Abstract
Phosphatidic acid (PA) and phosphoinositides are metabolically interconverted lipid second messengers that have central roles in many growth factor (GF)-stimulated signalling pathways. Yet, little is known about the mechanisms that coordinate their production and downstream signalling. Here we show that the phosphatidylinositol (PI)-transfer protein Nir2 translocates from the Golgi complex to the plasma membrane in response to GF stimulation. This translocation is triggered by PA formation and is mediated by its C-terminal region that binds PA in vitro. We further show that depletion of Nir2 substantially reduces the PI(4,5)P2 levels at the plasma membrane and concomitantly GF-stimulated PI(3,4,5)P3 production. Finally, we show that Nir2 positively regulates the MAPK and PI3K/AKT pathways. We propose that Nir2 through its PA-binding capability and PI-transfer activity can couple PA to phosphoinositide signalling, and possibly coordinates their local lipid metabolism and downstream signalling.
Keywords: EGFR signalling, HAD, lipid second messengers, PA, phosphoinositide signalling
INTRODUCTION
The conversion of membrane phospholipids into intracellular lipid second messengers is an early response to many growth factor (GF) receptors (GFRs) activation [1]. Among the different lipid second messengers, phosphoinositides (PIns) and PA activate several signalling pathways [2]. PI(4,5)P2, for example, is rapidly hydrolysed by phospholipase Cγ (PLCγ), which triggers the production of inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) and consequently activates the MAP-kinase (MAPK) (ERK1/ERK2) pathway. PI(3,4,5)P3 is produced by PI3 kinase and activates the AKT-downstream pathways [3]. Many GFRs, including epidermal growth factor receptor (EGFR), also activate phospholipase D (PLD1 and PLD2), which in turn hydrolyses phosphatidylcholine (PC) to produce PA [4]. PA can bind and activate various signalling proteins including Raf [5], the Ras guanine nucleotide-exchange factor Sos [6], and mTOR [7], and consequently stimulates the Ras-MAPK and mTOR pathways, respectively.
Remarkably, PA also activates type I PI4P5-kinase [8], which phosphorylates PI4P to PI(4,5)P2 [9], while PI(4,5)P2 enhances PLD activity [10]. This positive feedback loop demonstrates the interdependence of PA and PI(4,5)P2 synthesis at the plasma membrane (PM) [11]. Yet, activation of many GFRs, including EGFR, triggers the hydrolysis of PI(4,5)P2 and concomitantly the production of PA [11]. PI(4,5)P2 hydrolysis leads to DAG production, which can be phosphorylated by DAG-kinase to produce PA, while PA can be dephosphorylated by PA phosphatase (PAP) to produce DAG (supplementary Fig S1 online) [12, 13]. Both PA and PIns can trigger independent signalling cascades that can subsequently converge and modulate various cellular responses. Hence, the interplay between PA and PIns synthesis/turnover is critical for maintaining signalling capacity. However, at present it is unclear how the levels of PA and PIns as well as their downstream signalling events are coordinated. Here we found that the PI-transfer protein Nir2 is also a PA-binding protein that positively regulates PIns signalling. We propose that Nir2 can link PA to GF-stimulated PIns-signalling through its PI-transfer activity and its PA-binding capability.
RESULTS AND DISCUSSION
