Abstract
EphB4 is a member of the large Eph receptor tyrosine kinase family. By interacting with its preferred ligand ephrin-B2, which is also a transmembrane protein, EphB4 plays a role in a variety of physiological and pathological processes ranging from bone remodeling to cancer malignancy. EphB4-ephrin-B2 binding occurs at sites of contact between cells. Ephrin-B2 causes EphB4 clustering and increased kinase activity to generate downstream signals that affect cell behavior. Previous work identified a high-affinity antagonistic peptide that targets EphB4, named TNYL-RAW. This peptide is 15 amino acid long, has a molecular weight of ~1,700 Da and binds to the ephrin-binding pocket of EphB4. Here we report the structure-based design and chemical synthesis of two novel small molecules of ~600–700 Da, which were designed starting from the small and functionally critical C-terminal portion of the TNYL-RAW peptide. These compounds inhibit ephrin-B2 binding to EphB4 at low micromolar concentrations. Additionally, although the ephrin-B2 ligand can interact with multiple other Eph receptors besides EphB4, the two compounds retain the high selectivity of the TNYL-RAW peptide in targeting EphB4. TNYL-RAW peptide displacement experiments using the more potent of the two compounds, compound 5, suggest a competitive mode of inhibition. These EphB4 antagonistic compounds can serve as promising templates for the further development of small molecule drugs targeting EphB4.
Keywords: Small molecular inhibitors, Eph receptors, structure-based drug design, tumor angiogenesis, protein-protein interactions
1. Introduction
The EphB4 receptor tyrosine kinase is a member of the large Eph receptor family, which is known to regulate biological processes in developing and adult tissues, and has also been implicated in cancer (1, 2). There are two classes of Eph receptors, A and B. The EphA receptors (EphA1–EphA10) bind to six glycosyl phosphatidyl-inositol (GPI)-linked ephrin-A ligands (ephrin-A1–ephrin-A6), while the EphB receptors (EphB1–EphB6) interact with three transmembrane ephrin-B ligands (ephrin-B1–ephrin-B3). EphB4 preferentially binds ephrin-B2, and this interaction results in transduction of “forward” signals through EphB4 and “reverse” signals through ephrin-B2.
In recent years, the EphB4 receptor tyrosine kinase has emerged as an important potential therapeutic target (1, 2). Despite the complex and not fully elucidated roles of EphB4 in cancer, increasing evidence suggests that targeting this receptor may be useful to inhibit cancer growth and tumor angiogenesis. EphB4 is highly expressed in a variety of tumors, such as breast (3–5), colon (6, 7) bladder (8), prostate (9, 10) and ovarian cancers (11), melanoma (12), and others. EphB4 can support tumor cell survival in many of these cancers (3, 11, 13). For instance, in mouse melanoma cells ephrin-B2-induced EphB4 signaling results in increased cell migration and invasiveness (12, 14) while in MCF7 breast cancer cells it promotes growth and Erk1/2 activation (15). In addition, EphB4 forward signaling has been proposed to play a role in tumor angiogenesis (16–19). On the other hand, activation of ephrin-B2 reverse signaling by EphB4 can regulate endothelial cell migration and proliferation, the assembly of endothelial cells and their supporting mural cells as well as vascular endothelial growth factor-mediated angiogenesis, including tumor angiogenesis (16, 20–25).
Several strategies for developing EphB4 targeted anti-cancer therapies have shown promising results thus far. Knockdown of EphB4 using antisense oligonucleotides and siRNAs can reduce survival, proliferation, migration and invasion in several cancer cell types (3, 11, 13). Two recently reported antibodies (MAb47 and MAb131) that bind to EphB4 with high affinity show efficacy in inhibiting primary tumor development and metastasis in mouse models (25). Whereas MAb131 works mainly by inducing EphB4 degradation, MAb47 was suggested to work by inhibiting interaction of EphB4 with ephrin-B2 or other proteins. Furthermore, the monomeric EphB4 ectodomain can inhibit tumorigenesis presumably by blocking EphB4-ephrin-B2 forward and reverse signaling, resulting in inhibition of angiogenesis (19, 26, 27). Therefore, inhibitors of EphB4 such as small molecules and peptides that are able to block the interaction of the receptor with ephrin-B2, are likely to be useful therapeutic tools in cancer. So far there have been no reports of small molecules or peptidomimetics that selectively inhibit EphB4-ephrin-B2 binding. Although small molecule inhibitors of the kinase domain of EphB4 have been identified, these compounds target the ATP binding site and therefore their selectivity tends to be low (28). Additionally, EphB4 kinase inhibitors will not be effective in preventing ephrin-B2 reverse signaling (5, 28). In this study, we have identified small molecule peptidomimetic inhibitors that are highly selective for EphB4 and inhibit its binding to ephrin-B2 at low micromolar concentrations.
