Abstract
Mammalian skin incorporates a local equivalent of the hypothalamic-pituitary-adrenal (HPA) axis that is critical in coordinating homeostatic responses against external noxious stimuli. Ultraviolet radiation B (UVB) is a skin-specific stressor that can activate this cutaneous HPA axis. Since C57BL/6 (B6) and DBA/2J (D2) strains of mice have different predispositions to sensorineural pathway activation, we quantified expression of HPA axis components at the gene and protein levels in skin incubated ex vivo after UVB or sham irradiation. Urocortin mRNA was up-regulated after all doses of UVB with a maximum level at 50 mJ/cm2 after 12 h for D2 and at 200 mJ/cm2 after 24 h for B6. Proopiomelanocortin mRNA was enhanced after 6 h with the peak after 12 h and at 200 mJ/cm2 for both genotypes of mice. ACTH levels in tissue and media increased after 24 h in B6 but not in D2. UVB stimulated β-endorphin expression was higher in D2 than B6. Melanocortin receptor 2 mRNA was stimulated by UVB in a dose-dependent manner, with a peak at 200 mJ/cm2 after 12 h for both strains. The expression of Cyp11a1 mRNA — a key mitochondrial P450 enzyme in steroidogenesis, was stimulated at all doses of UVB irradiation, with the most pronounced effect after 12–24 h. UVB radiation caused, independently of genotype, a dose-dependent increases in corticosterone production in the skin, mainly after 24 h of histoculture. Thus, basal and UVB stimulated expression of the cutaneous HPA axis differs as a function of genotype: D2 responds to UVB earlier and with higher amplitude than B6, while B6 shows prolonged (up to 48 h) stress response to a noxious stimulus such as UVB.
Keywords: ultraviolet B radiation, HPA axis, cutaneous stress, C57Bl/6, DBA2J
1. Introduction
Mammalian skin incorporates the major functional elements of the hypothalamic-pituitary-adrenal (HPA) axis, and this cutaneous HPA axis influences local homeostasis [reviewed in (Slominski et al., 2007; Slominski et al., 2012; Slominski et al., 2013b)]. In the mouse skin the activity of proopiomelanocortin (POMC), one of the main components of the HPA axis, is coupled to the phase of hair cycle (Slominski et al., 1992). This includes hair cycle dependent expression of POMC transcripts, of prohormone proconvertases 1/3 (PC1/3) and 2 (PC2) with final processing of POMC to either ACTH or melanocyte stimulating hormone (MSH) and β-endorphin (β-END) peptides (Slominski et al., 1992; Slominski et al., 1996b; Ermak and Slominski, 1997; Slominski et al., 1998; Mazurkiewicz et al., 2000; Slominski et al., 2000c). Importantly, physiological immune functions and histology of murine skin dramatically change during hair cycling and the local melanogenic activity is strictly coupled to the anagen phase of hair growth (Slominski and Paus, 1993; Stenn and Paus, 2001).
Human skin does express an equivalent of the HPA axis that is highly organized and follows the central HPA axis organizational structure, in which corticotropin releasing hormone (CRH) or urocortin (UCN1) produced by skin cells and/or released by nerve endings interact with CRH receptor type 1 (CRH-R1) to produce POMC-derived β-END and ACTH peptides (Slominski et al., 2001; Slominski et al., 2006; Slominski et al., 2013b), with the latter stimulating local production of cortisol (COR) or corticosterone (CORT) (Slominski and Mihm, 1996; Ito et al., 2005; Slominski et al., 2005b; Slominski et al., 2005c; Cirillo and Prime, 2011; Hannen et al., 2011; Vukelic et al., 2011; Slominski et al., 2013b). It has been proposed that cutaneous CRH, β-END, ACTH and glucocorticoids (GCs) may not only regulate local homeostasis, but might also affect central body homeostasis through signals send via the neural and vascular connections [reviewed in (Slominski and Wortsman, 2000; Slominski et al., 2000c; Slominski et al., 2012)]. Ultraviolet B (UVB, λ 290–320 nm) acts as a skin-specific stressor capable of activating different elements of the local HPA axis in human skin (Slominski et al., 1996a; Chakraborty et al., 1999; Zbytek et al., 2006; Skobowiat et al., 2011; Slominski et al., 2012; Skobowiat et al., 2013).