Nir2 translocates from the Golgi to the PM
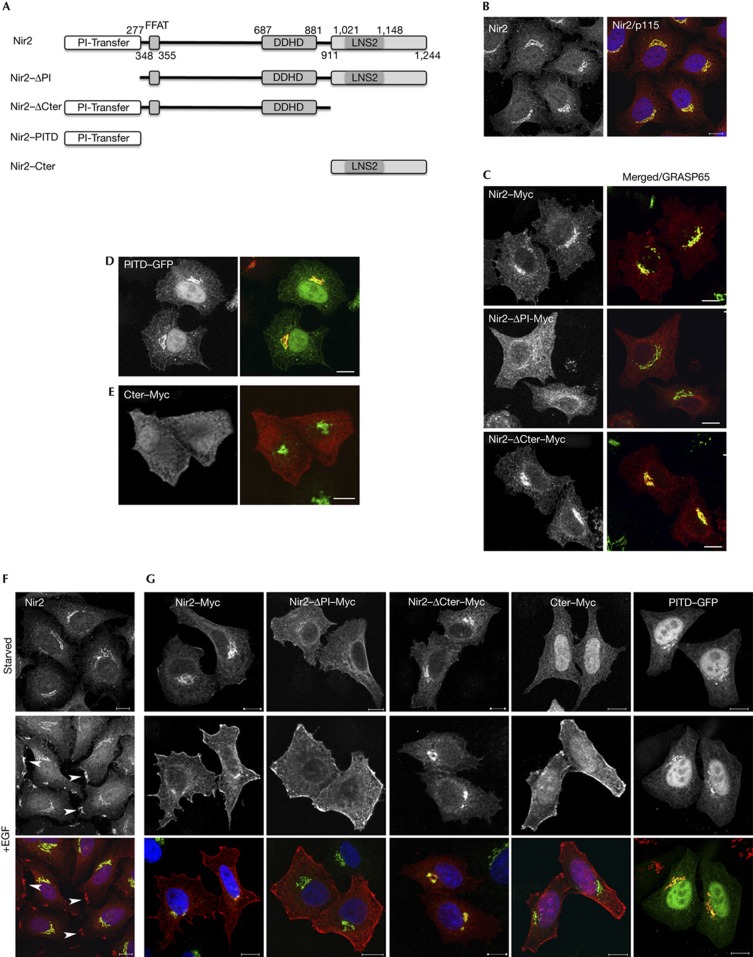
We previously showed that Nir2 localizes mainly to the Golgi apparatus in interphase cells [14, 15]. The mechanisms that govern its Golgi localization, however, remain unclear, as Nir2 lacks an obvious Golgi targeting motif. To define the domain that mediates its Golgi localization, we examined the localization of truncated Nir2 mutants by immunofluorescence analysis. Four mutants were established: a mutant lacking the N-terminal PI-transfer domain (ΔPI), a mutant deleted of the C-terminal domain (ΔCter) and mutants that consist of only the PI-transfer domain (PITD) or the C-terminal region (Cter) (Fig 1A). As shown, endogenous Nir2 (Fig 1B) as well as ectopically expressed wild-type (WT) Nir2–Myc (Fig 1C) localized mainly to the Golgi complex of HeLa cells. Deletion of the PITD, but not of the C-terminal region, markedly reduced its Golgi localization. Furthermore, the PITD fused to green fluorescent protein (GFP) was localized in the Golgi complex (Fig 1D), whereas the C-terminal domain (Cter–Myc) was distributed throughout the cytosol (Fig 1E). These results indicate that the Golgi localization of Nir2 is mainly mediated by its PITD.
Figure 1.
The PITD is essential for the Golgi localization of Nir2, whereas the C-terminal region mediates its PM targeting. (A) Domain organization of Nir2 and its truncated mutants. The N-terminal PITD, the FFAT motif that mediates its interaction with VAP-A/B proteins, the DDHD domain and the C-terminal region containing the LNS2 (Lipin/Nde1/Smp2) domain. (B) Endogenous Nir2 (red) is localized mainly to the Golgi apparatus of HeLa cells as shown by its co-localization with the Golgi marker p115 (green). (C–E) The Golgi localization of Myc-tagged Nir2 protein and its indicated mutants was assessed by colocalization with the indicated Golgi markers (p115 and GRASP65) in HeLa cells. Scale bar, 10 μm. (F,G) The localization of endogenous Nir2 (F) or ectopically expressed Myc-tagged Nir2 and its indicated truncated mutants (G) in serum-starved or EGF-treated (10 min, 100 ng/ml) HeLa cells was examined by double immunostaining with anti-Nir2 (F) or anti-Myc (G) antibodies (red) together with anti-p115 antibody (green). Localizations at PM patches are marked by arrowheads (F). Scale bar, 10 μm. EGF, epidermal growth factor; GFP, green fluorescent protein; PITD, phosphatidylinositol-transfer domain; PM, plasma membrane.
Although Nir2 localizes to the Golgi under steady-state conditions, our previous studies indicate that Nir2 can also be found in other subcellular locations, including the cleavage furrow and midbody during cytokinesis [14], and lipid droplets under certain metabolic conditions [16]. We noticed that the stimulation of serum-starved HeLa cells with EGF triggered a rapid translocation of endogenous Nir2 to the PM, where it was concentrated in membrane patches (Fig 1F). Similar results were obtained in MCF7 cells (supplementary Fig S2A online). WT Nir2–Myc as well as the ΔPI and the Nir2–Cter mutants were also detected at the PM shortly after EGF stimulation. The ΔCter mutant and the PITD, however, were retained in the Golgi in both serum-starved and EGF-stimulated HeLa cells (Fig 1G), suggesting that the C-terminal domain of Nir2 mediates its interaction with the PM in response to EGF stimulation.
