results show that the proportions of short propriospinal neurons and spinal motoneurons that are contacted by CST collaterals are increased more than 4- and 10-fold, respectively in animals injected with rAAV-STAT3 (Fig 3I–L). The finding that neuronal growth initiation by STAT3 is sufficient to induce the de novo formation of these midline crossing circuits indicates that the guidance signals that attract newly formed collaterals are endogenously present in the denervated spinal cord. It is interesting to note in this context that sustained STAT3 expression is not the only way to induce the remodelling of unlesioned CST fibres. Indeed previous studies have, for example, shown that the overexpression of the neuronal calcium sensor1 in projection neurons [14], or of neurotrophin 3 in the spinal target area [15] can induce sprouting and midline crossing of unlesioned CST fibres. Notably, a recent study further indicates that in primates, the CST can spontaneously extend midline crossing collaterals after spinal cord injury [16].
Figure 3.
Sustained STAT3 expression induces de novo formation of midline crossing circuits following pyramidotomy. (A) Schematic representation of the analysis of CST remodelling after unilateral pyramidotomy and injection of Control rAAV or rAAV-STAT3. (B–D) Confocal images of midline crossing fibres in mice injected with Control rAAV (B) or rAAV-STAT3 (C,D) and perfused 3 w (B,C) or 12 w (D) following pyramidotomy. Arrows indicate midline crossing fibres, arrowheads indicate examples of fibres that have already crossed the midline. (E,F) Quantification of the number of fibres exiting ipsilateral to the pyramidotomy from the main CST (E) and crossing the spinal midline (F) in mice injected with Control rAAV (grey bars) or rAAV-STAT3 (red bars) and perfused 1, 3 or 12 w (n=7–10 mice per group) following pyramidotomy. (G) Quantification of the percentage of midline crossing fibres that project to the contralateral (denervated) laminae VI to IX in mice injected with Control rAAV (grey bars) or rAAV-STAT3 (red bars) and perfused 3 or 12 w following pyramidotomy (n=7–10 mice per group). (H) Quantification of the density of midline crossing fibres in the contralateral (denervated) side of the spinal cord at different distances from the midline in mice injected with Control rAAV (grey line) or rAAV-STAT3 (red line) and perfused 12 w following pyramidotomy (n=7–8 mice per group). (I,K) Confocal images (single planes) of contacts between midline crossing forelimb CST collaterals (red) and a short propriospinal neuron (I, green) or a motoneuron (K, green) in mice injected with rAAV-STAT3 and perfused 12 w following the pyramidotomy. (J,L) Quantification of the percentage of short propriospinal neurons (J) and motoneurons (L) contacted by midline crossing forelimb CST collaterals in mice injected with Control rAAV (grey bars) or rAAV-STAT3 (red bars) and perfused 12 w following injury (n=7–8 mice per group). All bars and error bars in this figure represent mean±s.e.m. Statistical analysis was performed using a two-way ANOVA followed by Bonferroni test for E–G, and a one-way repeated ANOVA followed by Tukey test in H and t-tests for J, L. *P<0.05; **P<0.01; ***P<0.001. Scale bars equal 200 μm in D (also for B,C) and 25 μm in I and 10 μm in K. ANOVA, analysis of variance; CST, corticospinal tract; rAAV, recombinant adeno-associated viruses; SC, spinal cord; STAT3, signal transducer and activator of transcription 3.