C57BL/6 (B6) and DBA/2J (D2) are among the most widely used inbred strains and are exploited in many areas of research. Both have been sequenced and they are known to differ at ~5 million SNPs, indels, and CNVs (Wang et al., 2010; Porcu et al., 2011). Both strains are recessive homozygotes for the non-agouti allele (a/a) of the agouti signaling protein (Asip). Concerning pigmentary phenotype, both strains produce solely eumelanin. However, the amount of eumelanin pigment produced by D2 is lower than in B6, because of the loss of function mutations in Myo5a and Tyrp1 genes (Bennett and Lamoreux, 2003). Interestingly, POMC−/− B6 mice do produce exclusively eumelanin, indicating that POMC-derived peptides in both strains having the Asip a/a genotype play different role in skin physiology than strict regulation of melanin pigmentation (Slominski et al., 2004b; Slominski et al., 2005a). Over the last decade we have expanded a large family of inbred strains made by crossing B6 with D2. This family of BXD strains have strikingly different phenotypes (Andreux et al., 2012) including changes in melanin pigmentation (www.genenetwork.org). Since UVB light is the major cutaneous stressor, positive regulator of melanin pigmentation and inducer of local neuroendocrine activities (Slominski et al., 2000c; Slominski et al., 2004b; Slominski et al., 2012), we investigated the different responses of B6 and D2 parental genotypes to UVB in terms of induction of cutaneous HPA axis.
2. Material and methods
2.1. Animals
C57BL/6 (B6) and DBA/2J (D2) females were purchased from the Jackson Laboratory, and bred at UTHSC, Memphis, TN in a specific pathogen-free (SPF) facility. Animals were weaned at 25 days of age and housed in same sex cages with 2–5 mice per cage until the day of sacrifice. Animals had free access to standard laboratory chow and water and were maintained on a 12:12 light/dark cycle. Room temperature ranged from 20 to 24 C. Four B6 and four D2 mice were sacrificed at 60 days of age by cervical dislocation. All procedures involving mouse tissue were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center, Memphis TN.
2.2. Irradiation
Back skin was shaved with animal clipper, the epidermal surface was disinfected with 70% ethanol, and the skin dissected out and collected as described previously (Slominski et al., 1992; Slominski et al., 1994; Mazurkiewicz et al., 2000). Skin from each animal was subdivided into two parts: the control and experimental samples and placed onto humid (PBS), Waltham paper covered, 100 mm petri dishes. Experimental samples underwent appropriate UVB (290–320 nm) irradiation, while the control samples (sham-treated) the same way, except the irradiation (they stayed covered in alumni foil in humid chamber). Irradiation was performed with USHIO G15T8E bulb equipped with Kodacell filter which cuts of any shorter wavelengths than 290 nm (Skobowiat et al., 2013). The times [s] of UVB irradiation were calculated based on formula: Time=Dose/Intensity, [Jcm−2/Wcm−2]. Doses of UVB irradiation included Ctr (sham) = 0 mJ/cm2, 50 mJ/cm2 and 200 mJ/cm2, and their absolute and erythemic values are listed in Table 1. The UV dosimetry was performed by Dr. R.M. Sayre from the Rapid Precision Testing Laboratories, Cordova, TN with the use of a scanning double monochromater spectroradiometer (model OL 756; Optronic Laboratories, Orlando, FL) as described in (Skobowiat et al., 2011; Skobowiat et al., 2013). After irradiation, all skin parts were punched (6mm) to obtain 28 punches from each skin part. Seven punches per well were seated and 2 ml of Williams E medium (Sigma, St. Louis, MO) was added which was corticosterone- and serum-free but supplemented with 5μg/ml insulin and 1% Antibiotic-antimycotic solution. The skin samples were cultured in 6 well plates for 6, 12, 24 and 48 h, at 5 % CO2, 37 C. After defined incubation times, the 1.5 mL of medium and 2 skin punches for RNA, 3 for ELISA, and 2 for IHC were collected and frozen separately in −80 °C (see Fig. 1 for experimental layout).
Table 1.
Doses of UVB waveband administered in this study. Kodacel filter, which cuts off any UVC, was applied.
| Waveband | Control (Sham-treated) mJ/cm2 | Absolute Doses mJ/cm2 | Erythemic Doses SED |
|---|---|---|---|
| UVB | 0.0 | 50 | 1.3 |
| 200 | 5 |
Fig. 1.
Experiment design layout.
2.3. Real time RT-PCR (RT-PCR)
Total RNA was extracted with high Pure RNA Tissue Kit according to the manufacturer instructions (Roche, Indianapolis, IN). One microgram of total RNA was reverse transcribed into cDNA with high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Primers used for PCR amplification are listed in Table 2. The PCR reactions were performed in triplicate with SYBR Green I Master Mix (Roche, Manheim, Germany). The data were collected on the Light Cycler 480 from Roche. The amount of amplified product for each gene was compared to that for reference gene (β-actin) using a comparative ΔΔCT method and presented as a fold change ± SD.
Table 2.