PA triggers the PM translocation of Nir2
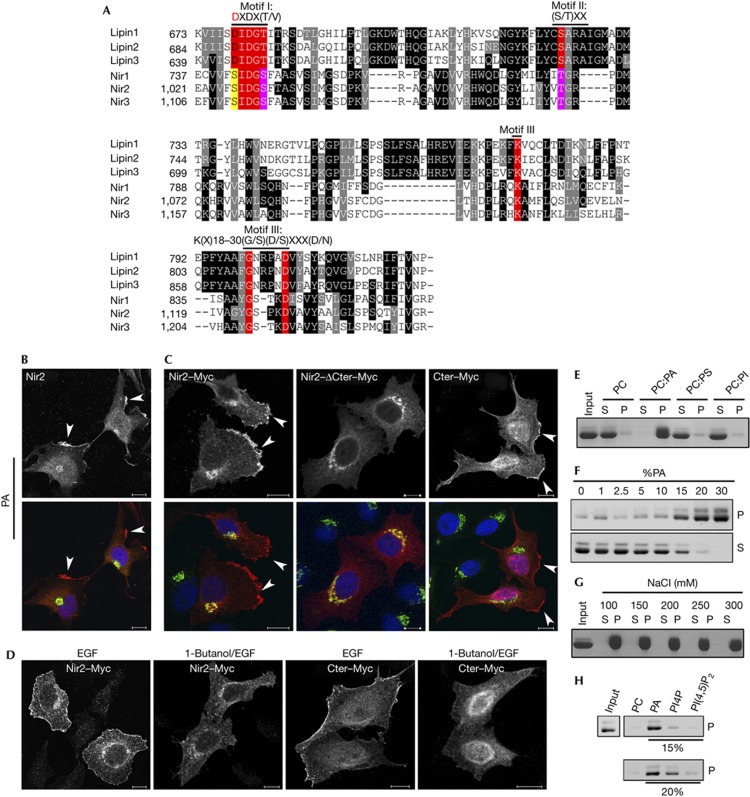
The C-terminal of Nir2 (aa 911–1244) is a highly conserved region that exhibits a strong sequence similarity (∼67%) with the C-terminal regions of its closely related proteins; Nir1 and Nir3, as well as with the Drosophila rdgB homologue (∼56%) [17]. This region contains an LNS2 (Lipin/Nde1/Smp2) domain (aa 1,021–1,148 in Nir2; Pfam: PF08235), which was identified in the lipin proteins as an haloacid dehalogenase (HAD)-like domain possessing a Mg2+-dependent PAP activity [18, 19]. The HAD superfamily is one of the largest and most ubiquitous enzyme families, of which ATPases and phosphatases are most prevalent [20]. The HAD superfamily is characterized by three sequence motifs (Fig 2A): the DXDX(T/V) motif contains an absolutely conserved aspartate residue (underlined) that is critical for the catalytic activity [21]. This motif is conserved within the lipin proteins and is required for their PAP activity [22]. A similar motif is also found in Nir proteins, but the conserved aspartate residue is substituted by a serine (Fig 2A), suggesting that Nir2 lacks an intrinsic PAP activity, but rather binds to PA.
Figure 2.
Phosphatidic acid (PA) triggers the translocation of Nir2 to the plasma membrane (PM) through the C-terminal region that binds to PA. (A) Alignment of the LNS2 (Lipin/Nde1/Smp2) domains of the human lipin and Nir proteins (accession numbers are given in supplementary information online). The alignment was obtained by Clustal W. Black and grey backgrounds represent degree of similarity (black; identity: 14.4%, grey; high similarity: 20.92%), numbers represent aa residues. The three conserved motifs in the haloacid dehalogenase superfamily: Motif I, DXDX(T/V), contains a conserved aspartate residue (red), which acts as nucleophile to form an acylphosphate intermediate in the proposed reaction mechanism for phosphotransferases [20, 21]. In Motif II, S/TXX, the conserved Ser/Thr is involved in hydrogen bonding to the phosphoryl oxygen, whereas Motif III, K(X)18-30(G/S)(D/S)XXX(D/N), is involved in phosphoryl oxygen hydrogen bonding and coordination of the magnesium ion [20]. (B,C) Serum-starved HeLa cells expressing endogenous Nir2 (B) or the indicated Myc-tagged Nir2 proteins (C) were stimulated with PA (100 μM) for 30 min. The cells were then fixed and double immunostained with anti-Nir2 (B) or anti-Myc (C) antibodies (red) together with anti-p115 antibody (green). The localization of endogenous Nir2 (B) as well as Nir2–Myc and its mutants (C) is shown. PM localization is marked by arrowheads. (D) Effect of 1-butanol on the translocation of Nir2. Serum-starved HeLa cells were preincubated with 0.3% 1-butanol for 30 min, and then stimulated with EGF (100 ng/ml) for 10 min in the presence of 1-butanol. The cells were fixed, immunostained with anti-Myc antibody and analysed by confocal microscope. Scale bar, 10 μm. (E) The C-terminal region of Nir2 binds to PA in vitro. The binding of recombinant C-terminal region of Nir2 to multilamellar vesicles consisting of PC alone, PC:PA (2:1), PC:PS (2:1) or PC:PImix (5:1) was assessed by liposome sedimentation assay (Methods). P and S indicate pellet and supernatant fraction, respectively. (F–H) Binding of recombinant C-terminal region of Nir2 to multilamellar vesicles containing either the indicated percentage of PA (F), 33% PA in the presence of the increasing concentrations of NaCl (G), or to vesicles containing 15% or 20% of PA, PI4P or PI(4,5)P2 (H) was examined as described in E.
As Nir2 translocates to the PM in response to EGF through its C-terminal region, and EGF activates PLD, which triggers PA production, we first examined whether exogenous PA can mimic the effect of EGF and induce the translocation of Nir2 to the PM. Serum-starved HeLa cells were treated with PA (100 μM) for 30 min and the localization of endogenous Nir2 was examined by immunofluorescence analysis. As shown, PA could trigger the translocation of endogenous Nir2 to PM patches (Fig 2B). PA also triggered the translocation of WT Nir2–Myc and its C-terminal region to the PM, but not of the ΔCter mutant (Fig 2C). These results indicate that the C-terminal mediates the translocation of Nir2 to the PM in response to both EGF and PA treatments.