Even though protein-protein interactions typically involve large interfaces and are perceived as difficult to inhibit by using small molecules (29, 30), new strategies have been successfully used by many groups over the past decade to modulate protein-protein interactions with synthetic peptides and small molecules (30–33). This has led to the growing field of drug discovery to target protein-protein interfaces. In the case of Eph receptors, a number of small molecule antagonists preferentially targeting EphA2 and EphA4, or a larger subset of Eph receptors, have been identified (29, 34–37). More selective peptide inhibitors of interactions between Eph receptors and ephrins have also been identified (28), including a 15 amino acid peptide (TNYL-RAW, amino acid sequence TNYLFSPNGPIARAW) that selectively targets EphB4 by binding to the ephrin-binding pocket of the receptor (38). The shorter peptide TNYL (TNYLFSPNGPIA), which inhibits ephrin-B2 binding to EphB4 with an IC50 value of ~150 µM, was identified by using phage display. Addition of the C-terminal RAW motif to TNYL, based on the sequence alignment of multiple EphB4-binding peptides identified in the same phage display screen, then yielded the TNYL-RAW peptide, which inhibits EphB4 binding with an IC50 of ~15 nM and has a low nanomolar binding affinity (38–40). Thus, the C-terminal RAW sequence is critical for the high-affinity binding of TNYL-RAW to the EphB4 receptor.
2. Materials and Methods
2.1 Reagents
Tental Gel S RAM resin (1% divinylbenzene, 100–200 mesh, 0.24 mmol/g substitution), amino acids, piperidine, trifluoroacetic acid and adamantane carboxylic acid were purchased from Sigma Aldrich (Steinheim, Germany). 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU), N-hydroxybenzotrizaole (HOBt) and N,N-diisopropylcarbodiimide were purchased from Anaspec (Fremont, CA). Anhydrous solvents were used for all the reactions. The reactions were carried out in peptide reaction vessels of 15 mL capacity, which were rotated on Fisher Isotemp Hybridization.
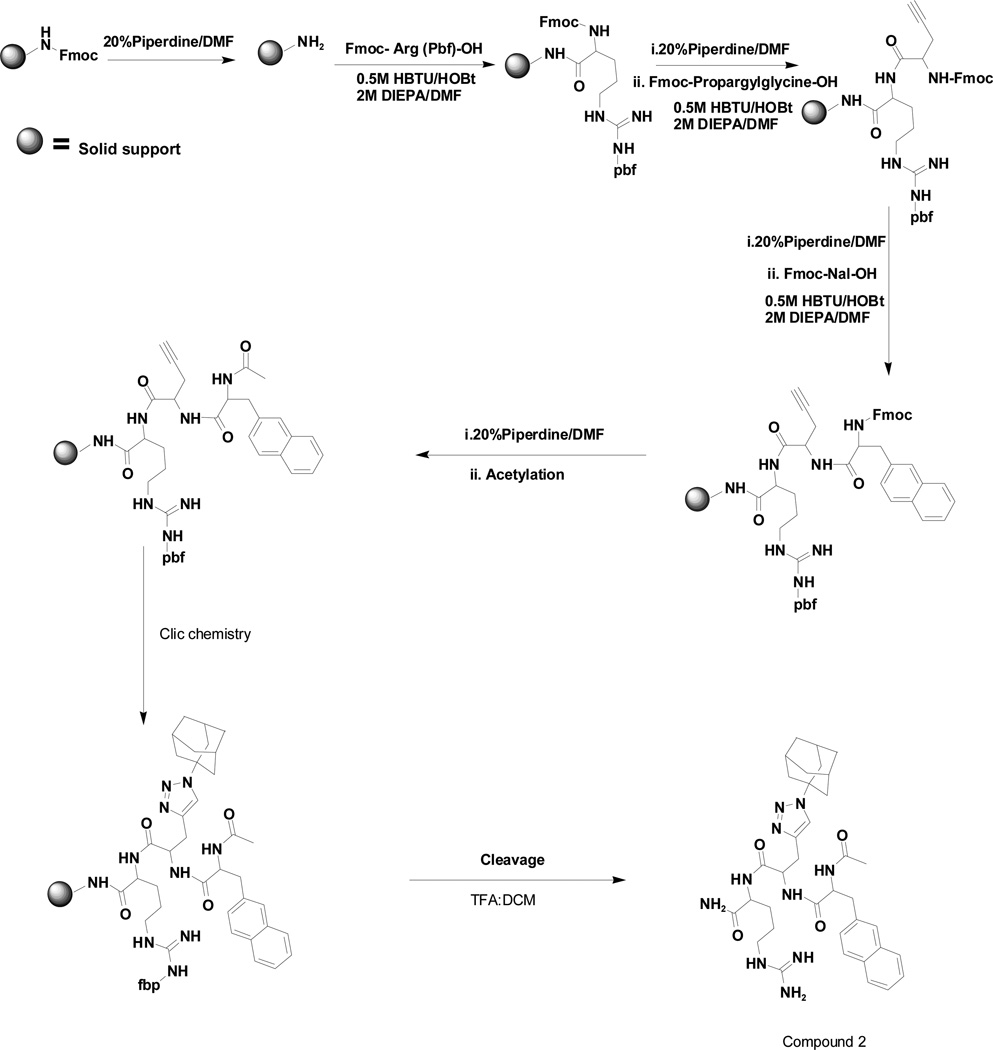
2.2 Peptide synthesis
Linear tripeptides were synthesized by removing the Fmoc group of Tanta Gel S RAM resin (0.24 mmol/g) by treatment with 20% piperidine in DMF (5 mL) for 5 and 15 min, followed by standard peptide couplings. Once synthesized, the N terminus of the tri-peptides (naphthylalanine/5-phenyl furoic acid-propargylglycine/Dap(Dde)/Lys(Dde)-Arg-resin) was acetylated with acetic anhydride, dichloromethane and pyridine (1:8:8) at room temperature for 30 min. This was confirmed by ninhydrine test. The side chain alkyne of propargylglycine was reacted with azido adamantane by using click chemistry to generate compound 2. Compounds 3–5 were synthesized by removing the side chain 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl (Dde) group of diaminopropionic acid (Dap) or Lys and then coupled with adamantane carboxylic acid. The final compounds were cleaved from the resin and the cleavage mixture was evaporated. This step was followed by addition of diethyl ether and centrifugation. The crude compounds were purified by using reverse-phase HPLC using a Xbridge™ BEH130 C-18 (4.6 × 250 mm) column with a flow rate of 1 ml/min. The following solvents were used: A, water with 0.1% TFA; and B, 10% water in CH3CN with 0.1% TFA in a linear gradient of 0% to 100% B over 25 min. The synthetic scheme for compound 2 is shown in Scheme 1(reviewer 1). The compounds were further purified by using Xbridge™ BEH130 prep C-18 (10 × 250 mm) column with a flow rate of 5 mL/min and characterized by MALDI-TOF-MS. The purity of the compounds were >95% (HPLC and MALDI-TOF characterization of compounds 1–5 are shown in the Appendix). The TFA salts of compounds 1–5 were used for the experiments.
Scheme 1.
Synthetic route for compound 2.
2.3 Computer modeling
The structure of the receptor solved at 1.65-Å resolution was obtained from crystal structure of EphB4 in complex with the antagonist peptide TNYL-RAW (PDB entry 2BBA). The receptor file was converted to a PDBQT file, and a 60 × 60 × 60 grid box with a grid spacing of 0.375 Å was defined to cover the whole pocket in which the TNYL-RAW binding site is located. The molecular docking study of compound 5 with EphB4 was performed using the Autodock4 program and AutoDockTools-1.5.2. We performed 100 docking runs, and selected the top 3 ranked models based on lowest binding energies. Because the top two models were not part of clusters of similar conformations (with an RMSD tolerance of 2.0 Å), we chose the model with the third lowest binding energy as the best binding model.
2.4 IC50 determination
Protein A-coated wells (Pierce Biotechnology, Rockford, IL) were incubated with 1 µg/mL EphB4 Fc (R&D Systems, Minneapolis, MN) in TBST (150 mM NaCl, 50 mM Tris-HCl, pH 7.5 containing 1 mM CaCl2 and 0.01% Tween 20) for 1 hour. EphB4 Fc-coated wells were then rinsed with TBST, and incubated for 1 hour with different concentrations of the compounds and cell culture medium containing ephrin-B2 AP diluted in TBST at a final concentration of 0.02 nM in a total volume of 50 µL. Cell culture medium containing ephrin-B2 AP was obtained by transiently transfecting HEK293 cells with an ephrin-B2 AP plasmid and alkaline phosphatase activity was determined as previously described (41–43). After washing away the unbound peptide and ephrin, bound ephrin-B2 alkaline phosphatase (AP) was detected using 1 mM pNPP (Pierce Biotechnology, Rockford, IL) as the substrate. Data were fitted using non linear regression and IC50 values were calculated using the program Prism (GraphPad Software Inc., San Diego).