List of primers used for real time RT-PCR. Primers were designed with Roche software at https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp.
| Target Name | Gene Symbol | Forward sequence 5′-3′ | Reverse sequence 5′-3′ |
|---|---|---|---|
| Urocortin 1) | Ucn1 | cgcgctcctcttgctgttag | atggacagtggagggtcgtc |
| Proopiomelanocortin | Pomc | agtgccaggacctcacca | cagcgagaggtcgagtttg |
| Melanocortin receptor 22) | Mc2R | aaatgattctgctgcttccaa | tggtgtttgccgttgactta |
| Cholesterol side-chain cleavage enzyme | Cyp11a1 | aagtatggccccatttacagg | tggggtccacgatgtaaact |
| β-actin | β-actin | ctaaggccaaccgtgaaaag | accagaggcatacagggaca |
2.4. ELISA/EIA
Skin samples (3 punches from each skin part; 6 per animal) together with media were homogenized with T-PER® buffer (Thermo Sci., Rockford, IL) supplemented with protease inhibitor cocktail (10μl/1ml; Sigma, St. Louis, MO), by the use of homogenizer (Polytron PT-MR2100, Swiss) and stand for 30 min, 4 C and centrifuged at 12,000 g, 25 min, 4 C. Supernatants containing extracted proteins were normalized, i.e. adjusted to the same concentration (2 μg/μl of total protein) after calculation with BCA assay (Thermo Sci., Rockford, IL). ELISA/EIA was performed according to the manufacturer’s directions, respectively for ACTH (Alpco Imm., #21-ACTHU-E01, Salem, NH) and CORT (Enzo Life Sci., ADI-900–097, Plymouth Meeting, PA). ACTH and CORT OD’s were read with spectrometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA) and the concentrations were calculated from the 4 parametric standard curve with the software (SoftMax Pro, Molecular Devices, Sunnyvale, CA) and presented in pg/mL for ACTH and in ng/mL for CORT.
2.5. Immunohistochemistry
Skin samples (2 punches per skin; 4 per animal) were fixed in 4% buffered (pH = 7.4) paraformaldehyde for 4 h. After extensive rinsing, punches were submerged in 18 % sucrose, frozen and cut on cryostat (Leica 3050, Bannockburn, IL) with the technique described by Ralf Paus (Paus et al., 1999). Ten μm sections were mounted onto silanized slides (Dako, Carpinteria, CA), rinsed several times with PBS and subjected to a single immunofluorescence. Blocking was performed with 5% donkey serum, 0.1% BSA, 0.3% Triton X-100 diluted in PBS for 1 h, RT. Following extensive rinsing in PBS, the primary antibodies diluted in the same blocking solution were applied overnight, RT. Next day, tissues were rinsed and the biotinylated donkey anti-rabbit IγG were applied for 1 h. Following rinsing in PBS, the streptavidine–CY3 (red) fluorophore was applied for 50 min. Afterward rinsing, sections were submerged in fluorescent mounting medium (Dako, Carpinteria, CA) and topped with cover glass. Negative controls were performed the same way except applying the primary antibodies (omission) or with the use of serum from non-immunized rabbits (replacement). At least 6 sections per skin scrap, i.e. 12 per animal of each primary antibody were studied under fluorescent microscope (Leica, Digital DM4000B, Buffalo Grove, IL) equipped with the filter capable for visualization of λ excitation/emission 550/570 nm (red), and conjugated to a digital camera. Pictures were further analyzed for quantitative intensity of immunocomplexes (6 measurements per assessment) with the use of ImageJ software (National Health Institute). List of primary and secondary antibodies used are provided in Table 3.
Table 3.
List of primary, secondary antibodies and reagents used for IHC.
| Antigen | Host | Titer | Source |
|---|---|---|---|
| Urocortin | rabbit | 1:2,000 | Sigma-Aldrich, MO |
| β-END | rabbit | 1:500 | Dr. Allen, USA |
| P450scc | rabbit | 1:1,000 | Dr. Tuckey, Australia |
| Biotin. anti-rabbit IγG | donkey | 1:1,000 | Jackson ImmunoRes., West Grove, PA |
| Streptavidin-CY3 | – | 1:12,000 |
2.6. Statistics
Data are presented as means ± SD and were analyzed with Student’s t-test (for 2 groups) or (for more than 2 groups) with one-way ANOVA Dunnett’s multiple comparison post hoc test using Prism 4.00 (GraphPad Software, San Diego, CA). Statistically significant differences are denoted by * (t-test) and # (ANOVA); where p<0.5 (*,#), p<0.01 (**,##) and p<0.001 (***,###).