To examine whether the translocation of Nir2 to the PM in response to EGF treatment is dependent on PA production, we pretreated serum-starved HeLa cells with 1-butanol, a PLD inhibitor [23], and then stimulated the cells with EGF. As shown, 1-butanol inhibited the translocation of WT Nir2 and its C-terminal domain to the PM in response to EGF treatment (Fig 2D), suggesting that EGF induces the production of PA and consequently the PM translocation of Nir2.
The results shown in Fig 2A–D indicate that the C-terminal region of Nir2 can bind to PA. To explore this possibility, we expressed the C-terminal region of Nir2 as a recombinant protein in bacteria, and examined its ability to bind to PA in vitro by liposome sedimentation assays. The recombinant purified His-tagged protein was incubated with multilamellar vesicles consisting of PC alone, or PC and either PA, PS or PIns mix as indicated. Binding was observed only to liposomes containing PA (Fig 2E). The binding was saturated at 20–25% PA (Fig 2F) and was not influenced by increasing the salt concentration of up to 300 mM (Fig 2G). A weak binding to PI4P, but not to PI(4,5)P2, was also detected possibly due to the mono-phosphate group common to both PA and PI4P (Fig 2H). These observations imply that the LNS2 domain that is known to bind a phosphoryl-group [20] mediates the PA binding in Nir2.
Nir2 modulates EGFR-mediated signalling pathways
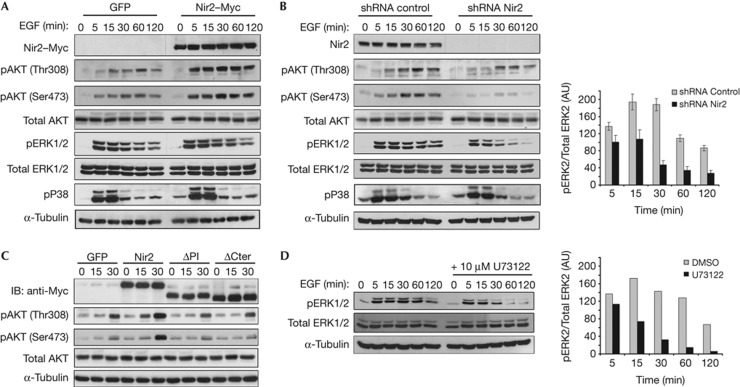
The translocation of Nir2 to the PM in response to EGF treatment implies that Nir2 participates in EGFR signalling. We, therefore, examined the influence of Nir2 overexpression or its downregulation by short hairpin RNA (shRNA) on EGFR-mediated downstream signals in HeLa (Fig 3) and MCF7 cells (supplementary Fig S2B online). Overexpression of Nir2 substantially enhanced the phosphorylation of AKT on both Thr308 and Ser473, but had a minor or no detectable effect on ERK1/2 or p38MAPK phosphorylation, respectively (Fig 3A). However, downregulation of Nir2 expression markedly reduced the phosphorylation of AKT as well as of ERK1/2. The effect of Nir2 shRNA on ERK1/2 phosphorylation was more profound at 30–120 min following EGF stimulation. Yet, Nir2 depletion had no effect on p38MAPK phosphorylation (Fig 3B). Similar effects on these downstream pathways were obtained with at least two more Nir2 shRNAs using lentivirus infection (supplementary Fig S3B online). These Nir2 shRNAs caused neither Golgi fragmentation nor inhibition of Golgi-mediated trafficking (supplementary Fig S3A,C,D online) as opposed to the Nir2 short interfering RNA that we used previously [15], possibly due to the higher potency and transient effect of the short interfering RNA.
Figure 3.
Nir2 modulates specific signalling pathways downstream to EGFR. HeLa cells were infected with lentiviruses expressing GFP, the Myc-tagged Nir2 (A), control shRNA or Nir2 shRNA (#1) (B). The cells were serum starved for 18 h and then stimulated with EGF (100 ng/ml) for the indicated time periods. Total cell lysates were analysed for phosphorylation of the indicated proteins by western blotting (WB) using the corresponding antibodies. Reproducible results were obtained in at least four experiments. (C) HeLa cells were infected with lentiviruses encoding GFP, Myc-tagged WT Nir2, or its ΔPI or ΔCter mutants. Three days later, the cells were serum starved for 18 h and then stimulated with EGF for the indicated time periods, lysed and immunoblotted with antibodies against the indicated proteins. Shown are representative results of at least four experiments. (D) Serum-starved HeLa cells were pretreated with the PLCγ inhibitor U73122 (10 μM, 30 min), and then stimulated with EGF for the indicated time periods. Total cell lysates were examined for ERK1/2 phosphorylation by WB using anti-pERK1/2 antibody. A.U., arbitrary unit; DMSO, dimethyl sulphoxide; EGF, epidermal growth factor; GFP, green fluorescent protein; IB, immunoblot; PITD, phosphatidylinositol-transfer domain; PM, plasma membrane; shRNA, short hairpin RNA.
To better characterize the stimulatory effect of Nir2 overexpression on EGF-induced AKT phosphorylation, we examined the influence of its truncated ΔPI and ΔCter mutants. Neither the ΔPI nor the ΔCter mutant enhanced the phosphorylation of AKT in response to EGF (Fig 3C), suggesting that the stimulatory effect of Nir2 on AKT phosphorylation requires both the translocation of Nir2 to the PM and its PI-transfer activity.