Midline crossing circuits improve functional recovery
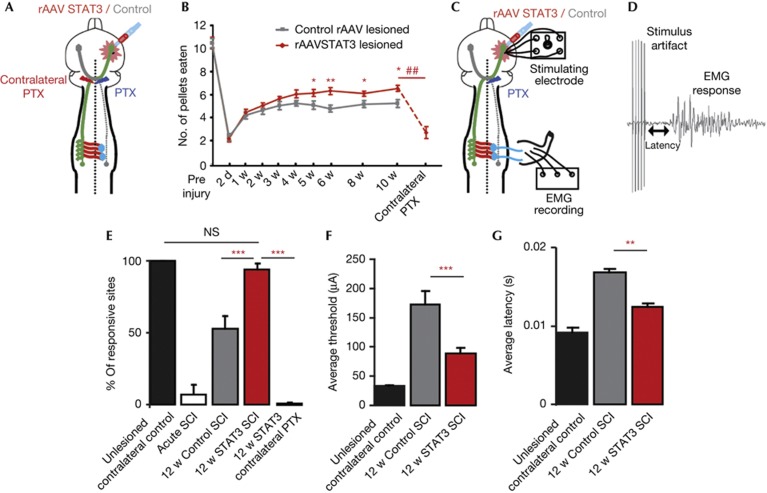
To investigate whether the newly formed midline crossing CST circuits induced by sustained STAT3 expression can foster functional recovery, we performed the following analyses after unilateral pyramidotomy. First, we used the staircase test, which measures the capability of mice to remove sugar pellets placed on different stairs of a staircase, to evaluate skilled forelimb grasping as previously described [17]. While mice from both experimental groups showed similarly impaired forelimb grasping immediately after injury, mice injected with rAAV-STAT3 performed significantly better in the staircase reaching task compared with mice injected with Control rAAV from 5 weeks after pyramidotomy onwards (Fig 4A,B). To assess the contribution of midline crossing circuits to this recovery, we followed the recovery process for 10 weeks and then lesioned the contralateral CST on the level of the pyramids. This lesion removes all midline crossing CST connections and leads to a complete reversal of functional recovery (Fig 4B). To further confirm the contribution of midline crossing circuits to functional recovery, we recorded forelimb flexor electromyographs (EMGs) after intracortical stimulations (Fig 4C,D) [4]. In unlesioned animals, EMG responses could be elicited in 100% of the cases by stimulation of the contralateral forelimb motor cortex (n=24 stimulations, Fig 4E). In the days following pyramidotomy, this response was basically abolished (Fig 4E). However at 12 weeks following pyramidotomy, nearly all sites in the ipsilateral cortex of animals treated with rAAV-STAT3 elicited EMG responses (Fig 4E). This finding is consistent with the idea that newly formed midline crossing CST fibres mediate this recovery. To confirm the contribution of new CST connections below the level of the pyramids to the recovery, we performed an additional pyramidotomy of the intact side in rAAV-STAT3-treated mice that had previously recovered responsiveness to stimulation. In these mice, the second pyramidotomy completely abolishes the response to cortical stimulation (Fig 4E). Further analysis of the cortical stimulation parameters revealed lower stimulation thresholds and shorter latencies to an EMG response in mice 12 weeks after injection with rAAV-STAT3 compared with mice injected with Control rAAV (Fig 4F,G).
Figure 4.
Sustained STAT3 expression promotes functional recovery following pyramidotomy. (A) Schematic representation of the pyramidotomy paradigm used for assessing behavioural recovery. (B) Quantification of the number of pellets eaten by mice injected with Control rAAV (grey line, n=23) or rAAV-STAT3 (red line, n=28) at different test intervals up to 10 w following pyramidotomy and at 3 days following lesion of the contralateral CST tract (contralateral PTX, n=13). (C) Schematic representation of the cortical stimulations and EMG recordings that we used to quantify circuit reconnection after pyramidotomy. (D) Trace of a forelimb EMG recording after cortical stimulation. (E) Quantification of the percentage of responsive sites contralateral to the lesion in unlesioned mice (black bar, n=6 mice) and ipsilateral to the lesion acutely following pyramidotomy (white bar) and 12 w following pyramidotomy in mice injected with Control rAAV (grey bar) or rAAV-STAT3 (red bar, n=6–8 mice per group). A contralateral pyramidotomy 12 w following the initial lesion and injection of rAAV-STAT3 abolishes the ipsilateral responses (red bar, contralateral PTX; n=5 mice). (F,G) Quantification of the stimulation thresholds (F) and latencies (G) of the forelimb responses in unlesioned mice (black bars, contralateral to lesion) in mice injected with Control rAAV (grey bars, ipsilateral to the lesion) or rAAV-STAT3 (red bars, ipsilateral to the lesion) at 12 w following pyramidotomy (n=5–8 mice per group). All bars and error bars in this figure represent mean±s.e.m. Statistical analysis was performed using a repeated one-way ANOVA followed by Tukey test in B and a one-way ANOVA followed by Tukey test in E, F, G. *P<0.05; **P<0.01; ***P<0.001. ##P<0.01 10 w re-lesion vs 10 w. ANOVA, analysis of variance; CST, corticospinal tract; EMG, electromyography; SCI, spinal cord injury; STAT3, signal transducer and activator of transcription 3.
Taken together, our electrophysiological and behavioural analyses indicate that the midline crossing CST circuits induced by sustained expression of STAT3 are functional and contribute to improved recovery of forelimb function after injury. While the viral gene transfer strategy used in this study is not directly translatable to a clinical setting as treatment was initiated before injury, we believe that strategies that enhance endogenous remodelling processes have considerable therapeutic potential. This has been documented by a number of recent studies in rodents [18, 19] as well as first reports in humans [20]. The recruitment of unlesioned fibres to the remodelling process, for example, by induction of sustained STAT3 expression, should thus be a promising complement to these strategies and help advance therapeutic concepts that improve functional recovery in neurological conditions in which trauma, inflammation or ischaemia cause permanent axon damage.