3. Results
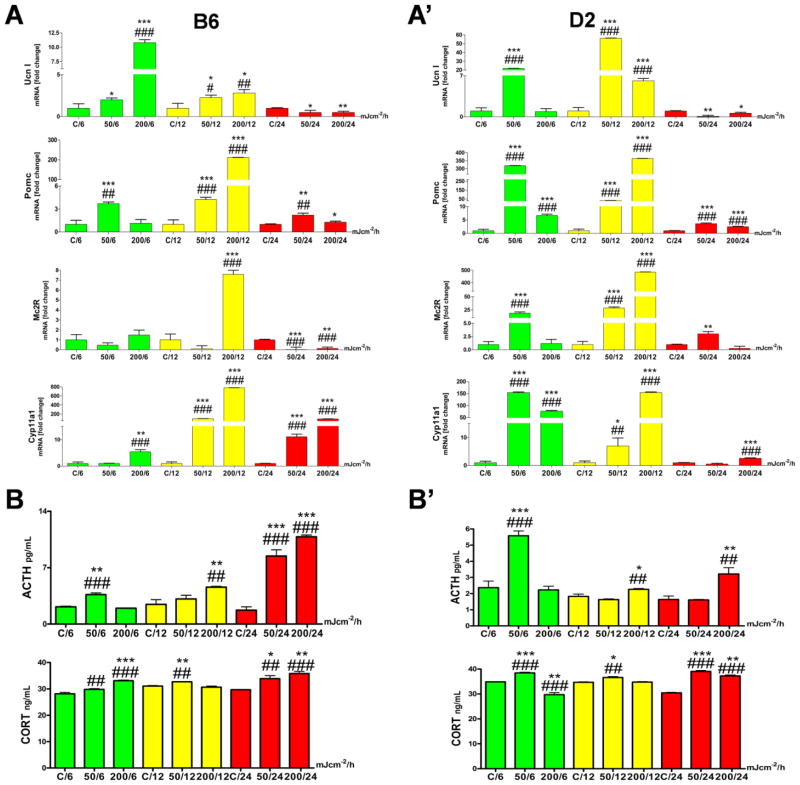
Ex-vivo UVB irradiation up-regulated the HPA axis elements in murine skin independently of the strain. Although the pattern was similar, the amplitude of changes and time of reactions differed between B6 and D2 (Figures 2 and 3).
Fig. 2.
Comparison of the UVB induced changes in the expression of cutaneous HPA axis elements between B6 and D2 mice.
Panel A. The qPCR analysis comparing the mRNA expression of Ucn1, Pomc, Mc2R and Cyp11a1 between control and UVB-irradiated skin histocultured for 6, 12 and 24 h is presented separately for B6 (A) and D2 (A′). UVB radiation stimulated genes involved in the cutaneous HPA axis expression independently of the mouse strain. D2 responded to UVB earlier and with higher amplitude than the B6 mice, which in turn, showed prolonged stress response to this noxious stimulus. All data are presented as a fold change vs. control at 6, 12 and 24 h time points, based on ΔΔct method between reference (β-actin) and target gene expression level. Statistically differences were obtained by comparison variability inside group means to between group means (Anova, marked as #) and with t-test (marked with *) where every UVB irradiated sample mean was compared to appropriate time point control mean (run in triplicate). Samples from 6 h time point are presented in green, from 12 h in yellow and 24 h in red.
Panel B. The immunoenzymatic assay presents the concentration of peptide (ACTH) and final steroid product (CORT) of HPA axis activation in murine skin. B) Results for B6, B′) for D2.
ACTH (ELISA, in pg/mL) and CORT (EIA, in ng/mL) concentrations measured in skin scraps extracted together with media after appropriate tissue culture incubation. UVB stimulated the cutaneous ACTH and CORT production in a similar pattern in both strains; however the calculated amount of ACTH was higher in B6 than in D2. Samples from 6 h time point are presented in green, from 12 h in yellow and 24 h in red. Statistical differences were calculated using t-test (marked with *) and Anova Dunnet’s test (marked with #), where p<0.05 (*,#), p<0.01 (**,##), and p<0.001 (***,###).
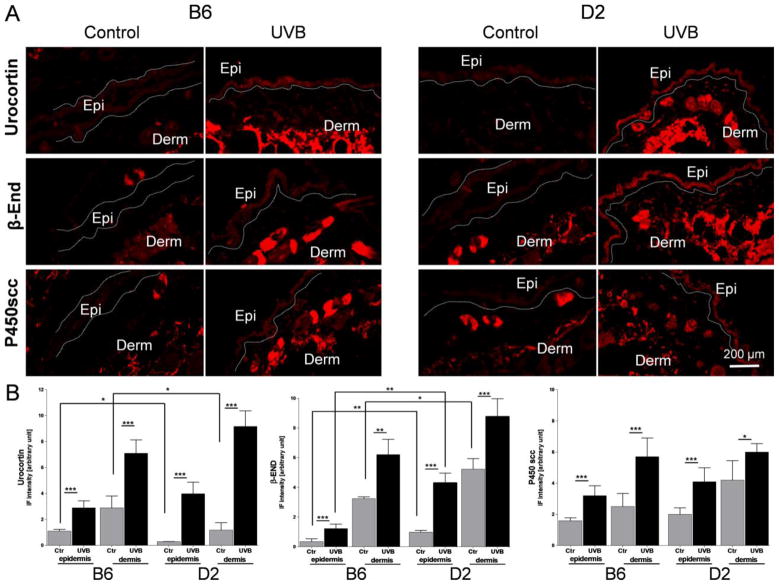
Fig. 3.