Nir2 regulates PI(4,5)P2 and PI(3,4,5)P3 production
The enhanced effects of Nir2 overexpression on AKT phosphorylation, and the inhibitory effects of its shRNA on both AKT and ERK1/2 phosphorylation, indicate that Nir2 regulates several signalling pathways downstream of EGFR. Phosphorylation of AKT is absolutely dependent on PI(3,4,5)P3 production, whereas ERK1/2 phosphorylation can be activated by several upstream pathways, including the PLCγ-mediated PI(4,5)P2 hydrolysis pathway [3]. Inhibition of this pathway by the PLC inhibitor U73122 substantially reduced the phosphorylation of ERK1/2 at 30–120 min following EGF-treatment (Fig 3D), resembling the effect of Nir2 depletion on ERK1/2 phosphorylation (Fig 3B). These results indicate that Nir2 regulates the levels of PI(4,5)P2 and PI(3,4,5)P3 and consequently their downstream signalling pathways.
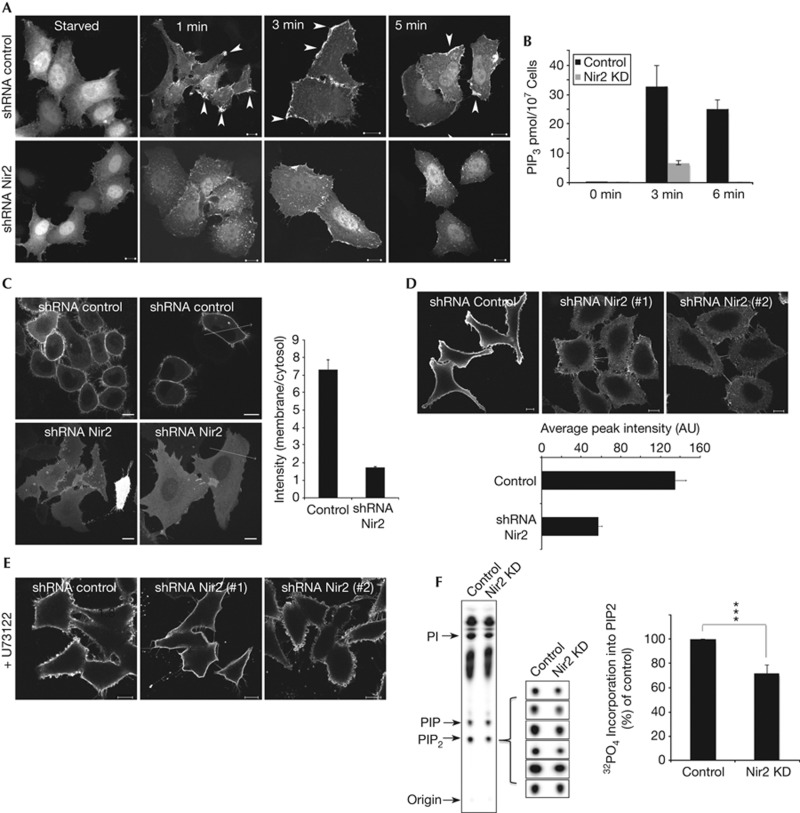
To monitor the level of PI(3,4,5)P3 in response to EGF stimulation, we used the PH domain of AKT fused to GFP as a reporter [24, 25]. GFP–PH–AKT was visualized in the cytosol and nuclei of serum-starved HeLa cells. EGF treatment triggered a rapid translocation of this reporter to the PM of the control cells; within 1 min GFP–PH–AKT was detected in PM patches. Its PM localization was further enhanced at 3 min, and then slightly declined at 5 min (Fig 4A). In contrast, the recruitment of GFP–PH–AKT to the PM of Nir2-depleted HeLa cells (Fig 4A) or MCF7 cells (supplementary Fig S2C online) was much less profound at all the examined time points, suggesting that Nir2 positively regulates PI(3,4,5)P3 production in response to EGF stimulation. Indeed, quantitative analysis of EGF-induced PI(3,4,5)P3 production utilizing a competitive enzyme-linked immunosorbent assays clearly showed the inhibitory effect of Nir2 depletion on PI(3,4,5)P3 production (∼32 pmol PI(3,4,5)P3/107 control cells as compared with ∼6.5 pmol/107 Nir2-depleted HeLa cells at 3 min following EGF treatment; Fig 4B).
Figure 4.