2.5 Receptor selectivity
Protein A-coated wells (Pierce Biotechnology, Rockford, IL) were incubated with 1 µg/mL EphA Fc or EphB Fc (modified according reviewer 1) receptors (R&D Systems, Minneapolis, MN) in TBST for 1 hour. The wells were then incubated for 1 hour with either 0.01 nM ephrin-A5 AP (produced as described above for ephrin-B2 AP) for the EphA receptors, or 0.02 nM ephrin-B2 AP for the EphB receptors in the presence or absence of 200 µM compound 2 or 100 µM compound 5. After washing away unbound ephrin and compound, the amount of bound ephrin was detected by measuring alkaline phosphatase activity as described above.
2.6 Inhibition of TNYL-RAW binding to EphB4 by compound 5
Curves for the binding of biotinylated TNYL-RAW to EphB4 Fc, which was immobilized on protein A-coated plates as described above, were obtained in the presence of several concentrations of compound 5. Bound biotinylated TNYL-RAW was detected with horseradish peroxidase (HRP)-conjugated streptavidin (1:2,000 dilution in TBST, Pierce Biotechnology, Rockford, IL). The binding curves were fitted to the Michaelis-Menten equation: B = Bmax [S]/(Kd + [S]), where [S] is the concentration of TNYL-RAW and Kd is the dissociation constant, using non linear regression and the program Prism (GraphPad Software Inc., San Diego).
3. Results and Discussion
The crystal structure of the ephrin-binding domain of human EphB4 in complex with the antagonistic TNYL-RAW peptide shows that the RAW motif binds to a hydrophobic cavity within the ephrin-binding pocket, near the EphB4 G–H loop (44). Arginine (R13) forms a hydrogen bond with E43 in the receptor, whereas tryptophan (W15) is buried in the hydrophobic cavity of the ephrin-binding pocket, stabilized between the J–K and G–H loops of the receptor, and forms hydrophobic interactions with L95, L100, P101 and K149 of EphB4.
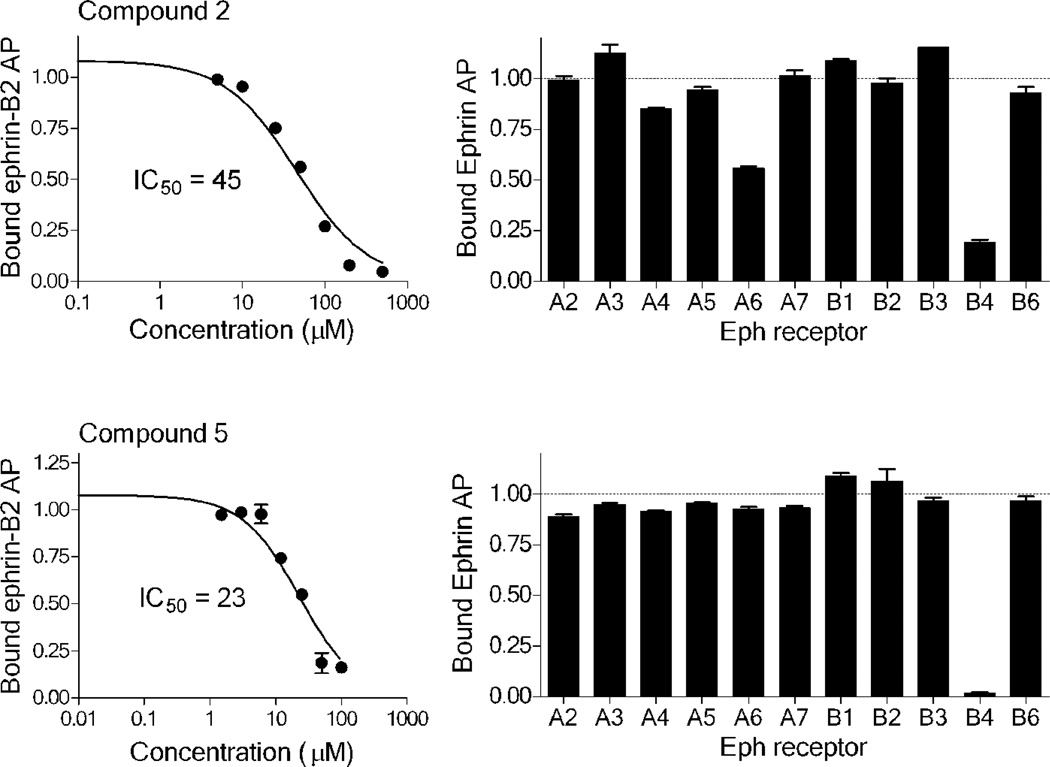
Based on the information from binding studies of TNYL-RAW and its crystal structure in complex with EphB4, we selected the tri-peptide Arg-Ala-Trp (RAW) and its reverse sequence Trp-Ala-Arg (WAR) as a starting point to design much smaller peptidomimetics of TNYL-RAW (Table 1). First, we synthesized peptidomimetic analogs of the WAR sequence (compounds 1 and 2, Table 1). To favor binding in the same hydrophobic binding pocket of EphB4 where TNYL-RAW is known to bind, we replaced the tryptophan with the more hydrophobic naphthylalanine (compound 1) and to further increase the hydrophobicity of the molecule, we replaced the alanine with propargylglycine and used click chemistry to introduce triazolo adamantane to generate compound 2 (Scheme 1) (45) The RAW and WAR