Panel A The immunohistochemical detection (immunofluorescent CY3 red fluorophore) of urocortin, β-endorphin (β-END) and P450 cholesterol side-chain cleavage enzyme (P450scc; CYP11A1) performed on cryosectioned skin 24 h after UVB radiation (400 mJ/cm2) with corresponding controls (for antibodies characteristics please see Table 3).
All antigens under investigation were higher expressed after UVB treatment compared to control (sham-treated) scraps. The distribution pattern of immunopositive signals was similar between B6 and D2. Ctr=Control, UVB=UVB irradiated skin, Epi=epidermis, Derm=dermis.
Panel B. Comparison of immunofluorescent signal (IF) intensity for Urocortin, β-END and P450scc between B6 and D2 measured separately in epidermis and dermis with Image J, NIH. 6 measurements per tissue area and 6 biopsies per antigen were investigated and the average results compared between conditions and strains are presented on the graphs. The average IF signal intensities were higher after UVB exposure compared to sham treated (Ctr) samples for all antigens under investigation. The constitutive Urocortin expression was slightly lower in D2 compared B6 while β-End IF signal levels were higher in D2 than B6. The statistical differences were measured with t-test and marked with *, where p<0.05 (*), p<0.01 (**), and p<0.001 (***).
First we examined the UVB induced changes in gene expression (Fig. 2). Ucn1 (urocortin) mRNA was up-regulated after all doses of UVB with a peak at 50 mJ/cm2 for D2 and 200 mJ/cm2 for B6 at incubation time of 6–12 h (Fig. 2A, A′). After 24 h the Ucn1 gene was slightly down-regulated compared to untreated controls. Pomc mRNA was augmented already at 6 h (50 mJ/cm2) with the peak after 12 h for the dose of 200 mJ/cm2 for both strains (Fig. 2A, A′). Longer incubation, 24 h, also enhanced mRNA expression, but the fold change was lower compared to the earlier (12 h) time point. There were tangible differences in Mc2R mRNA (receptor for ACTH) expression between B6 and D2. While D2 reacted in a dose-dependent manner in a fast (6 h) and long lasting mood (up to 24 h), the B6 responded only to one dose, i.e., 200 mJ/cm2, 12 h (Fig. 2A, A′). The gene Cyp11a1, encoding the cholesterol P450 side-chain cleavage enzyme (P450scc), a key mitochondrial enzyme which starts steroidogenesis, was stimulated earlier (6–12 h) and with higher amplitude in D2 compared to B6 where the delayed and prolonged (12–24 h) up-regulation of Cyp11a1 mRNA has been observed (Fig. 2A, A′).
Next, we measured endogenous and UVB-induced production of ACTH and CORT (Fig. 2). ACTH peptide level increased slightly after 50 mJ/cm2 of UVB irradiation, similarly in both strains, but the higher amounts were produced after dose of 200 mJ/cm2 followed 24 h of murine skin culture (Fig. 2B, B′). The production of final product of GC pathway in mouse – CORT followed the dose-dependent enhancement after UVB irradiation in B6 (Fig. 2B, B′). In D2, the dose of 50 mJ/cm2 is more effective than 200 mJ/cm2 in stimulation of CORT synthesis at 6 h after UVB exposure. However, after 24 h UVB-induced accumulation of CORT in culture media was similar in both mouse strains, being significantly higher than in controls (Fig. 2B, B′).
Finally, we applied IHC for in situ expression and relative expression of UCN1, β-END and P450SCC antigens. Thus, UVB radiation stimulated significantly expression of these antigens in both epidermal and dermal compartments including hair follicles with sebaceous glands and dermal mesenchymal cells in both strains (Fig. 3A). The relative quantification of immunopositive signal intensity surprisingly revealed that endogenous expression levels of UCN1 were significantly lower in D2 than B6 (respectively, in epidermis 0.29 ± 0.01 vs. 1.1 ± 0.14 and dermis (1.18 ± 0.57 vs. 2.89 ± 0.54) (Fig. 3B). Interestingly, UVB was more potent in stimulation of this antigen in D2 than in B6 as measured by differences in ratios of UVB/control (respectively, in epidermis 13.7 vs. 2.62 and dermis 7.77 vs. 2.45). Furthermore, levels of β-END expression were higher in D2 than B6 (respectively, in epidermis 0.98 ± 0.12 vs. 0.34 ± 0.18 and dermis 5.23 ± 0.71 vs. 3.24 ± 0.12) (Fig. 3B), while the ratios of UVB/control were similar in D2 and B6 (respectively, 4.38 vs. 3.49 in epidermis and 1.67 vs. 1.92 in dermis). The constitutive P450scc expression levels were similar in B6 and D2 in epidermis 1.59 ± 0.19 vs. 2.00 ± 0.42 and dermis 2.50 ± 0.84 vs. 4.20 ± 1.25, respectively (Fig. 3B). Also, there were no statistically significant differences in the UVB induced stimulation of P450scc between both mouse strains.