Methods
Animals. To delete STAT3 expression in cortical projection neurons, we crossed STAT3fl/fl mice [13], in which deletion of the STAT3 gene depends on Cre-mediated excision of loxP sites, and Emx-Cre mice [12], in which regulatory elements of the Emx1 gene drive Cre expression in the forebrain starting at E12.5. Adult female mice homozygous for the floxed STAT3 allele and either expressing Cre (STAT3-deficient group) or Cre-negative (STAT3-competent group) were used for experiments. For all other experiments, we used adult female C57/Bl6 mice (6–12 weeks old). All animal experiments were performed in accordance with regulations of the animal welfare act and protocols approved by the Regierung von Oberbayern. For further methods, see supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank A. Schmalz and G. Heitmann for excellent technical assistance, D. Matzek for animal husbandry, S. McMullan for helpful advice on EMG recordings and S. Akira for providing the STAT3fl/fl mice. Work in F.M.B’s lab is supported by the Deutsche Forschungsgemeinschaft (SFB 870) and by the Federal Ministry of Education and Research (BMBF, Independent Groups in the Neurosciences). Work in M.K.’s lab is supported by the DFG (SFB 870 and SFB-Tr 128), the BMBF (Competence Network Multiple Sclerosis), the European Research Council under the European Union’s Seventh Framework Program (FP/2007-2013; ERC Grant Agreement n. 310932), the Hertie Foundation and the Verein ‘Therapieforschung für MS-Kranke e.V.’
Author contributions: M.K. and F.M.B. conceived the experiments. C.L. performed the analysis of axon growth, remodelling and behavioural recovery. C.L. and A.J. performed and evaluated immunohistochemistry experiments. P.M.B. conducted the electrophysiological analysis. C.L, P.M.B., M.K. and F.M.B. wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Dietz V Wirz M Colombo G & Curt A (1998) Locomotor capacity and recovery of spinal cord function in paraplegic patients: a clinical and electrophysiological evaluation. Electroencephalogr Clin Neurophysiol 109: 140–153 [DOI] [PubMed] [Google Scholar]
- Bareyre FM Kerschensteiner M Raineteau O Mettenleiter TC Weinmann O & Schwab ME (2004) The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7: 269–277 [DOI] [PubMed] [Google Scholar]
- Courtine G Song B Roy RR Zhong H Herrmann JE Ao Y Qi J Edgerton VR & Sofroniew MV (2008) Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 14: 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K Pedersen V Schwab ME & Brösamle C (2001) Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770 [DOI] [PubMed] [Google Scholar]
- Weidner N Ner A Salimi N & Tuszynski MH (2001) Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci USA 98: 3513–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C Guo X Kerschensteiner M & Bareyre FM (2012) Single collateral reconstructions reveal distinct phases of corticospinal remodeling after spinal cord injury. PLoS One 7: e30461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K Tedeschi A Park KK & He Z (2011) Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci 34: 131–152 [DOI] [PubMed] [Google Scholar]
- Bareyre FM Garzorz N Lang C Misgeld T Büning H & Kerschensteiner M (2011) In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci USA 108: 6282–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE Cho Y Beirowski B Milbrandt J Cavalli V & DiAntonio A (2012) Dual leucine zipper kinase is required for retrograde signalling and axonal regeneration. Neuron 74: 1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J Cafferty WBJ McMahon SB & Thompson SWN (2005) Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci 25: 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F et al. (2011) Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480: 372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA Talley T Qiu M Puelles L Rubenstein JL & Jones KR (2002) Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci 22: 6309–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa A Takeda K Kudo S Maeda T Kagayama M & Akira S (2003) Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J Immunol 171: 6198–6205 [DOI] [PubMed] [Google Scholar]
- Yip PK Wong LF Sears TA Yáñez-Muñoz RJ & McMahon SB (2010) Cortical overexpression of neuronal calcium sensor-1 induces functional plasticity in spinal cord following unilateral pyramidal tract injury in rat. PLoS Biol 8: e1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L Baumgartner BJ Hill-Felberg SJ McGowen LR & Shine HD (2003) Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J Neurosci 23: 1424–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES et al. (2010) Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci 13: 1505–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey ML Barritt AW Yip PK Davies M Hamers FPT McMahon SB & Bradbury EJ (2005) Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp Neurol 195: 524–539 [DOI] [PubMed] [Google Scholar]
- Girgis J Merrett D Kirkland S Metz GAS Verge V & Fouad K (2007) Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain 130: 2993–3003 [DOI] [PubMed] [Google Scholar]
- van den Brand R et al. (2012) Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336: 1182–1185 [DOI] [PubMed] [Google Scholar]
- Harkema S et al. (2011) Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.