Nir2 regulates the levels of PI(4,5)P2 and PI(3,4,5)P3 at the PM. (A) The production of PI(3,4,5)P3 in response to EGF is markedly reduced in Nir2-depleted cells. Control or Nir2-depleted HeLa cells were transiently transfected with a vector encoding the PH–AKT–GFP fusion protein. Twenty-four hours later, the cells were serum starved for 16 h and then stimulated with EGF (100 ng/ml) for the indicated time points. The cells were fixed and examined by confocal microscope. Localization of PI(3,4.5)P3 in control cells is marked by arrowheads. Scale bar, 10 μm. (B) Quantitative analysis of EGF-induced PI(3,4,5)P3 production in control and Nir2-knockdown HeLa cells was performed as described in METHODS. The results are the mean values±s.d. of two experiments that were performed in triplicates. (C) Control and Nir2-depleted HeLa cells were transfected with a vector encoding the PH–PLCδ–GFP fusion protein. The distribution of this reporter was examined in live cells. The relative fluorescence intensity along the marked lines was measured (Zeiss LSM510 software), and the ratio between the signals at the PM and the cytosol was calculated for 50 cells and is shown in the graph. (D) The level of PI(4,5)P2 at the PM of control and Nir2-depleted HeLa cells was determined by immunostaining using anti-PI(4,5)P2 antibody. The intensity of the signals at the PM was measured as described in B, and the mean values±s.d. of 200 cells are shown in the attached graph. (E) The PLCγ inhibitor U73122 restored the PI(4,5)P2 levels in the PM of Nir2-depleted cells. Control and Nir2-depleted HeLa cells were treated with 10 μM U73122 for 30 min, and then fixed, immunostained with anti-PI(4,5)P2 antibody, and analysed by confocal microscope. (F) Synthesis of PI(4,5)P2 in control or Nir2-KD HeLa cells was measured as described in METHODS. Shown is a representative TLC along with the PI(4,5)P2 signals of six additional experiments and a graph that summarizes the mean values±s.d. of seven experiments that were performed in duplicates. Statistical significance was assessed by Student’s t-test. ***P<0.005. A.U., arbitrary unit; EGF, epidermal growth factor; GFP, green fluorescent protein; KD, knockdown; PI, phosphatidylinositol; PLCγ, phospholipase Cγ; PM, plasma membrane; shRNA, short hairpin RNA; TLC, thin layer chromatography.
We next examined the level of PI(4,5)P2, a precursor for PI(3,4,5)P3. To monitor the PI(4,5)P2 levels at the PM, we used the PH domain of PLCδ fused to GFP as a reporter [26], and examined its distribution in HeLa cells and in MCF7 cells by live-cell imaging. As shown, GFP–PH–PLCδ was localized mainly to the PM of control cells, but could hardly be detected at the PM of Nir2-depleted HeLa cells (Fig 4C) or MCF7 cells (supplementary Fig S2D online). The relative fluorescence intensity between the PM and the cytosol was ∼4.5 times higher in the control cells as compared with Nir2-depleted cells (n=50), suggesting that depletion of Nir2 substantially reduces the PI(4,5)P2 levels at the PM. Immunostaining with anti-PI(4,5)P2 antibodies (Fig 4D) confirmed these results and showed the remarkable effect of Nir2 depletion on PI(4,5)P2 level at the PM.
The PI(4,5)P2 level at the PM is regulated by several pathways that control its production and turnover, including its hydrolysis by PLCγ. Strikingly, the PLC inhibitor U73122 restored the PI(4,5)P2 level at the PM of Nir2-depleted cells (Fig 4E), but had no effect on the PI(4,5)P2 level of cells overexpressing the inositol 5-phosphatase Synaptojanin (supplementary Fig S4A online). These results indicate that Nir2 regulates a PI(4,5)P2 pool that is utilized for PLC hydrolysis. Yet, it could be that depletion of Nir2 accelerates PI(4,5)P2 hydrolysis by PLCγ, or alternatively, reduces the production of PI(4,5)P2. In both cases, U73122 would restore the levels of PI(4,5)P2. However, the first possible mechanism predicts an enhanced PLCγ-mediated ERK1/2 phosphorylation, whereas our results indicate that depletion of Nir2 attenuates ERK1/2 phosphorylation similarly to the U73122 effect (Fig 3B,D). Our results, therefore, indicate that Nir2 is essential for maintaining PI(4,5)P2 level at the PM, possibly by positively regulating PI(4,5)P2 production. Indeed, metabolic labelling experiments utilizing [32P]Orthophosphate clearly showed that PI(4,5)P2 synthesis was significantly reduced (by ∼28%) in Nir2-depleted HeLa cells (Fig 4F).