4. Discussion
In this paper we show for the first time that UVB can stimulate expression of local HPA axis including stimulation of Ucn1, Pomc and POMC-derived ACTH and β-END, Mc2R and P450scc expression with increased production of CORT in the mouse skin incubated ex-vivo. Furthermore, there was a different pattern of endogenous or UVB-induced expression of selected elements of the “cutaneous HPA axis” for B6 and D2 strains. These studies are in agreement with previous data showing that mammalian skin and skin cells cultured in vitro express POMC [reviewed in (Slominski et al., 2000c; Slominski et al., 2012)] and CRH (reviewed in (Slominski et al., 2001; Slominski et al., 2006)) signaling systems, and steroidogenic activities [reviewed in (Slominski et al., 1996c; Slominski et al., 2004c; Slominski et al., 2007; Slominski et al., 2013a)] substantiating the concept on cutaneous HPA axis acting as one of the coordinators of skin responses to stress (Slominski and Mihm, 1996; Slominski et al., 2007; Slominski et al., 2012). They also show that UCN1, POMC and POMC-derived peptides are expressed in D2, similarly to that in B6 skin reported previously (Slominski et al., 1992; Slominski et al., 1996b; Ermak and Slominski, 1997; Slominski et al., 1998; Slominski et al., 1999a; Mazurkiewicz et al., 2000; Slominski et al., 2000b; Slominski et al., 2004a), however, with noted differences, see discussion below. Importantly, they show, for the first time, that mouse skin does express steroidogenic activity, which is similar to human skin (Slominski et al., 1996c; Slominski et al., 1999b; Slominski et al., 2004c; Ito et al., 2005; Slominski et al., 2005b; Slominski et al., 2005c; Cirillo and Prime, 2011; Hannen et al., 2011; Skobowiat et al., 2011; Vukelic et al., 2011; Slominski et al., 2013a) and rat skin (Slominski et al., 2000a).
Noticeable, the D2 had lower endogenous expression of UCN1 than B6, however, it was more sensitive to UVB stimulatory effect both at gene and protein levels, e.g., gene induction in D2 was seen at lower dose (50 mJ/cm2) of UVB than in B6, the latter required higher dose for maximal stimulation (200 mJ/cm2). Similar results were reflected at the protein level, where UVB stimulated UCN1 expression at the higher level in D2 than in B6. It must be noted, that mouse skim, in contrast to human skin, does not express CRH gene, making UCN1 as the leading molecule of the cutaneous HPA axis trigger (Slominski et al., 2001; Slominski et al., 2004a). Accordingly, we failed to detect CRH mRNA in D2 (not shown), which is similar to B6 (Slominski et al., 1996b; Slominski et al., 2001). Thus, in mouse skin UCN1 or CRH (released by nerve endings) would activate CRH-R1 leading to increased Pomc gene expression, with subsequent mRNA processing, production of POMC precursor protein and its enzymatic cleavage with proconvertases (PC1/3; PC2) to yield ACTH and β-END peptides (scheme of processing of POMC is in Suppl. Fig. 1).