peptides as well as compound 1 do not show any detectable inhibition of EphB4-ephrin-B2 binding (Table 1). In contrast, compound 2 inhibits EphB4-ephrin-B2 binding with an IC50 ~ 40 µM (Table 1 and Figure 1). Importantly, compound 2 retains selectivity for EphB4, showing only substantial inhibition of EphA6 among the other Eph receptors tested when used at a concentration of 200 µM (Figure 1). Therefore, we identified compound 2 as our lead compound and proceeded with structure-activity relationship analysis to improve its affinity and selectivity for EphB4.
Table 1.
Chemical structures and IC50 values for inhibition of EphB4-ephrin-B2 binding
| Compound | MW | Structure | IC50 (µM) |
|---|---|---|---|
| RAW | 430 | 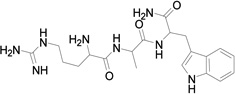 |
>100 |
| WAR | 430 | 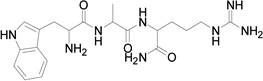 |
>100 |
| 1 | 442 | 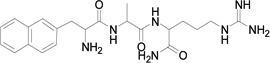 |
>100 |
| 2 | 685 |  |
41 ± 2.2 (n = 3) |
| 3 | 703 | 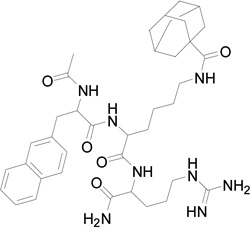 |
>100 |
| 4 | 661 | 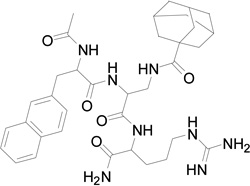 |
>100 |
| 5 | 592 | 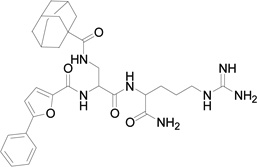 |
18 ± 2.8 (n = 4) |
Figure 1.
Potency and Eph receptor selectivity of compounds 2 and 5. The left panels show curves for inhibition of ephrin-B2 AP binding to immobilized EphB4 Fc by compounds 2 and 5 and the calculated IC50 values. The right panels show the levels of bound ephrin-A5 AP (for EphA Fc receptors) or ephrin-B2 AP (for EphB Fc receptors) in the presence of 200 µM compound 2 or 100 µM compound 5 normalized to the levels in the absence of compound. Error bars represent standard errors from duplicate measurements (compound 2) or triplicate measurements (compound 5). The curves for IC50 determination were repeated in 3 independent experiments for compound 2 and in 4 independent experiments for compound 5 (see Table 1). The Eph receptor selectivity experiments were repeated twice with similar results.
We replaced the triazoloadamantane with adamantane carboxylic acid attached to a butylamine linker (compound 3) or adamantane carboxylic acid (compound 4). Compounds 3 and 4 did not show any measureable activity (Table 1). Further replacement of the adamantane group with different hydrophobic groups like biphenyl, pyrene and 4-(4-fluorophenoxy) phenyl (not shown in Table 1) all resulted in compounds with undetectable inhibition of EphB4-ephrin-B2 interaction. However, replacing the naphthylalanine in compound 4 with 5-phenyl furoic acid in compound 5 resulted in higher potency than compound 2, with an IC50 value of 18 µM. Compound 5 is also very selective and inhibits ephrin binding only to EphB4 among the Eph receptors tested (Figure 1).