Nir2 regulates PI(4,5)P2 via its lipid transfer/binding capability
To better understand the mechanism by which Nir2 regulates PI(4,5)P2 level at the PM, we examined whether the WT Nir2, its truncated ΔPI and ΔCter mutants, as well as the Nir2(S164A) mutant, which apparently lacks a PI/PC-transfer activity in vitro (supplementary Fig S4B online), could restore the PI(4,5)P2 levels in Nir2-depleted cells. As shown, neither the ΔPI and the ΔCter truncated mutants, nor the Nir2(S164A) mutant restored the PI(4,5)P2 level at the PM of Nir2-depleted cells (Fig 5A). Remarkably, the Nir2(S164A) mutant also did not enhance the phosphorylation of AKT in response to EGF (supplementary Fig S4C online). Hence, we identified a point mutation (S164A) which is crucial for both PI-transfer activity and for PI(4,5)P2 production. We then asked whether a point mutation that affects PA binding would also impair PI(4,5)P2 production. On the basis of the structural data and conserved motifs in HAD superfamily [20], we established point mutants of Nir2 within its LNS2 domain: a single mutant D1128A and a triple mutant S1026/D1028/D1128A. As shown, the D1128A mutation substantially reduced the binding of the C-terminal to liposomes containing PA in vitro (Fig 5B). Furthermore, this point mutation markedly reduced the translocation of Nir2 to the PM in response to either PA or EGF. The effect was even more profound with the triple mutant (Fig 5C). Most importantly, both the single and the triple mutants failed to restore the PI(4,5)P2 levels at the PM of Nir2-depleted cells (Fig 5D). These results strongly indicate that both the PI-transfer activity and the translocation of Nir2 to the PM are essential for maintaining PI(4,5)P2 level at the PM. The PI-transfer activity might be required for transport of PI, a precursor of PI(4,5)P2 synthesis, or alternatively, could positively regulate metabolic enzymes that contribute to PI(4,5)P2 synthesis. At present, we are unable to distinguish between these possibilities. Nevertheless, our data strongly indicate that Nir2 positively regulates PI(4,5)P2 and PI(3,4,5)P3 production and their downstream signalling events. Strikingly, rdgB, the Drosophila homologue of Nir2, is also required for PI(4,5)P2 production in response to light. Mutations in rdgB impair PI(4,5)P2 regeneration and cause light-dependent retinal degeneration [27, 28]. Our studies indicate that similarly to Nir2, rdgB is also a dual lipid-binding protein that possibly couples PA to PI(4,5)P2 regeneration during phototransduction. Further studies in flies would clarify this hypothesis.
Figure 5.
Mutants defective in either the PI-transfer activity or the PA-binding capability failed to restore the PI(4,5)P2 levels in Nir2-depleted cells. (A) Nir2-depleted HeLa cells were transiently transfected with vectors encoding the indicated proteins. Twenty-four hr later, the cells were fixed and either immunostained with anti-PI(4,5)P2 antibody (for GFP) or double immunostained with anti-PI(4,5)P2 and anti-Myc antibodies. Shown are representative images demonstrating the effects of GFP and the WT Nir2. Scale bar, 10 μm. The intensity of PI(4,5)P2 staining at the PM of ∼150 transfected cells with the indicated Nir2 constructs was measured and the mean values±s.d. of three experiments are shown in the attached graph. (B). Liposome sedimentation assay was performed with either a recombinant WT C-terminal region of Nir2 or the D1128A mutant. The D1128A mutant failed to bind or binds very weakly liposomes containing 20% or 30% PA, respectively. (C) The D1128A mutation and more profoundly the triple S1026/D1028/D1128A mutations impaired the translocation of Nir2 to the PM in response to EGF (100 ng/ml, 15 min) or PA (100 μm, 30 min) treatment. Shown are representative confocal images. Scale bar, 10 μm. (D) WT Nir2–Myc or the indicated mutants were transiently expressed in Nir2-depleted cells (marked by arrowheads) and their influence on PI(4,5)P2 levels was assessed as described in A. Representative images are shown along with a graph demonstrating the mean values±s.d. of PI(4,5)P2 intensity at the PM of∼100 transfected cells. A.U., arbitrary unit; EGF, epidermal growth factor; GFP, green fluorescent protein; PA, phosphatidic acid; PI, phosphatidylinositol; PM, plasma membrane; WT, wild type.
METHODS
Liposome sedimentation assays. The C-terminal region of Nir2 was expressed as His-tagged protein in bacteria (BL21) and purified on Ni-NTA agarose beads (Qiagen) according to the manufacturer’s instructions. Liposome sedimentation assays were performed essentially as described previously [29], and in supplementary information online.
Measurement of PIP3 content. The PIP3 content of control and Nir2-depleted HeLa cells was determined by the PIP3 Mass Elisa Kit (Echelon Biosciences Inc.) according the manufacturer’s instructions, as described in supplementary information online.
Measurement of PIP2 production. For measuring PIP2 production, control and Nir2-depleted HeLa cells (4 × 106) were metabolically labelled for 1 h with 100 μCi [32P]Orthophosphate/ml (PerkinElmer) in phosphate-free media. Acidic lipids were extracted, separated by thin layer chromatography, visualized by phosphorimager and PI(4,5)P2 synthesis was quantified by densitometry analysis (Image J). Further details are described in supplementary information onlineOther methods are described in supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
Sima Lev is the incumbent of the Joyce and Ben B. Eisenberg Chair of Molecular Biology and Cancer Research. We thank Karen N. Allen and Lucia Rameh for productive discussion and excellent suggestions. This work was supported by the Israel Science Foundation, grant no. 1223/12, the Israel Cancer Association and the Women’s Health research centre.