Since the skin was exposed to the UVB light, after dissection from killed animals, we conclude that the skin of D2 shows lowered threshold sensitivity in terms of UCN1 stimulation in comparison to B6, which is similar to lower threshold sensitivity in this species observed in opioid management (Eastwood and Phillips, 2012). Furthermore, on the level of gene expression, D2 responded more rapidly than B6 in Pomc, Mc2R and Cyp11a1 genes expression (Fig. 2A, A′). However, both strains reacted similarly in terms of general induction ACTH and CORT production with D2 showing nevertheless higher level of sensitivity at lower doses and shorter time of UVB exposure (Fig. 2B, B′). This shows qualitative similarity in response to UVB exposure with quantitative differences indicating higher sensitivity of D2 to induction of HPA axis parameters. It has to be noted, that expression of Pomc gene in mice fluctuates along the hair-cycle stage, reaching highest levels in anagen (Slominski et al., 1992; Slominski et al., 1998; Mazurkiewicz et al., 2000). In our experiments we used mice at telogen stage of hair cycle, where the Pomc expression is the lowest and restricted to the exon 3 (Ermak and Slominski, 1997). Therefore, changes observed after UVB radiation are considered as stress induced and independent of hair cycling. Furthermore, they are independent of the systemic responses, since the skin from killed animals, which was dissected from the body, was used for the experiments. The quantitative differences in stimulation of local HPA axis (Pomc, ACTH and CORT) are consistent with observed increased expression of UCN1 after UVB, which could act on CRH-R1 to activate the HPA mode (Slominski et al., 2012). However, the direct action on CRH-R2 that is independent of HPA axis may also be considered (Gerber and Bale, 2012). In addition, β-END expression showed striking differences between both strains with higher endogenous immunopositive signals in D2 than for B6, which might be related to a different behavioral reaction to stress between D2 and B6 (Paterson et al., 2010; Williams and Mulligan, 2012). We suggest that above differences in cutaneous opioid activities between both species would be related to genetically predetermined endogenous production of β-END. Therefore, endogenous and UVB-induced production of UCN1 and β-END may also affect pathways that are independent of the HPA axis but related to local activation of opioid peptides (Slominski et al., 2011; Slominski et al., 2012) and opioid receptors (Bigliardi et al., 2009).
Since both strains show different responses to cutaneous carcinogens and irritating factors (http://www.informatics.jax.org/faq/STRN_imgpop.shtml), the challenge for future research is to carefully dissect whether these differences are linked to differential activation of the local HPA axis. In addition, B6 and D2 strains, while showing different phenotypes and behavioral responses (Paterson et al., 2010; Andreux et al., 2012; Eastwood and Phillips, 2012; Williams and Mulligan, 2012), are also a source of B6 X D2 - derived isogenic but diverse strains of mice (BXD type) that are deposited in an open-access web service for systems genetics (www.genenetwork.org). Accordingly, linking different phenotypes with the differential local neuroendocrine responses will be a subject of our future studies based on the above BXD progeny. In this context, the significant differences in quantitative expression of the cutaneous HPA axis between B6 and D2 strains (both endogenous and UVB-induced) make this model a promising tool in dissecting genetic traits and networks linked to local regulation of UCN1, POMC and steroidogenic activity.
5. Conclusion
We demonstrated expression of different HPA axis elements, including UCN1, POMC-derived ACTH and β-END as well as P450scc and production of CORT in B6 and D2 murine skin. We expect that differences in endogenous or UVB-induced expression of the selected elements of local neuroendocrine pathways in skin are determined by the genetic background of these two mouse strains.
Supplementary Material
Highlights.
UVB radiation induces the “cutaneous HPA axis” independently of the mouse strain
UVB stimulates steroidogenesis in mouse skin independently of the mouse strain
Expression of “cutaneous HPA axis” differs between C57BL/6 and DBA/2J strains
DBA/2J appears is more sensitive in UVB induction of HPA elements than C57BL/6
UVB stimulated β-endorphin expression is higher in DBA/2J than in C57BL/6
Acknowledgments
The authors are indebted to Dr. R.M. Sayre from Rapid Precision Testing Laboratories, Cordova, TN, for performing the dosimetry of UVB radiation. This study was supported in part by grants from National Science Foundation (# IOS-0918934) and National Institutes of Health (#1R01AR056666–01A2) to AS.
Abbreviations
- HPA axis
hypothalamic-pituitary-adrenal axis
- B6
C57BL/6J
- D2
DBA/2J
- UVR
ultraviolet radiation
- CRH
corticotropin releasing hormone
- ACTH
adrenocorticotropin
- GC
glucocorticoids
- GR
glucocorticoid receptor
- β-END
β-endorphin
- POMC
proopiomelanocortin
- UCN1
urocortin
- COR
cortisol
- CORT
corticosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cezary Skobowiat, Email: cskobowi@uthsc.edu.
Reza Nejati, Email: mrnejati@yahoo.com.
Lu Lu, Email: lulu@uthsc.edu.
Robert W. Williams, Email: rwilliams@uthsc.edu.