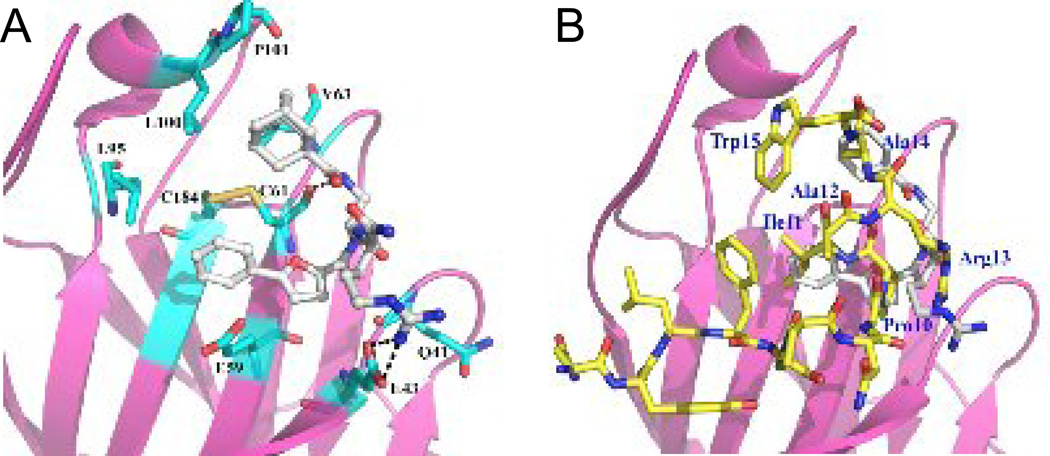
Compound 5 appears to be a competitive inhibitor of TNYL-RAW binding to EphB4 (Figure 2), which suggests that the compound binds in the same pocket of EphB4 as TNYL-RAW and ephrin-B2. Further analysis of the binding using molecular modeling also supports this notion (Figure 3A). In the model, the backbone of compound 5 forms a hydrogen bond with the backbone of C61 in EphB4 and the arginine guanidium group of compound 5 occupies the same binding position as the guanidium from R13 of TNYL-RAW, forming a hydrogen bond with the EphB4 E43 side chain. The adamantane group occupies a hydrophobic pocket composed of the hydrophobic side chains of EphB4 V63, L100 and P101. The model shows that compound 5 fits in the ephrin binding pocket of EphB4 very well. Superposition of compound 5 with the TNYL-RAW peptide from the crystal structure of the complex with EphB4 (Figure 3B) shows that the guanidium groups of compound 5 and TNYL-RAW R13 overlap, suggesting that they are engaged in similar interactions with the EphB4 receptor. However, the interaction with E43 is unlikely to account for the selective EphB4 targeting of compound 5 and TNYL-RAW because this glutamic acid is conserved in four of the five EphB receptors (EphB1 through EphB4). On the other hand, the hydrophobic benzene ring of compound 5 superimposes with the I11 side chain of TNYL-RAW. Thus, our model suggests that compound 5 only partially mimics the position of the RAW sequence from the TNYL-RAW peptide within the ephrin-binding pocket of the EphB4 receptor, although the crystal structure of the complex will be necessary to conclusively define the positioning of compound 5 bound to EphB4.
Figure 2.
Compound 5 competitively inhibits TNYL-RAW binding to EphB4. Curves for the binding of the biotinylated TNYL-RAW peptide to immobilized EphB4 Fc in the presence of the indicated concentrations of compound 5. Kd, dissociation constant; Bmax, maximal binding. This experiment was repeated twice with similar results.
Figure 3.
Molecular modeling of compound 5 in the EphB4 ephrin-binding pocket. (A) The EphB4 receptor is shown as a pink ribbon with residues important for TNYL-RAW binding indicated in cyan and labeled. Compound 5 is shown as gray sticks, and forms hydrogen bonds with EphB4 E43 and C61. Yellow indicates the disulfide bond between EphB4 C61 and C184. (B) Superposition of compound 5 with the TNYL-RAW peptide from the crystal structure of the complex with EphB4 (PDB entry 2BBA). Compound 5 is shown in gray sticks and TNYL-RAW in yellow sticks.
In conclusion, we have designed and synthesized two small molecules that selectively inhibit ephrin-B2 binding to EphB4 at low micromolar concentrations. The most potent is compound 5, which shows competitive binding against TNYL-RAW suggesting that it binds in the same ephrin-binding pocket of EphB4 as the peptide and the natural ligand ephrin-B2. This is the first report of small peptidomimetics (molecular weight less than 700 Da) that selectively inhibit the protein-protein interface between EphB4 and ephrin-B2. Compound 5 can be useful either as a probe in screens for other small molecule EphB4 antagonists or as a starting point for designing more potent small molecule derivatives that inhibit ephrin-B2 binding to this receptor.