Author contributions: S.K., A.K., M.M., N.G. and S.L. performed the experiments and analysed the data. O.K., M.S. and O.L. prepared reagents. S.L. designed the study and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Gips SJ, Kandzari DE, Goldschmidt-Clermont PJ (1994) Growth factor receptors, phospholipases, phospholipid kinases and actin reorganization. Semin Cell Biol 5: 201–208 [DOI] [PubMed] [Google Scholar]
- Toker A (2002) Phosphoinositides and signal transduction. Cell Mol Life Sci 59: 761–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney TD, Katan M (2010) Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer 10: 342–352 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K, Daum G (1999) Phosphatidic acid, a key intermediate in lipid metabolism. Eur J Biochem 266: 1–16 [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Shome K, Vasudevan C, Stolz DB, Sung TC, Frohman MA, Watkins SC, Romero G (1999) Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J Biol Chem 274: 1131–1139 [DOI] [PubMed] [Google Scholar]
- Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D (2007) Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol 9: 706–712 [DOI] [PubMed] [Google Scholar]
- Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J (2001) Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294: 1942–1945 [DOI] [PubMed] [Google Scholar]
- Jenkins GH, Fisette PL, Anderson RA (1994) Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem 269: 11547–11554 [PubMed] [Google Scholar]
- Rameh LE, Tolias KF, Duckworth BC, Cantley LC (1997) A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390: 192–196 [DOI] [PubMed] [Google Scholar]
- Divecha N, Roefs M, Halstead JR, D’Andrea S, Fernandez-Borga M, Oomen L, Saqib KM, Wakelam MJ, D’Santos C (2000) Interaction of the type Ialpha PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4,5-bisphosphate .in the regulation of PLD2 activity. EMBO J 19: 5440–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S (2009) Phosphatidic acid regulation of phosphatidylinositol 4-phosphate 5-kinases. Biochim Biophys Acta 1791: 905–912 [DOI] [PubMed] [Google Scholar]
- Lung M, Shulga YV, Ivanova PT, Myers DS, Milne SB, Brown HA, Topham MK, Epand RM (2009) Diacylglycerol kinase epsilon is selective for both acyl chains of phosphatidic acid or diacylglycerol. J Biol Chem 284: 31062–31073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman GM, Han GS (2009) Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J Biol Chem 284: 2593–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Argov R, Dahan N, Ramachandran S, Amarilio R, Shainskaya A, Lev S (2004) Mitotic phosphorylation of the peripheral Golgi protein Nir2 by Cdk1 provides a docking mechanism for Plk1 and affects cytokinesis completion. Mol Cell 14: 319–330 [DOI] [PubMed] [Google Scholar]
- Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S (2005) Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol 7: 225–234 [DOI] [PubMed] [Google Scholar]
- Litvak V, Shaul YD, Shulewitz M, Amarilio R, Carmon S, Lev S (2002) Targeting of Nir2 to lipid droplets is regulated by a specific threonine residue within its PI-transfer domain. Curr Biol 12: 1513–1518 [DOI] [PubMed] [Google Scholar]
- Lev S, Hernandez J, Martinez R, Chen A, Plowman G, Schlessinger J (1999) Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol Cell Biol 19: 2278–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han GS, Wu WI, Carman GM (2006) The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J Biol Chem 281: 9210–9218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K (2007) Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem 282: 3450–3457 [DOI] [PubMed] [Google Scholar]
- Allen KN, Dunaway-Mariano D (2004) Phosphoryl group transfer: evolution of a catalytic scaffold. Trends Biochem Sci 29: 495–503 [DOI] [PubMed] [Google Scholar]
- Collet JF, Stroobant V, Van Schaftingen E (1999) Mechanistic studies of phosphoserine phosphatase, an enzyme related to P-type ATPases. J Biol Chem 274: 33985–33990 [DOI] [PubMed] [Google Scholar]
- Mietkiewska E, Siloto RM, Dewald J, Shah S, Brindley DN, Weselake RJ (2010) Lipins from plants are phosphatidate phosphatases that restore lipid synthesis in a pah1Delta mutant strain of Saccharomyces cerevisiae. FEBS J 278: 764–775 [DOI] [PubMed] [Google Scholar]
- Munnik T, Arisz SA, De Vrije T, Musgrave A (1995) G Protein Activation Stimulates Phospholipase D Signaling in Plants. Plant Cell 7: 2197–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watton SJ, Downward J (1999) Akt/PKB localisation and 3' phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol 9: 433–436 [DOI] [PubMed] [Google Scholar]
- Lin C, Ear J, Pavlova Y, Mittal Y, Kufareva I, Ghassemian M, Abagyan R, Garcia-Marcos M, Ghosh P (2011) Tyrosine phosphorylation of the Galpha-interacting protein GIV promotes activation of phosphoinositide 3-kinase during cell migration. Sci Signal 4: ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T (1998) Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol 8: 343–346 [DOI] [PubMed] [Google Scholar]
- Harris WA, Stark WS (1977) Hereditary retinal degeneration in Drosophila melanogaster. A mutant defect associated with the phototransduction process. J Gen Physiol 69: 261–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Niemeyer B, Colley N, Socolich M, Zuker CS (1995) Regulation of PLC-mediated signalling in vivo by CDP-diacylglycerol synthase. Nature 373: 216–222 [DOI] [PubMed] [Google Scholar]
- Lee SH, Jin JB, Song J, Min MK, Park DS, Kim YW, Hwang I (2002) The intermolecular interaction between the PH domain and the C-terminal domain of Arabidopsis dynamin-like 6 determines lipid binding specificity. J Biol Chem 277: 31842–31849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.