References
- Andreux PA, Williams EG, Koutnikova H, Houtkooper RH, Champy MF, Henry H, Schoonjans K, Williams RW, Auwerx J. Systems genetics of metabolism: the use of the BXD murine reference panel for multiscalar integration of traits. Cell. 2012;150:1287–99. doi: 10.1016/j.cell.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DC, Lamoreux ML. The color loci of mice--a genetic century. Pigment Cell Res. 2003;16:333–44. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, Bigliardi-Qi M. Opioids and the skin--where do we stand? Exp Dermatol. 2009;18:424–30. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Bolognia J, Sodi S, Ichihashi M, Pawelek JM. UV light and MSH receptors. Ann N Y Acad Sci. 1999;885:100–16. doi: 10.1111/j.1749-6632.1999.tb08668.x. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- Eastwood EC, Phillips TJ. Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict Biol. 2012 doi: 10.1111/adb.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak G, Slominski A. Production of POMC, CRH-R1, MC1, and MC2 receptor mRNA and expression of tyrosinase gene in relation to hair cycle and dexamethasone treatment in the C57BL/6 mouse skin. J Invest Dermatol. 1997;108:160–5. doi: 10.1111/1523-1747.ep12332925. [DOI] [PubMed] [Google Scholar]
- Gerber AR, Bale TL. Antiinflammatory treatment ameliorates HPA stress axis dysfunction in a mouse model of stress sensitivity. Endocrinology. 2012;153:4830–7. doi: 10.1210/en.2012-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. 2011;404:62–7. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Tojo K, Inada Y, Takada Y, Sakamoto M, Lam M, Claycomb WC, Tajima N. Regulation of urocortin I and its related peptide urocortin II by inflammatory and oxidative stresses in HL-1 cardiomyocytes. J Mol Endocrinol. 2009;42:479–89. doi: 10.1677/JME-08-0151. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–4. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz JE, Corliss D, Slominski A. Spatiotemporal expression, distribution, and processing of POMC and POMC-derived peptides in murine skin. J Histochem Cytochem. 2000;48:905–14. doi: 10.1177/002215540004800703. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Iwunze M, Davis SF, Malekiani SA, Hanania T. Comparison of the predictive validity of the mirror chamber and elevated plus maze tests in mice. J Neurosci Methods. 2010;188:62–70. doi: 10.1016/j.jneumeth.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Song SC, Harenza JL, Lu L, Wang X, Williams RW, Miles MF, Morrow AL. Genetic analysis of the neurosteroid deoxycorticosterone and its relation to alcohol phenotypes: identification of QTLs and downstream gene regulation. PLoS One. 2011;6:e18405. doi: 10.1371/journal.pone.0018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsin Y, van Wijk D, Saravia FE, Oitzl MS, De Nicola AF, de Kloet ER. Adrenal hypersensitivity precedes chronic hypercorticism in streptozotocin-induced diabetes mice. Endocrinology. 2008;149:3531–9. doi: 10.1210/en.2007-1340. [DOI] [PubMed] [Google Scholar]
- Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–93. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996a;399:175–6. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Slominski A, Botchkareva NV, Botchkarev VA, Chakraborty A, Luger T, Uenalan M, Paus R. Hair cycle-dependent production of ACTH in mouse skin. Biochim Biophys Acta. 1998;1448:147–52. doi: 10.1016/s0167-4889(98)00124-4. [DOI] [PubMed] [Google Scholar]
- Slominski A, Botchkareva NV, Botchkarev VA, Chakraborty A, Luger T, Uenalan M, Paus R. ACTH production in C57BL/6 mouse skin. Ann N Y Acad Sci. 1999a;885:448–50. doi: 10.1111/j.1749-6632.1999.tb08709.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Chassalevris N, Mazurkiewicz J, Maurer M, Paus R. Murine skin as a target for melatonin bioregulation. Exp Dermatol. 1994;3:45–50. doi: 10.1111/j.1600-0625.1994.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochimica et biophysica acta. 1996b;1289:247–51. doi: 10.1016/0304-4165(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996c;81:2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- Slominski A, Gomez-Sanchez C, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochimica et biophysica acta. 2000a;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999b;455:364–6. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–51. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia. 1992;48:50–4. doi: 10.1007/BF01923606. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004a;145:941–50. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005a;146:1245–53. doi: 10.1210/en.2004-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab. 2000b;85:815–23. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004b;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000c;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. Journal of Steroid Biochemistry and Molecular Biology. 2013a doi: 10.1016/j.jsbmb.2013.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005b;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005c;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–48. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, RCT A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004c;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytej B, DJT, Theoharides CT, Rivier J. Skin stress response system: central role of CRF. Endocrine Reviews. 2013b doi: 10.1210/er.2012-1092. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, Szczesniewski A, Tobin DJ. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131:613–22. doi: 10.1038/jid.2010.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–75. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Agarwala R, Capra J, Chen Z, Church D, Ciobanu D, Li Z, Lu L, Mozhui K, Mulligan M, Nelson S, Pollard K, Taylor W, Thomason D, Williams R. High-throughput sequencing of the DBA/2J mouse genome. BMC Bioinformatics. 2010;11:O7. [Google Scholar]
- Williams RW, Mulligan MK. Genetic and molecular network analysis of behavior. Int Rev Neurobiol. 2012;104:135–57. doi: 10.1016/B978-0-12-398323-7.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–47. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.