Supplementary Material
Acknowledgments
This work is supported by DoD grant W81XWH-07-1-0462 (ZH and EBP) and NIH grants P01CA138390 and CA116099 (EBP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signaling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, Weaver FA, Gill PS. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 5.Noren N, KP EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67:3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- 6.Batlle EBJ, Begthel H, Jonkeer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, Gallinger S, Pals S, Clevers H. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson SA, Slomka S, Douglas EL, Hewett PJ, Hardingham JE. Receptor protein tyrosine kinase EphB4 is up-regulated in colon cancer. BMC Mol Biol. 2001;2:15. doi: 10.1186/1471-2199-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, Hassanieh L, Smith DL, Buscarini M, Broek D, Quinn DI, Weaver FA, Gill PS. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–780. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- 9.Lee YC, Perren JR, Douglas EL, Raynor MP, Bartley MA, Bardy PG, Stephenson SA. Investigation of the expression of the EphB4 receptor tyrosine kinase in prostate carcinoma. BMC Cancer. 2005;5:1. doi: 10.1186/1471-2407-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, Quinn DI, Henshall SM, Sutherland RL, Pinski JK, Daneshmand S, Buscarini M, Stein JP, Zhong C, Broek D, Roy-Burman P, Gill PS. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 11.Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, Weaver FA, Sood AK, Gill PS. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer. 2007;96:1083–1091. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang NY, Pasquale EB, Owen LB, Ethell IM. The EphB4 Receptor-tyrosine Kinase Promotes the Migration of Melanoma Cells through Rho-mediated Actin Cytoskeleton Reorganization. J Biol Chem. 2006;281:32574–32586. doi: 10.1074/jbc.M604338200. [DOI] [PubMed] [Google Scholar]
- 13.Masood R, Kumar SR, Sinha UK, Crowe DL, Krasnoperov V, Reddy RK, Zozulya S, Singh J, Xia G, Broek D, Schonthal AH, Gill PS. EphB4 provides survival advantage to squamous cell carcinoma of the head and neck. Int J Cancer. 2006;119:1236–1248. doi: 10.1002/ijc.21926. [DOI] [PubMed] [Google Scholar]
- 14.Yang NY, Lopez-Bergami P, Goydos JS, Yip D, Walker AM, Pasquale EB, Ethell IM. The EphB4 receptor promotes the growth of melanoma cells expressing the ephrin-B2 ligand. Pigment Cell Melanoma Res. 2010;23:684–687. doi: 10.1111/j.1755-148X.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Z, Carrasco R, Kinneer K, Sabol D, Jallal B, Coats S, Tice DA. EphB4 promotes or suppresses Ras/MEK/ERK pathway in a context-dependent manner: Implications for EphB4 as a cancer target. Cancer Biol Ther. 2012;13:630–637. doi: 10.4161/cbt.20080. [DOI] [PubMed] [Google Scholar]
- 16.Fuller T, Korff T, Kilian A, Dandekar G, Augustin HG. Forward EphB4 signaling in endothelial cells controls cellular repulsion and segregation from ephrinB2 positive cells. J Cell Sci. 2003;116:2461–2470. doi: 10.1242/jcs.00426. [DOI] [PubMed] [Google Scholar]
- 17.Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Martiny-Baron G, Korff T, Schaffner F, Esser N, Eggstein S, Marme D, Augustin HG. Inhibition of tumor growth and angiogenesis by soluble EphB4. Neoplasia. 2004;6:248–257. doi: 10.1593/neo.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci USA. 2004;101:5583–5588. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, Tosato G. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 24.Steinle JJ, Meininger CJ, Chowdhury U, Wu G, Granger HJ. Role of ephrin B2 in human retinal endothelial cell proliferation and migration. Cell Signal. 2003;15:1011–1017. doi: 10.1016/s0898-6568(03)00072-x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 26.Krasnoperov V, Kumar SR, Ley E, Li X, Scehnet J, Liu R, Zozulya S, Gill PS. Novel EphB4 monoclonal antibodies modulate angiogenesis and inhibit tumor growth. Am J Pathol. 2010;176:2029–2038. doi: 10.2353/ajpath.2010.090755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kertesz N, Krasnoperov V, Reddy R, Leshanski L, Kumar SR, Zozulya S, Gill PS. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 2006;107:2330–2338. doi: 10.1182/blood-2005-04-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scehnet JS, Ley EJ, Krasnoperov V, Liu R, Manchanda PK, Sjoberg E, Kostecke AP, Gupta S, Kumar SR, Gill PS. The role of Ephs, Ephrins, and growth factors in Kaposi sarcoma and implications of EphrinB2 blockade. Blood. 2009;113:254–263. doi: 10.1182/blood-2008-02-140020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noberini R, Lamberto I, Pasquale EB. Targeting Eph receptors with peptides and small molecules: Progress and challenges. Semin Cell Dev Biol. 2012;1:51–57. doi: 10.1016/j.semcdb.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry DC, Vassilev LT. Targeting protein-protein interactions for cancer therapy. J Mol Med. 2005;83:955–963. doi: 10.1007/s00109-005-0705-x. [DOI] [PubMed] [Google Scholar]
- 31.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z. Structural chemistry and therapeutic intervention of protein-protein interactions in immune response, human immunodeficiency virus entry, and apoptosis. Pharmacol Ther. 2000;86:201–215. doi: 10.1016/s0163-7258(00)00052-8. [DOI] [PubMed] [Google Scholar]
- 33.Huang Z. The chemical biology of apoptosis. Exploring protein-protein interactions and the life and death of cells with small molecules. Chem Biol. 2002;9:059–072. doi: 10.1016/s1074-5521(02)00247-8. [DOI] [PubMed] [Google Scholar]
- 34.Arkin MR, Whitty A. The road less traveled: modulating signal transduction enzymes by inhibiting their protein-protein interactions. Curr Opin Chem Biol. 2009l;13:284–290. doi: 10.1016/j.cbpa.2009.05.125. [DOI] [PubMed] [Google Scholar]
- 35.Tognolini M, Giorgio C, Hassan Mohamed I, Barocelli, Calani L, Reynaud E, Danhles O, Borges G, Crozier A, Brighenti F, Del Rio D. Perturbation if the EphA2-Ephrin A1 System in Human Prostate Cancer Cells by Colonic (Poly) phenol Catabolites. J Agric Food Chem. 2012;60:8877–8884. doi: 10.1021/jf205305m. [DOI] [PubMed] [Google Scholar]
- 36.Tognolini M, Incerti M, Hassan-Mohamed I, Giorgio C, Russo S, Bruni R, Lelli B, Bracci L, Noberini R, Pasquale EB, Barocelli E, Vicini P, Mor M, Lodola A. Structure-activity relationships and mechanism of action of Eph-ephrin antagonists: interaction of cholanic acid with the EphA2 receptor. Chem Med Chem. 2012;7:1071–1083. doi: 10.1002/cmdc.201200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petty A, Myshkin E, Qin H, Guo G, Miao H, Tochtrop GP, Hsieh JT, Page P, Liu L, Lindner DJ, Acharya C, MacKerell AD, Jr, Ficker E, Song J, Wang B. A Small Molecule Agonist of EphA2 Receptor Tyrosine Kinase Inhibits Tumor Cell Migration In Vitro and Prostate Cancer Metastasis In Vivo. PLoS One. 2012;7:e42120. doi: 10.1371/journal.pone.0042120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noberini R, Koople M, Lamberto I, Pasquale EB. Inhibition of Eph receptor-ephrin ligand interaction by tea polyphenols. Pharmacol Res. 2012;66:363–373. doi: 10.1016/j.phrs.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280:17301–17311. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- 40.Noberini R, Mitra S, Salvucci O, Valencia F, Duggineni S, Prigozhina N, Wei K, Tosato G, Huang Z, Pasquale EB. PEGylation potentiates the effectiveness of an antagonistic peptide that tragets the EphB4 receptor with nanomolar affinity. PLoS One. 2012;6:e29611. doi: 10.1371/journal.pone.0028611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- 42.Cullen BR. Utility of the secreted placental alkaline phosphatase reporter enzyme. Methods Enzymol. 2000;326:159–164. doi: 10.1016/s0076-6879(00)26053-9. [DOI] [PubMed] [Google Scholar]
- 43.Flanagan JG, Cheng HJ, Feldheim DA, Hattori M, Lu Q, Vanderhaeghen P. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods in Enzymology. 2000;327:19–35. doi: 10.1016/s0076-6879(00)27264-9. [DOI] [PubMed] [Google Scholar]
- 44.Chrencik E, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB, Kuhn P. Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14:321–330. doi: 10.1016/j.str.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.